In this issue of Blood, Iqbal et al created a novel mouse model with a strong expression of green fluorescence protein (GFP) in monocytes, tissue resident macrophages, and inflammatory macrophages, and may provide an important tool for future studies focusing on macrophage biology.1 Several transgenic mice with expression of fluorescent proteins in myeloid cells exist, among them the CCR2-RFP and the CX3CR1GFP mouse. However, both of these mice have several limitations: they are knock-in constructs under control of chemokine receptors with potential effects on monocyte mobilization from the bone marrow, recruitment to sites of inflammation, or survival.4,5 Alteration of chemokine receptor expression during macrophage differentiation may affect expression of fluorescent proteins and thus render macrophages nonfluorescent.
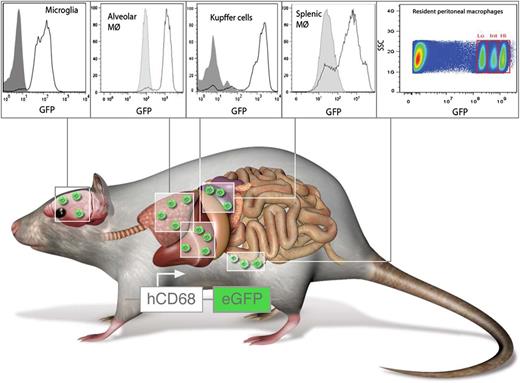
hCD68-GFP labels resident and inflammatory macrophages. Mice expressing GFP under control of the human CD68 promoter carry resident macrophages with high levels of GFP in the spleen, liver, lungs, brain, and peritoneum. In addition, circulating monocytes are GFP+ and retain high levels of GFP even after transmigration and differentiation toward macrophages. Panels from Figure 2 and Figure 5 in the article by Iqbal et al beginning on page e33. Professional illustration by Luk Cox, Somersault1824.
hCD68-GFP labels resident and inflammatory macrophages. Mice expressing GFP under control of the human CD68 promoter carry resident macrophages with high levels of GFP in the spleen, liver, lungs, brain, and peritoneum. In addition, circulating monocytes are GFP+ and retain high levels of GFP even after transmigration and differentiation toward macrophages. Panels from Figure 2 and Figure 5 in the article by Iqbal et al beginning on page e33. Professional illustration by Luk Cox, Somersault1824.
Here, the authors report a mouse expressing GFP under the control of the human CD68 promoter (see figure). The authors convincingly show that resident macrophages in the spleen, liver, brain, and lungs express high levels of GFP (see figure). Interestingly, they also identify 3 populations of resident peritoneal macrophages carrying different GFP levels. This raises the question that if these represent functional subpopulations, this may require further attention. In contrast to existing monocyte-reporter mice, the design of the transgenic hCD68-GFP mouse supposedly does not affect monocyte trafficking or survival. In addition, the authors provide experimental evidence that emigrated monocytes in hCD68-GFP mice retain high levels of GFP expression even after differentiation into macrophages; this was not the case in CX3CR1GFP mice. In conclusion, the hCD68-GFP mouse may be superior in studying several aspects of monocyte trafficking and macrophage behavior in vivo.
Recruitment and activation of macrophages are key aspects in many inflammatory diseases. In particular, circumstances of chronic inflammation visualization of macrophage origin, behavior, and activation might harbor the key to development of novel therapeutic approaches.6 During atherosclerosis—a chronic inflammation of the arterial vessel wall—blood monocytes are recruited to the inflamed vessel wall,7 where they give rise to inflammatory macrophages and foam cells. However, recent reports suggest that lesional macrophages might also arise from differentiated fetal macrophages that persist from early embryonic development and self-renewal throughout adulthood.8 Given recently established protocols for stable intravital visualization of inflammatory myeloid cell behavior in high resolution,9 the hCD68-GFP mouse might offer a powerful tool to further dissect aspects of macrophage origin and activation in atherosclerosis and similar chronic inflammatory processes. In addition, these mice might also afford direct visualization of macrophage egress from established atherosclerotic lesions.
Although it is not described in the study, the results suggest that the human CD68 promoter may be a good driver of Cre-recombinase expression to specifically delete floxed alleles in macrophages. The widely used Lysm-cre mouse is clearly not ideal for macrophage-specific deletion, and there are many reports of leaky or inefficient deletion. Thus the human CD68 promoter may be a better driver of macrophage-specific Cre expression than other currently available Cre lines. However, the problem with deriving myeloid-specific Cre-expressing mice using this expression cassette seems to be a transient expression of the hCD68 promoter in hematopoietic stem cells and progenitor cells that leads to deletion of floxed transgenes in nearly all hematopoietic cell lineages.10 To circumvent this issue, one would need to employ an inducible construct. However, given the shortage of macrophage-specific deletion strategies, this might be a worthwhile investment for future development.
Although the study by Iqbal et al does not present any particularly novel information on myeloid cell biology, it provides a fresh tool that clearly adds to the existing arsenal of transgenic mice and may hence be interesting to many investigators interested in myeloid cell origin, trafficking, differentiation, and fate. Widespread distribution and testing of this mouse line in studies on fate mapping, steady-state trafficking, and inflammation will shed light on the suitability and superiority of this strain over existing ones and likely help deliver exciting data in emerging questions of macrophage biology.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal