Key Points
Survival in ET is superior to that of PV, regardless of mutational status, but remains inferior to the sex- and age-matched US population.
JAK2/CALR/MPL mutational status is prognostically informative in PMF, regarding overall and leukemia-free survival.
Abstract
Janus kinase 2 (JAK2) mutations define polycythemia vera (PV). Calreticulin (CALR) and myeloproliferative leukemia virus oncogene (MPL) mutations are specific to JAK2-unmutated essential thrombocythemia (ET) and primary myelofibrosis (PMF). We examined the effect of these mutations on long-term disease outcome. One thousand five hundred eighty-one patients from the Mayo Clinic (n = 826) and Italy (n = 755) were studied. Fifty-eight percent of Mayo patients were followed until death; median survivals were 19.8 years in ET (n = 292), 13.5 PV (n = 267; hazard ratio [HR], 1.8; 95% confidence interval [CI], 1.4-2.2), and 5.9 PMF (n = 267; HR, 4.5; 95% CI, 3.5-5.7). The survival advantage of ET over PV was not affected by JAK2/CALR/MPL mutational status. Survival in ET was inferior to the age- and sex-matched US population (P < .001). In PMF (n = 428), but not in ET (n = 576), survival and blast transformation (BT) were significantly affected by mutational status; outcome was best in CALR-mutated and worst in triple-negative patients: median survival, 16 vs 2.3 years (HR, 5.1; 95% CI, 3.2-8.0) and BT, 6.5% vs 25% (HR, 7.6; 95% CI, 2.8-20.2), respectively. We conclude that life expectancy in morphologically defined ET is significantly reduced but remains superior to that of PV, regardless of mutational status. In PMF, JAK2/CALR/MPL mutational status is prognostically informative.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2615.
Disclosures
The authors, Associate Editor Jacob M. Rowe, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Compare survival outcomes in essential thrombocythemia vs polycythemia vera, based on a large, retrospective cohort study.
Describe the associations of JAK2/CALR/MPL mutational status in primary myelofibrosis with overall and leukemia-free survival.
Distinguish the clinical and research implications of the findings of this large, retrospective cohort study on survival outcomes in myeloproliferative neoplasms.
Release date: October 16, 2014; Expiration date: October 16, 2015
Introduction
Janus kinase 2 (JAK2) mutations are present in >95% of patients with polycythemia vera (PV) and also constitute the most frequent mutation (∼60% incidence) in essential thrombocythemia (ET) and primary myelofibrosis (PMF).1 Calreticulin (CALR) exon 9 insertions/deletions represent the second most frequent mutation in ET and PMF; their mutational frequency is estimated between 15% and 32% in ET and 25% and 35% in PMF.2-8 Myeloproliferative leukemia virus oncogene (MPL) mutations are also specific to ET and PMF and occur with mutational frequencies of 4% in ET and ∼8% in PMF.1 JAK2, CALR, and MPL mutations are for the most part mutually exclusive and their pathogenetic contribution is currently believed to include upregulation of JAK2 signaling.9
The original descriptions of CALR mutations in myeloproliferative neoplasms (MPN) suggested a significant impact on disease phenotype and outcome.2,3 In 1 of these 2 seminal reports,2 CALR-mutated patients with ET, compared with their JAK2-mutated counterparts, displayed higher platelet count, lower leukocyte count, lower hemoglobin level, lower risk of thrombosis, and better survival. The authors also reported higher platelet count, lower leukocyte count, and better survival in CALR-mutated PMF patients, compared with JAK2-mutated cases.2 In the second study,3 CALR-mutated ET patients were associated with higher platelet count, lower hemoglobin level, and higher incidence of fibrotic transformation, compared with JAK2-mutated cases.3
Subsequent reports did not find significant difference in survival or risk of fibrotic transformation between CALR- and JAK2-mutated ET.4,5,7 In PMF, the favorable impact of CALR mutations on survival2 was confirmed in a subsequent study,6 which also disclosed the prognostically detrimental effect of “triple-negative” mutational status (ie, wild type for JAK2, CALR and MPL). The latter study6 and subsequent reports10,11 showed further prognostic modifications by ASXL1 mutational status and number of prognostically detrimental mutations. Mutant CALR vs JAK2 mutations in PMF were also associated with younger age, lower hemoglobin level, lower risk disease, and lower frequency of spliceosome mutations.6 More recent reports have suggested differential phenotypic and prognostic effects from distinct mutant CALR variants, including an association of type 2 vs type 1 variants with higher platelet count in ET12 and worse prognosis in PMF.13
JAK2 mutations in ET are often accompanied by distinct biological and clinical characteristics,1 suggesting the possibility of a JAK2 mutation-associated disease continuum that is phenotypically patterned by additional genetic or biologic changes. Consistent with this concept, some have argued that PV and JAK2-mutated ET are different phases of the same disease and share similar natural history, including survival.5,14 The current study addresses this issue by comparing long-term survival and blast transformation (BT) rates between PV, ET, and PMF, in the context of their specific JAK2/MPL/CALR mutational status; we also examined the impact of JAK2/MPL/CALR mutational status on long-term disease outcome in ET and PMF.
Methods
The current study was approved by the institutional review boards of Mayo Clinic (Rochester, MN), University of Florence (Florence, Italy), and Papa Giovanni XXIII Hospital (Bergamo, Italy) and conducted in accordance with the Declaration of Helsinki. To obtain mature survival data, study eligibility criteria included a diagnosis date prior to 2006 for ET and PV and 2011 for PMF. In addition, for ET and PMF, only those patients whose mutational status, in terms of JAK2, CALR, or MPL, was known were included in the current study. The performance of mutation screening was solely based on availability of archived DNA and no other selection bias was introduced. Diagnoses of ET, PV, PMF, and BT were according to the 2008 World Health Organization (WHO) criteria.15,16 Previously published methods were used for CALR, JAK2, and MPL mutation analyses.6
All statistical analyses considered clinical and laboratory parameters obtained at diagnosis or within 1 year of diagnosis. Differences in the distribution of continuous variables between categories were analyzed by either Mann-Whitney or Kruskal-Wallis test. Patient groups with nominal variables were compared by χ2 test. All patients, including those from the Mayo Clinic and the 2 Italian centers, were followed until death, leukemic transformation, or last clinic visit if they were currently alive. In addition, follow-up information on the patients who are alive was updated by directly contacting patients or their physicians. Survival analysis was considered from the date of diagnosis to date of death (uncensored) or last contact (censored). Leukemia-free survival (LFS) calculations considered BT as the uncensored variable. Survival curves were prepared by the Kaplan-Meier method and compared by the log-rank test. Observed survival in ET was compared with the expected survival of the age- and sex-matched US total population. The rate of BT was calculated as the cumulative incidence of transformation, accounting for the competing risk of death.17 The Cox proportional hazard regression model was used for multivariable analysis. P values < .05 were considered significant. All analyses were conducted using the Stat View (SAS Institute) or SAS version 9.2 (SAS Institute) statistical packages.
Results
One thousand five hundred eighty-one patients were included in the current study and were recruited from the Mayo Clinic (n = 826) and 2 centers from Italy (n = 755; 598 from Florence and 157 from Bergamo). The comparison of survival between ET, PV, and PMF was first analyzed using the Mayo cohort and the results validated using the Italian cohort; the 2 cohorts were considered together for examining the prognostic implications of mutational status in ET and PMF, to optimize the sample sizes for patients with the less frequent mutational categories.
The 826 Mayo patients included 292 with ET, 267 PV, and 267 PMF (Table 1). The 755 patients from Italy included 284 with ET (median age, 54 years; