Key Points
Single-agent bortezomib produces durable hematologic responses and promising long-term overall survival in relapsed AL patients.
Once-weekly bortezomib is better tolerated and produces similar responses to twice-weekly bortezomib in relapsed AL patients.
Abstract
CAN2007 was a phase 1/2 study of once- and twice-weekly single-agent bortezomib in relapsed primary systemic amyloid light chain amyloidosis (AL) amyloidosis. Seventy patients were treated, including 18 and 34 patients at the maximum planned doses on the once- and twice-weekly schedules. This prespecified final analysis provides mature response and long-term outcomes data after 3-year additional follow-up since the last report. In the once-weekly 1.6 mg/m2 and twice-weekly 1.3 mg/m2 bortezomib groups, final hematologic response rates were 68.8% and 66.7%; 80% of patients in each group sustained their response for ≥1 year. One-year progression-free rates were 72.2% and 76.8%. Median overall survival (OS) was 62.1 months and not reached; 4-year OS rates were 75.0% and 63.0%. Low baseline difference in κ/λ free light-chain level was associated with higher hematologic complete response rates and longer OS. At data cutoff, 40 (57%) patients had received subsequent therapy, including 19 (27%) retreated with bortezomib, 11 (58%) of whom achieved complete or partial hematologic responses. Four patients received prolonged bortezomib for between 3.5 and 5.6 years, with no new safety concerns, highlighting the feasibility of long-term therapy. Single-agent bortezomib produced durable hematologic responses and promising long-term OS in relapsed AL amyloidosis. This trial was registered at www.clinicaltrials.gov as #NCT00298766.
Introduction
Primary systemic amyloid light chain amyloidosis (AL) is a protein misfolding disorder in which a clonal plasma cell dyscrasia results in excessive production of abnormal immunoglobulin light chains.1-4 Extracellular deposition and toxicity of these fibril-forming abnormal light chains can lead to end-organ damage, particularly in the kidneys and heart,1-4 and death. Suppression of the plasma cell dyscrasia and reduction or elimination of the production of amyloidogenic immunoglobulin light chains is the aim of AL treatment.1 Amyloid deposits can be resorbed and organ function restored on elimination of plasma cells secreting the abnormal light chains.1 Achievement of a hematologic response to therapy, particularly complete response (CR), is associated with improved organ function and prolonged survival.5-8
The plasma cell dyscrasia in AL is similar to that in multiple myeloma (MM).3,9 Thus, therapies that are effective in MM are typically used to treat AL.1 High-dose melphalan followed by stem cell transplantation (HDM-SCT) is highly effective in AL; however, many patients may be transplantation ineligible10,11 due to age, poor performance status, and multiple organ involvement. Despite improvements in the management of transplantation in AL patients in recent years, the observed transplantation-associated mortality is still higher in AL than MM.12 Therefore, alternative therapies are needed for transplantation-ineligible AL patients; such therapies may also benefit transplantation-eligible patients.
The proteasome inhibitor bortezomib is approved in the United States for the treatment of MM and for the treatment of mantle cell lymphoma after ≥1 prior therapy.13 It has been suggested that the pathology of AL makes cells highly sensitive to the effects of proteasome inhibition.4 Several retrospective analyses5,14-18 and case series studies19-22 have shown that bortezomib-based therapy is active in patients with previously untreated and relapsed AL. CAN2007 was the first prospective phase 1/2 study of single-agent bortezomib in relapsed AL. Phase 1 findings23,24 and primary phase 2 efficacy and safety results from the study after 10.2- to 37.7-month median follow-up across dose groups have been reported previously.25 Here we report long-term outcome data at study closure after 51.8-month median follow-up (median, 46.1-66.1 months across dose groups), and highlight a small subset of patients who received prolonged bortezomib treatment of up to 66.8 months (5.6 years).
Patients and methods
Patients
Eligibility criteria have been reported previously23,25 and are summarized in the supplemental Appendix available on the Blood Web site. In brief, patients ≥18 years of age with confirmed AL diagnosis, who had previously been treated and required further treatment of AL due to persistent clonal disease, were eligible. Review boards at all participating institutions approved the study, which was conducted according to the Declaration of Helsinki and International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided informed consent.
Study design
CAN2007 was a nonrandomized, noncomparative, phase 1/2 study of weekly (QW) and twice-weekly (BIW) single-agent bortezomib in patients with relapsed AL (NCT00298766). The study was conducted at 9 sites in Canada, France, Germany, Italy, Spain, and the United States between July 25, 2005, and March 9, 2009.23,25 Study design has been reported previously.23,25 During phase 1, patients sequentially received single-agent bortezomib at 0.7, 1.0, 1.3, or 1.6 mg/m2 QW and then at 0.7, 1.0, or 1.3 mg/m2 BIW.23 Patients on the QW schedule received bortezomib on days 1, 8, 15, and 22 in 35-day cycles. Patients on the BIW schedule received bortezomib on days 1, 4, 8, and 11 in 21-day cycles. As the maximum tolerated dose of bortezomib was not reached on either schedule, the prespecified maximum planned dose levels of 1.6 mg/m2 QW and 1.3 mg/m2 BIW were expanded during phase 2.25 The planned treatment duration was 8 cycles; prolonged treatment beyond 8 cycles was permitted for patients showing ongoing clinical benefit. Patients were followed every 6 weeks until disease progression (defined per criteria reported in Gertz et al 2005)26 and every 3 months for survival during long-term follow-up.
Objectives
The primary phase 2 objective was to evaluate the safety of QW and BIW single-agent bortezomib in relapsed AL. Secondary objectives included determining hematologic response rate (CR plus partial response [PR]) and duration of response (DOR). Exploratory objectives included assessing the rate of organ response, overall survival (OS), and the value of frequent free light chain (FLC) measurements during cycle 1 and their relationship to hematologic response. The specific objectives of this final analysis, conducted after data cutoff for study closure, were to determine final hematologic response rates, final DOR, time to hematologic disease progression, OS, and subsequent AL therapy.
Assessments
Hematologic responses were determined during the rest period of each cycle according to established consensus criteria,26 as previously reported.25 Investigator-assessed responses were confirmed by an Independent Data Monitoring Committee and were based on central laboratory efficacy measurements. Best confirmed hematologic response was defined as the best hematologic response (CR or PR) achieved between cycle 1 and the time of first documented disease progression or, in the absence of progressive disease, the last response assessment confirmed by evaluation of the same category of response or better at the next visit. The overall hematologic response rate was defined as the rate of CR+PR (including unconfirmed responses) among patients with any postbaseline hematologic response assessment. Serum FLC assay and determination of the absolute value of the difference in κ and λ FLC levels (dFLC) were performed at screening, prior to each bortezomib administration within and during the rest period of cycle 1, during the rest period of each subsequent cycle, at the end-of-treatment (EOT) visit, and every 6 weeks prior to disease progression. Adverse events (AEs) were monitored throughout the study and graded according to National Cancer Institute–Common Terminology Criteria for Adverse Events v3.0.
Statistical analysis
Definitions of analysis populations and details of statistical analyses performed in this study are provided in the supplemental Appendix.
Results
Patients
As previously reported,25 70 patients were enrolled across 7 dose groups: 18 received bortezomib 1.6 mg/m2 QW, 34 received bortezomib 1.3 mg/m2 BIW, and 18 received bortezomib at lower doses QW or BIW. Patient demographics and baseline disease characteristics were reported previously.25 Among all 70 patients, median age was 60.5 years (range, 38-80 years), 27 (39%) patients were ≥65 years of age, 39 (56%) were men, and 63 (90%) were white. Thirty-one (44%) patients had ≥3 organs involved, with 51 (73%), 39 (56%), and 20 (29%) having renal, cardiac, and gastrointestinal involvement, respectively. Forty-one (59%) patients had a neurologic history, and 24 (34%) had reported cardiac history or baseline cardiac condition, including cardiac arrhythmias (n = 14), heart failure (n = 13), angina pectoris, and prior myocardial infarction (each n = 1).
Forty (57%), 20 (29%), and 10 (14%) patients had received 1, 2, and ≥3 prior lines of therapy for amyloidosis, respectively. Overall, 67 (96%) patients had received prior melphalan, with 40 (57%) having previously undergone HDM-SCT; 57 (81%) had received prior glucocorticoids, and 26 (37%) had received prior lenalidomide or thalidomide.
Patient characteristics were generally similar across dose groups, with the exception of a higher median age and higher proportion of patients ≥65 years of age in the 1.3 mg/m2 BIW group25 and a higher proportion of patients with ≥3 organs involved, gastrointestinal involvement, cardiac history, neurologic history, and prior HDM-SCT in the 1.6 mg/m2 QW group.25
Disposition, treatment exposure, and safety
Table 1 summarizes patient disposition and treatment exposure at the final analysis (August 28, 2012). Patients in the 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose groups received a median (range) of 8 (1-39), 6 (1-57), and 8 (3-57) cycles of bortezomib, respectively. Nine (50%), 9 (26%), and 10 (56%) patients, respectively, had completed the planned 8 cycles of treatment, and 9 (50%), 25 (74%), and 8 (44%) patients, respectively, had discontinued treatment prior to completing the planned 8 cycles. Four (6%) patients were receiving ongoing treatment after having received 39 to 57 cycles. Patient flow through the study is summarized in supplemental Figure 1.
Patient disposition and treatment exposure
| Parameter . | Bortezomib dose groups . | ||
|---|---|---|---|
| 1.6 mg/m2 QW (n = 18) . | 1.3 mg/m2 BIW (n = 34) . | Lower doses QW/BIW (n = 18) . | |
| Median number of cycles received, n (range) | 8 (1-39) | 6 (1-57) | 8 (3-57) |
| Mean actual dose received/planned dose of bortezomib, % | 89 | 81 | 98 |
| Received ≥ 8 cycles, n (%) | 10 (56) | 11 (32) | 11 (61) |
| Completed 8 cycles, n (%) | 9 (50) | 9 (26) | 10 (56) |
| Discontinued treatment, n (%) | 9 (50) | 25 (74) | 8 (44) |
| Adverse events | 5 (28) | 10 (29) | 3 (17) |
| Patient choice | 3 (17) | 4 (12) | 2 (11) |
| Disease progression | 1 (6) | 3 (9) | 0 |
| Clinical deterioration* | 0 | 2 (6) | 3 (17) |
| Death | 0 | 2 (6) | 0 |
| Other causes† | 0 | 4 (12) | 0 |
| Ongoing on treatment, n | 1 | 1 | 2‡ |
| Alive, n (%) | 11 (61) | 22 (65) | 9 (50) |
| Parameter . | Bortezomib dose groups . | ||
|---|---|---|---|
| 1.6 mg/m2 QW (n = 18) . | 1.3 mg/m2 BIW (n = 34) . | Lower doses QW/BIW (n = 18) . | |
| Median number of cycles received, n (range) | 8 (1-39) | 6 (1-57) | 8 (3-57) |
| Mean actual dose received/planned dose of bortezomib, % | 89 | 81 | 98 |
| Received ≥ 8 cycles, n (%) | 10 (56) | 11 (32) | 11 (61) |
| Completed 8 cycles, n (%) | 9 (50) | 9 (26) | 10 (56) |
| Discontinued treatment, n (%) | 9 (50) | 25 (74) | 8 (44) |
| Adverse events | 5 (28) | 10 (29) | 3 (17) |
| Patient choice | 3 (17) | 4 (12) | 2 (11) |
| Disease progression | 1 (6) | 3 (9) | 0 |
| Clinical deterioration* | 0 | 2 (6) | 3 (17) |
| Death | 0 | 2 (6) | 0 |
| Other causes† | 0 | 4 (12) | 0 |
| Ongoing on treatment, n | 1 | 1 | 2‡ |
| Alive, n (%) | 11 (61) | 22 (65) | 9 (50) |
KPS, Karnofsky performance status.
Clinical deterioration includes deterioration in KPS and organ function and deterioration in patients’ overall condition.
Other causes of discontinuation were attainment of CR after cycle 2, study drug intolerance, clinical progression on maintenance (treatment cycle 11), and plateaued hematologic response in cycle 4 (each n = 1).
One patient has progressed since data cutoff and discontinued treatment.
Twenty-eight (40%) patients had died at the final analysis compared with 11 (16%) in the previous report.25 Cause of death among the 17 additional patients who died since the previous report was progressive disease (n = 13), and pneumonia, infection, respiratory failure leading to cardiorespiratory arrest, and sudden cardiac death (each n = 1). Detailed phase 2 safety information was reported previously.25 Nervous system disorder AEs were comparable between the 41 patients with a neurologic history and the 29 without a history (supplemental Table 1). No substantive changes in the previously reported safety profile had occurred, as all but 4 patients receiving long-term bortezomib had completed therapy at the time of the prior report. Grade 3/4 AEs in these long-term exposure patients are detailed below.
Response to treatment
Table 2 summarizes hematologic response and outcomes in the 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose groups. Overall best confirmed hematologic response rates (CR+PR) were 68.8%, 66.7%, and 38.9% for the 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose groups, respectively. Overall response rates in the long-term extension phase did not change compared with the previously published results25 ; however, 1 patient in the lower-dose group who had previously achieved PR upgraded to CR. Eighty percent, 80.0%, and 83.3% of responders in the 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose groups, respectively, sustained their response for ≥1 year.
Hematologic response, disease progression, and overall survival
| Parameter . | Bortezomib dose groups . | ||
|---|---|---|---|
| 1.6 mg/m2 QW . | 1.3 mg/m2 BIW . | Lower doses QW/BIW . | |
| Hematologic response | n = 16 | n = 33 | n = 18 |
| Best confirmed hematologic response rate (CR+PR), n (%) | 11 (68.8) | 22 (66.7) | 7 (38.9) |
| CR rate, n (%) | 6 (37.5) | 8 (24.2) | 3 (16.7) |
| Median time to hematologic response, months (range) | n = 11 | n = 22 | n = 7 |
| To first response | 2.1 (0.9-6.9) | 0.7 (0.3-4.0) | 1.2 (0.6-4.8) |
| To best confirmed response | 3.2 (0.9-7.2) | 1.2 (0.3-7.6) | 1.2 (0.6-39.6) |
| 1-year DOR rate, %* | 80.0 | 80.0 | 83.3 |
| Hematologic disease progression | |||
| Median follow-up, months (range) | 21.6 (0-35) | 11.3 (0-18) | 9.9 (2-49) |
| Patients progressing, n | 4 | 5 | 4 |
| 1-year hematologic disease progression-free rate, % | 72.2 | 76.8 | 88.5 |
| OS | |||
| Median follow-up, months (range) | 51.8 (1-68) | 46.1 (1-61) | 66.1 (2-80) |
| Deaths, n | 7 | 12 | 9 |
| Median OS, months (95% CI) | 62.1 (50.1-NE) | NE (30.2-NE) | 63.2 (45.4-NE) |
| 4-year OS rate, % | 75.0 | 63.0 | 69.7 |
| Parameter . | Bortezomib dose groups . | ||
|---|---|---|---|
| 1.6 mg/m2 QW . | 1.3 mg/m2 BIW . | Lower doses QW/BIW . | |
| Hematologic response | n = 16 | n = 33 | n = 18 |
| Best confirmed hematologic response rate (CR+PR), n (%) | 11 (68.8) | 22 (66.7) | 7 (38.9) |
| CR rate, n (%) | 6 (37.5) | 8 (24.2) | 3 (16.7) |
| Median time to hematologic response, months (range) | n = 11 | n = 22 | n = 7 |
| To first response | 2.1 (0.9-6.9) | 0.7 (0.3-4.0) | 1.2 (0.6-4.8) |
| To best confirmed response | 3.2 (0.9-7.2) | 1.2 (0.3-7.6) | 1.2 (0.6-39.6) |
| 1-year DOR rate, %* | 80.0 | 80.0 | 83.3 |
| Hematologic disease progression | |||
| Median follow-up, months (range) | 21.6 (0-35) | 11.3 (0-18) | 9.9 (2-49) |
| Patients progressing, n | 4 | 5 | 4 |
| 1-year hematologic disease progression-free rate, % | 72.2 | 76.8 | 88.5 |
| OS | |||
| Median follow-up, months (range) | 51.8 (1-68) | 46.1 (1-61) | 66.1 (2-80) |
| Deaths, n | 7 | 12 | 9 |
| Median OS, months (95% CI) | 62.1 (50.1-NE) | NE (30.2-NE) | 63.2 (45.4-NE) |
| 4-year OS rate, % | 75.0 | 63.0 | 69.7 |
NE, not estimable.
Based on Kaplan-Meier analysis.
The relationship between baseline dFLC and hematologic response was explored in response-evaluable patients. Overall response rates/CR rates in patients with high vs low baseline dFLC (>124.2 vs ≤124.2 mg/L, cutoff per the median baseline dFLC) after cycles 1, 2, and 4, and at EOT were 39%/3% vs 40%/9%, 50%/8% vs 52%/18%, 70%/5% vs 54%/21%, and 43%/18% vs 58%/32%, respectively (Table 3). Similar results were obtained using baseline dFLC cutoffs of 180 (previously validated to be of prognostic importance in AL)27 and 50 mg/L (per the definition of measurable disease in AL)28 (supplemental Table 2).
Relationship between baseline dFLC and change in dFLC during cycle 1 and hematologic response rates after 1, 2, and 4 cycles and at the end of treatment
| Hematologic response rates . | High baseline dFLC* (>124.2 mg/L) . | Low baseline dFLC* (≤124.2 mg/L) . | High dFLC decrease during cycle 1† (>59%) . | Low dFLC decrease during cycle 1† (≤59%) . |
|---|---|---|---|---|
| After cycle 1 | ||||
| Patients evaluable, nঠ| 31 | 35 | 32 | 33 |
| Hematologic response rate, n (%) | 12 (39) | 14 (40) | 22 (69) | 4 (12) |
| CR | 1 (3) | 3 (9) | 3 (9) | 1 (3) |
| PR | 11 (35) | 11 (31) | 19 (59) | 3 (9) |
| After cycle 2 | ||||
| Patients evaluable, nঠ| 26 | 33 | 29 | 30 |
| Hematologic response rate, n (%) | 13 (50) | 17 (52) | 23 (79) | 7 (23) |
| CR | 2 (8) | 6 (18) | 6 (21) | 2 (7) |
| PR | 11 (42) | 11 (33) | 17 (59) | 5 (17) |
| After cycle 4 | ||||
| Patients evaluable, nঠ| 20 | 28 | 23 | 25 |
| Hematologic response rate, n (%) | 14 (70) | 15 (54) | 20 (87) | 9 (36) |
| CR | 1 (5) | 6 (21) | 4 (17) | 3 (12) |
| PR | 13 (65) | 9 (32) | 16 (70) | 6 (24) |
| End of treatment | ||||
| Patients evaluable, nঠ| 28 | 31 | 27 | 31 |
| Hematologic response rate, n (%) | 12 (43) | 18 (58) | 21 (78) | 9 (29) |
| CR | 5 (18) | 10 (32) | 11 (41) | 4 (13) |
| PR | 7 (25) | 8 (26) | 10 (37) | 5 (16) |
| Hematologic response rates . | High baseline dFLC* (>124.2 mg/L) . | Low baseline dFLC* (≤124.2 mg/L) . | High dFLC decrease during cycle 1† (>59%) . | Low dFLC decrease during cycle 1† (≤59%) . |
|---|---|---|---|---|
| After cycle 1 | ||||
| Patients evaluable, nঠ| 31 | 35 | 32 | 33 |
| Hematologic response rate, n (%) | 12 (39) | 14 (40) | 22 (69) | 4 (12) |
| CR | 1 (3) | 3 (9) | 3 (9) | 1 (3) |
| PR | 11 (35) | 11 (31) | 19 (59) | 3 (9) |
| After cycle 2 | ||||
| Patients evaluable, nঠ| 26 | 33 | 29 | 30 |
| Hematologic response rate, n (%) | 13 (50) | 17 (52) | 23 (79) | 7 (23) |
| CR | 2 (8) | 6 (18) | 6 (21) | 2 (7) |
| PR | 11 (42) | 11 (33) | 17 (59) | 5 (17) |
| After cycle 4 | ||||
| Patients evaluable, nঠ| 20 | 28 | 23 | 25 |
| Hematologic response rate, n (%) | 14 (70) | 15 (54) | 20 (87) | 9 (36) |
| CR | 1 (5) | 6 (21) | 4 (17) | 3 (12) |
| PR | 13 (65) | 9 (32) | 16 (70) | 6 (24) |
| End of treatment | ||||
| Patients evaluable, nঠ| 28 | 31 | 27 | 31 |
| Hematologic response rate, n (%) | 12 (43) | 18 (58) | 21 (78) | 9 (29) |
| CR | 5 (18) | 10 (32) | 11 (41) | 4 (13) |
| PR | 7 (25) | 8 (26) | 10 (37) | 5 (16) |
Groups were defined based on a median baseline dFLC of 124.2 mg/L.
Groups were defined based on a median percentage decrease in dFLC from baseline to the last visit of cycle 1 of 59%.
For the high vs low baseline dFLC comparison, evaluable responses available for 66 patients after cycle 1, 59 patients after cycle 2, 48 patients after cycle 4, and 59 patients at the end of treatment.
For the high vs low dFLC decrease comparison, evaluable responses available for 65 patients after cycle 1, 59 patients after cycle 2, 48 patients after cycle 4, and 58 patients at the end of treatment.
The relationship between percentage dFLC change from baseline to the last visit of cycle 1 and hematologic response was also assessed. Overall response rates/CR rates in patients with high vs low percentage decreases in dFLC during cycle 1 (>59% vs ≤59%, cutoff per the median dFLC decrease during cycle 1) after cycles 1, 2, and 4 and at EOT were 69%/9% vs 12%/3%, 79%/21% vs 23%/7%, 87%/17% vs 36%/12%, and 78%/41% vs 29%/13%, respectively (Table 3). Similar results were obtained when a cutoff of 50% decrease in dFLC during cycle 1 was applied (50% dFLC reduction being the definition of a PR according to recently updated AL response criteria)29 (supplemental Table 3).
Hematologic disease progression and overall survival
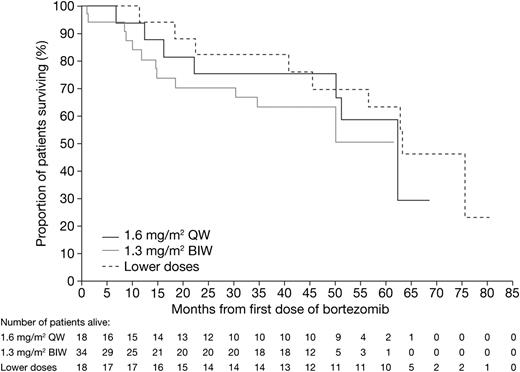
Time to hematologic disease progression and OS are summarized in Table 2. One-year hematologic disease progression-free rates in the 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose groups were 72.2%, 76.8%, and 88.5%, respectively. After a 51.8-month median follow-up for all patients, median OS was 62.7 months, and the 4-year OS rate was 67.3%. Median OS was 62.1 months, not reached, and 63.2 months in the 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose groups, respectively. Respective 4-year OS rates were 75.0%, 63.0%, and 69.7%. Kaplan-Meier distribution curves for OS by dose group are shown in Figure 1.
Kaplan-Meier analysis of overall survival in the bortezomib 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose QW/BIW groups.
Kaplan-Meier analysis of overall survival in the bortezomib 1.6 mg/m2 QW, 1.3 mg/m2 BIW, and lower-dose QW/BIW groups.
As with hematologic response, the relationship between baseline dFLC and change in dFLC from baseline to cycle 1 with OS was explored. In patients subgrouped according to a median baseline dFLC of 124.2 mg/L, high baseline dFLC was associated with inferior OS (median 50.1 vs 63.2 months with low baseline dFLC ≤ 124.2 mg/L, P = .0047; Figure 2A). Significantly shorter OS was also observed in patients with high baseline dFLC according to a cutoff of either 180 (median 45.4 vs 63.2 months with low baseline dFLC ≤ 180 mg/L, P = .0006; supplemental Figure 2A) or 50 mg/L (median 50.1 months vs not reached with low baseline dFLC ≤ 50 mg/L, P = .0165; supplemental Figure 2B). In contrast, the magnitude of the change in dFLC from baseline to end of cycle 1 did not appear to correlate with OS (median 56.6 vs 62.7 months with high [>59%] vs low [≤59%] dFLC decrease, P = .9856; Figure 2B). Similarly, no correlation with OS was observed when a cutoff of 50% decrease in dFLC from baseline to end of cycle 1 was applied (median 56.6 vs 62.7 months for patients with >50% vs ≤50% change in dFLC, respectively, P = .7158; supplemental Figure 3).
Kaplan-Meier analysis of OS. Overall survival was analyzed in patients grouped according to (A) high (>124.2 mg/L) vs low (≤124.2 mg/L) baseline dFLC or (B) high (>59%) vs low (≤59%) percentage decrease in dFLC during cycle 1. Cutoffs based on median baseline dFLC and median percentage dFLC decrease from baseline to the last visit of cycle 1, respectively.
Kaplan-Meier analysis of OS. Overall survival was analyzed in patients grouped according to (A) high (>124.2 mg/L) vs low (≤124.2 mg/L) baseline dFLC or (B) high (>59%) vs low (≤59%) percentage decrease in dFLC during cycle 1. Cutoffs based on median baseline dFLC and median percentage dFLC decrease from baseline to the last visit of cycle 1, respectively.
As baseline dFLC level appeared to predict for both hematologic CR rate and OS, the relationship between achievement of hematologic CR and OS was explored in a landmark analysis from EOT. There was a trend for longer OS in patients who achieved a hematologic CR vs <CR by EOT (median not reached vs 54.5 months; P = .134; supplemental Figure 4).
Subsequent therapy for AL
At data cutoff, 40 (57%) patients had received subsequent therapy for AL, and 6 (9%) had died due to disease progression prior to receiving subsequent therapy. Twenty-four (34%) patients remained alive and had not received subsequent therapy. In the 1.6 mg/m2 QW group, 7 (39%) patients had received subsequent therapy, 2 (11%) had died prior to subsequent therapy, and the median time to subsequent therapy/death was 50.1 months (95% confidence interval [CI], 11.4-not estimable). In the 1.3 mg/m2 BIW group, 21 (62%) patients had received subsequent therapy, 2 (6%) had died prior to subsequent therapy, and the median time to subsequent therapy/death was 17.2 months (95% CI, 12.7-30.4). In the lower doses group, 12 (67%) patients had received subsequent therapy, 2 (11%) had died prior to subsequent therapy, and the median time to subsequent therapy/death was 29.0 months (95% CI, 13.9-45.4).
Subsequent AL therapies received are summarized in Table 4. The most common agents included dexamethasone (n = 28, 40%), bortezomib (n = 19, 27%), lenalidomide (n = 18, 26%), cyclophosphamide (n = 9, 13%), melphalan (n = 8, 11%), and thalidomide (n = 7, 10%). Of 19 patients who received bortezomib-based retreatment, 11 achieved a hematologic CR (n = 6) or PR (n = 5), giving an overall hematologic response rate to bortezomib retreatment of 58%. Responses to initial bortezomib and to bortezomib retreatment are summarized in supplemental Table 4.
Subsequent therapies for AL amyloidosis
| Subsequent therapy for AL, n (%)*† . | Bortezomib dose groups . | Total (n = 70) . | ||
|---|---|---|---|---|
| 1.6 mg/m2 QW (n = 18) . | 1.3 mg/m2 BIW (n = 34) . | Lower doses QW/BIW (n = 18) . | ||
| Any subsequent therapy for AL | 7 (39) | 21 (62) | 12 (67) | 40 (57) |
| Median (95% CI) time to subsequent therapy, months | 50.1 (11.4-NE) | 17.2 (12.7-30.4) | 29.0 (13.9-45.4) | 22.7 (16.9-40.6) |
| Agents received in any subsequent line | ||||
| Dexamethasone | 6 (33) | 13 (38) | 9 (50) | 28 (40) |
| Bortezomib | 5 (28) | 10 (29) | 4 (22) | 19 (27) |
| Lenalidomide | 2 (11) | 9 (26) | 7 (39) | 18 (26) |
| Cyclophosphamide | 4 (22) | 3 (9) | 2 (11) | 9 (13) |
| Melphalan | 2 (11) | 3 (9) | 3 (17) | 8 (11) |
| Thalidomide | 3 (17) | 1 (3) | 3 (17) | 7 (10) |
| Prednisone | 1 (6) | 3 (9) | 0 | 4 (6) |
| Investigational drug | 0 | 2 (6) | 1 (6) | 3 (4) |
| Bendamustine | 1 (6) | 1 (3) | 0 | 2 (3) |
| Cisplatin | 1 (6) | 1 (3) | 0 | 2 (3) |
| Doxorubicin | 1 (6) | 1 (3) | 0 | 2 (3) |
| Etoposide | 1 (6) | 1 (3) | 0 | 2 (3) |
| HDM-SCT | 0 | 0 | 2 (11)‡ | 2 (3) |
| Subsequent therapy for AL, n (%)*† . | Bortezomib dose groups . | Total (n = 70) . | ||
|---|---|---|---|---|
| 1.6 mg/m2 QW (n = 18) . | 1.3 mg/m2 BIW (n = 34) . | Lower doses QW/BIW (n = 18) . | ||
| Any subsequent therapy for AL | 7 (39) | 21 (62) | 12 (67) | 40 (57) |
| Median (95% CI) time to subsequent therapy, months | 50.1 (11.4-NE) | 17.2 (12.7-30.4) | 29.0 (13.9-45.4) | 22.7 (16.9-40.6) |
| Agents received in any subsequent line | ||||
| Dexamethasone | 6 (33) | 13 (38) | 9 (50) | 28 (40) |
| Bortezomib | 5 (28) | 10 (29) | 4 (22) | 19 (27) |
| Lenalidomide | 2 (11) | 9 (26) | 7 (39) | 18 (26) |
| Cyclophosphamide | 4 (22) | 3 (9) | 2 (11) | 9 (13) |
| Melphalan | 2 (11) | 3 (9) | 3 (17) | 8 (11) |
| Thalidomide | 3 (17) | 1 (3) | 3 (17) | 7 (10) |
| Prednisone | 1 (6) | 3 (9) | 0 | 4 (6) |
| Investigational drug | 0 | 2 (6) | 1 (6) | 3 (4) |
| Bendamustine | 1 (6) | 1 (3) | 0 | 2 (3) |
| Cisplatin | 1 (6) | 1 (3) | 0 | 2 (3) |
| Doxorubicin | 1 (6) | 1 (3) | 0 | 2 (3) |
| Etoposide | 1 (6) | 1 (3) | 0 | 2 (3) |
| HDM-SCT | 0 | 0 | 2 (11)‡ | 2 (3) |
Therapies listed are not mutually exclusive; patients could have received >1 agent or combination of agents.
27 patients with subsequent therapy had discontinued initial bortezomib treatment in CAN2007 prior to disease progression.
Patients received their first HDM-SCT as a subsequent therapy.
Relationship between organ response and time to subsequent therapy and OS
The relationship between hematologic response and organ response was previously demonstrated in the primary analysis of the CAN2007 study.25 The proportion of patients achieving an organ response was higher in patients who achieved a hematologic response vs those who did not (32% vs 13%). In the present long-term follow-up analysis of CAN2007, the relationship between organ response and time to subsequent therapy and OS was explored. As shown in supplemental Figure 5A-B, median time to subsequent therapy (36 vs 26 months, P = .4078) and median OS (not reached vs 46 months, P = .04) appeared longer for patients who achieved an organ response compared with those who did not.
Long-term bortezomib exposure
In 1 center, 4 patients (3 with clinical benefit but no CR at cycle 8) received long-term bortezomib for between 3.5 and 5.6 years, including 1 patient each in the 1.6 mg/m2 QW and 1.3 mg/m2 BIW groups and 2 patients in the lower-dose group, who were treated at 1.3 mg/m2 QW and 0.7 mg/m2 BIW, respectively. Disease/treatment information for these patients is summarized in Table 5. At the final analysis, all 4 patients remained progression-free and were ongoing with bortezomib. Patient 2 progressed after final data cutoff and is now in remission on alternative therapy. There were no new safety concerns with extended bortezomib treatment, and no grade 3/4 peripheral neuropathy or second primary malignancies.
Disease and treatment information for the 4 patients who received long-term treatment with bortezomib in CAN2007
| Characteristic/parameter . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Gender | Male | Male | Female | Female |
| Age at study entry, years | 53 | 53 | 55 | 54 |
| Dose level, mg/m2 | 1.3 QW | 0.7 BIW | 1.6 QW | 1.3 BIW |
| KPS | 90 | 90 | 100 | 90 |
| AL type | IgA λ | IgG κ | IgG λ | λ light chain |
| Time since diagnosis, months | 16.0 | 38.0 | 14.0 | 44.0 |
| Organ involvement | 2: cardiac, renal | 1: GI* | 2: GI, other sites | 2: cardiac, GI |
| Prior therapy (best response achieved) | Melphalan plus ASCT (PR) | ASCT (PR) | ASCT (SD) | Cardiac allograft then ASCT (PR) |
| Cycles of bortezomib | 57 | 47 | 39 | 57 |
| Months/years on bortezomib | 66.8/5.6 | 52.0/4.3 | 44.7/3.7 | 41.4/3.5 |
| Planned cumulative dose, mg/m2 | 296.4 | 131.6 | 249.6 | 296.4 |
| Cumulative dose received, mg/m2 | 190.5 | 77.7 | 167.7 | 173.7 |
| % cumulative/planned dose | 64.3 | 59.0 | 67.2 | 58.6 |
| Dose intensity, mg/mg2/cycle | 3.3 | 1.7 | 4.3 | 3.0 |
| Grade 3/4 AEs, dose modifications | No grade 3/4 AEs | No grade 3/4 AEs | Grade 3 paralytic ileus, cycle 8, dose reduced to 1.3 then 1.0 mg/m2 | Grade 3 vasculitis, cycle 2 (dose reduced to 1.0 mg/m2, cycle 3); grade 3 volvulus, cycle 16 (dose reduced to 0.7 mg/m2); other grade 3 AEs: pneumonia, hemoptysis, and C difficile diarrhea |
| Adjusted to 1.3 mg/m2 Q2W, cycle 18, due to other reason | Adjusted to 0.7 mg/m2 QW, cycle 10, due to grade 2 pain in extremity | |||
| Hematologic response | CR | SD | PR | PR |
| Organ responses | Renal: response | Renal: response | Renal: NC | Renal: response |
| Cardiac: NC | Cardiac: NC | Cardiac: NC | Cardiac: NC | |
| Status | Ongoing, progression-free | Progressed, in remission on lenalidomide/dexamethasone | Ongoing, progression-free | Ongoing, progression-free |
| Characteristic/parameter . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Gender | Male | Male | Female | Female |
| Age at study entry, years | 53 | 53 | 55 | 54 |
| Dose level, mg/m2 | 1.3 QW | 0.7 BIW | 1.6 QW | 1.3 BIW |
| KPS | 90 | 90 | 100 | 90 |
| AL type | IgA λ | IgG κ | IgG λ | λ light chain |
| Time since diagnosis, months | 16.0 | 38.0 | 14.0 | 44.0 |
| Organ involvement | 2: cardiac, renal | 1: GI* | 2: GI, other sites | 2: cardiac, GI |
| Prior therapy (best response achieved) | Melphalan plus ASCT (PR) | ASCT (PR) | ASCT (SD) | Cardiac allograft then ASCT (PR) |
| Cycles of bortezomib | 57 | 47 | 39 | 57 |
| Months/years on bortezomib | 66.8/5.6 | 52.0/4.3 | 44.7/3.7 | 41.4/3.5 |
| Planned cumulative dose, mg/m2 | 296.4 | 131.6 | 249.6 | 296.4 |
| Cumulative dose received, mg/m2 | 190.5 | 77.7 | 167.7 | 173.7 |
| % cumulative/planned dose | 64.3 | 59.0 | 67.2 | 58.6 |
| Dose intensity, mg/mg2/cycle | 3.3 | 1.7 | 4.3 | 3.0 |
| Grade 3/4 AEs, dose modifications | No grade 3/4 AEs | No grade 3/4 AEs | Grade 3 paralytic ileus, cycle 8, dose reduced to 1.3 then 1.0 mg/m2 | Grade 3 vasculitis, cycle 2 (dose reduced to 1.0 mg/m2, cycle 3); grade 3 volvulus, cycle 16 (dose reduced to 0.7 mg/m2); other grade 3 AEs: pneumonia, hemoptysis, and C difficile diarrhea |
| Adjusted to 1.3 mg/m2 Q2W, cycle 18, due to other reason | Adjusted to 0.7 mg/m2 QW, cycle 10, due to grade 2 pain in extremity | |||
| Hematologic response | CR | SD | PR | PR |
| Organ responses | Renal: response | Renal: response | Renal: NC | Renal: response |
| Cardiac: NC | Cardiac: NC | Cardiac: NC | Cardiac: NC | |
| Status | Ongoing, progression-free | Progressed, in remission on lenalidomide/dexamethasone | Ongoing, progression-free | Ongoing, progression-free |
ASCT, autologous stem cell transplantation; GI, gastrointestinal; NC, no change; Q2W, every two weeks; SD, stable disease.
Biopsy-confirmed GI involvement in the ascending transverse and descending colons and stomach.
Discussion
This final analysis of CAN2007 provides mature data on response and outcomes in relapsed AL patients treated with single-agent bortezomib after an additional 3-year follow-up since the last publication.25 Our data indicate that bortezomib can produce durable hematologic responses and promising long-term OS in this patient population. Consistent with the previous report,25 single-agent bortezomib produced overall hematologic response rates of 68.8% and 66.7% in the 1.6 mg/m2 QW and 1.3 mg/m2 BIW groups, respectively. Responses were durable, with ≥80% of responding patients in all treatment groups sustaining hematologic response for a year or more. These response rates with single-agent bortezomib appear comparable or favorable in the context of other combination regimens in relapsed AL, including cyclophosphamide-thalidomide-dexamethasone,30 cyclophosphamide-lenalidomide-dexamethasone,31 and lenalidomide-dexamethasone,32 which produced hematologic response rates in the range of 62% to 74%; however, cross-study comparisons should be interpreted with caution due to the heterogeneous nature of the AL patient population.
After a 51.8-month median follow-up, the median OS for all patients was 62.7 months. OS with single-agent bortezomib in this study is encouraging with respect to that reported in studies of other combination regimens20,30,31 ; however, data exclusively in the relapsed AL setting are generally lacking. Forty (57%) patients received subsequent AL therapy, most commonly with dexamethasone, bortezomib, lenalidomide, cyclophosphamide, melphalan, or thalidomide. The notable OS reported here may therefore be due, in part, to additional AL therapies based on novel agents such as bortezomib, lenalidomide, and thalidomide, available to patients in more recent years, compared with the more limited treatment options available at the time of older studies that reported considerably shorter OS.33-36 The long-term follow-up of CAN2007 also allowed for analysis of time to subsequent AL therapy and OS in patients stratified by achievement of organ response. Extending on our previously published findings,25 achievement of organ response was found to positively impact time to subsequent therapy and OS in this study.
In light of previous studies demonstrating an association between FLC levels and hematologic response or long-term outcomes,29,37-40 measuring dFLC parameters during cycle 1 and assessing the relationship to hematologic response was included as an exploratory objective in this study. Higher overall and complete response rates at EOT were observed in patients with low vs high baseline dFLC (using 3 different cutoffs for patient dichotomization: 124.2 [median], 180 [validated as of prognostic importance],27 and 50 mg/L [definition of measurable disease]28 ) and high vs low percentage dFLC decrease at the end of cycle 1 (using cutoffs for patient dichotomization of 59% [median] and 50% [validated cutoff defining dFLC PR]29 ). The former association may reflect a deeper impact of treatment in patients with lower disease burden at baseline. Notably, a low baseline dFLC, but not a >50% or > 59% decrease in dFLC at the end of cycle 1, was predictive of improved OS. The latter result may be explained, in part, by the post-cycle 1 percentage dFLC reduction being too premature a measurement to predict for long-term survival. It is of note, however, that per recently updated and validated response criteria in AL, a 50% reduction in dFLC (corresponding to achievement of a PR) at 3 and/or 6 months after treatment initiation has been shown to confer an OS benefit.29
Although these efficacy data with single-agent bortezomib are promising, our findings may not be generalizable to the entire AL patient population. As previously reported,25 patients with the poorest prognosis and cardiac status (New York Heart Association classification III or IV, grade 2/3 atrioventricular block) were excluded to ensure a comprehensive characterization of the safety profile of bortezomib in AL. The relatively low median age, the high proportion of patients with prior HDM-SCT, and the long median time from AL diagnosis, plus the fact that all patients were at first or later relapse,41 are suggestive of a more robust patient population compared with the general AL patient population. Nevertheless, these data add to a growing body of evidence supporting the activity of bortezomib in AL treatment. Since commencement of CAN2007, bortezomib has been studied in various combination regimens in relapsed and previously untreated AL and demonstrated substantial activity, with hematologic response rates in the range of 54% to 94%.5,15-17,19,20,42 Based on data from this and other studies, bortezomib ± dexamethasone, bortezomib-melphalan-dexamethasone, and bortezomib-cyclophosphamide-dexamethasone are currently recommended (category 2A) as primary treatment options for AL in the US National Comprehensive Cancer Network guidelines. Further, local treatment guidelines in several European countries, including Holland and France, recognize bortezomib-based regimens as treatment options for AL.43,44
The optimal duration of bortezomib therapy for patients with relapsed AL, or with relapsed MM, has not been established in prospective trials. Although the phase 3 APEX trial, which demonstrated the superiority of bortezomib over high-dose dexamethasone for relapsed MM, mandated a fixed duration of therapy,45 the phase 2 SUMMIT and CREST trials allowed continuation of bortezomib in patients who, in the investigator’s opinion, would benefit from extended treatment.46 In addition, the subsequent phase 3 DOXIL-MMY-3001 study, which compared the combination of bortezomib and pegylated liposomal doxorubicin with bortezomib alone in relapsed or refractory MM, permitted continued bortezomib administration under similar circumstances.47 Although prolonged bortezomib therapy was shown to be tolerable, these trials were not designed to evaluate the efficacy of continued therapy. Four AL patients in this study received long-term bortezomib treatment of between 3.5 and 5.6 years, with no new safety concerns; of these, 3 remained progression-free and on bortezomib treatment at the time of manuscript writing. It is of note that, although all 4 long-term exposure patients showed clinical stability or improvement to initial therapy, 3 had only stable disease or PR as their best hematologic response. These data suggest the feasibility of single-agent bortezomib for long-term disease control in relapsed AL, particularly in patients who do not initially achieve a hematologic CR. In patients responding to treatment but with residual disease, continuation of therapy may lead to suppression of the culprit plasma cell clone and longer disease control. Indications for patients who might benefit most from prolonged bortezomib therapy and the optimal schedule on which long-term bortezomib should be administered cannot be surmised from this analysis. However, it should be noted that the 4 long-term patients were of relatively young age (53-55 years) at study entry and had all previously undergone HDM-SCT, perhaps reflecting their relative fitness and ability to tolerate prolonged bortezomib. In addition, all had experienced improvement or disease stabilization in ≥1 affected organ.
Additional follow-up since the previous report of the study25 allowed for collection of subsequent therapy data in patients with progressive disease following initial bortezomib treatment. Of 19 patients who received bortezomib retreatment, 11 (58%) achieved a hematologic response (CR or PR). These data suggest that bortezomib-based therapy may be feasible and active at >1 stage in the AL treatment paradigm.
In CAN2007, the potential utility of QW and BIW bortezomib administration was explored. Although the study was not designed to directly compare the 2 administration schedules, our data suggest that bortezomib 1.6 mg/m2 QW was well tolerated with fewer discontinuations (50% vs 74% for BIW), allowing for a higher proportion of patients to complete ≥8 treatment cycles than with bortezomib 1.3 mg/m2 BIW. These findings are in accordance with prior studies of QW vs BIW bortezomib administration in patients with MM or lymphoma, in which less intensive QW dosing in the context of combination therapy improved treatment tolerability without compromising efficacy.48-50 The feasibility of less intensive bortezomib dosing for relapsed AL is also indicated by long-term disease control in 2 lower-dose group patients who received prolonged bortezomib therapy in CAN2007. QW bortezomib administration may also offer a potential advantage over BIW dosing in terms of patient convenience. However, response was achieved more quickly with BIW bortezomib, suggesting that this could be the initial schedule for patients with a high disease burden who require rapid FLC reduction. On the other hand, QW bortezomib may be a preferred schedule in patients with less rapidly progressing disease or after an initial response is obtained. Other factors, such as the tolerability of the dose and planned duration of therapy, should also be taken into account when determining the optimal dose for a given patient.
In conclusion, single-agent bortezomib produced durable hematologic responses and promising long-term OS in relapsed AL patients. Prolonged therapy is feasible and can provide long-term disease control, potentially in patients who do not initially achieve a hematologic CR. Favorable hematologic response rates can also be achieved with bortezomib retreatment in this setting. Previous studies have demonstrated the feasibility and activity of bortezomib in combination regimens. In the future, bortezomib might prove useful as part of initial AL treatment and possibly as part of extended therapy after HDM-SCT or alternative nontransplantation therapies.
Presented in poster format at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 4, 2013.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients who participated in this study and their families, as well as the investigators and staff at all CAN2007 clinical sites for their valuable contribution to the study. The authors acknowledge Emma Landers, a medical writer with FireKite, part of KnowledgePoint360 group, an Ashfield Company, for writing support during the development of this manuscript, which was funded by Millennium: The Takeda Oncology Company and Janssen Global Services.
This study was supported by research funding from Millennium: The Takeda Oncology Company and Janssen Research and Development. G.M. and G.P. are supported by a grant from Associazione Italiana per la Ricerca sul Cancro Special Program Molecular Clinical Oncology 5 per mille n. 9965.
Authorship
Contribution: D.E.R., V.S., H.v.d.V., A.C., D.-L.E., and R.L.C. designed the trial; D.E.R., U.H., G.M., G.P., J.B., J.-P.F., H.H., L.H., V.K., R.A.V., C.E., D.-L.E., H.v.d.V., and A.C. interpreted the data; U.H., V.S., H.H., L.H., and R.L.C. collected the data; U.H., L.P., and H.v.d.V. analyzed the data; L.P. and H.v.d.V. performed the statistical analysis; and all authors contributed to the writing/review of the manuscript and approved the final version for publication.
Conflict-of-interest disclosure: D.E.R. receives honoraria from Janssen, Celgene, Otsuka, and Merck and research funding from Janssen, Millennium: The Takeda Oncology Company, Celgene, Merck, Novartis, Otsuka, and Bristol-Myers Squibb. U.H. receives honoraria from Janssen. V.S. receives research support from Celgene and Millennium: The Takeda Oncology Company. G.M. receives honoraria from Millennium: The Takeda Oncology Company. J.B. receives honoraria from, and has membership on an advisory board for, Janssen and Celgene and receives research funding from Janssen. H.H. receives research support from Celgene and Millennium: The Takeda Oncology Company. L.H. receives research support from Janssen and Millennium: The Takeda Oncology Company. V.K. receives honoraria from Celgene and Janssen. R.A.V. participates in a speakers bureau for Millennium: The Takeda Oncology Company. L.P. is employed by Janssen. C.E. and H.v.d.V. are employed by Janssen R&D and receive equity ownership in Johnson & Johnson. D.-L.E. is employed by Takeda Pharmaceuticals International Co. and receives equity ownership in Takeda and Johnson & Johnson. A.C. is a former employee of, formerly provided expert testimony for, and has equity ownership in Johnson & Johnson. R.L.C. receives research support from Millennium: The Takeda Oncology Company, Prothena, and Teva. G.P. and J.-P.F. declare no competing financial interests.
Correspondence: Donna E. Reece, Princess Margaret Cancer Center, 610 University Ave, Suite 5-207, Toronto, ON, Canada M5G 2M9; e-mail: donna.reece@uhn.ca.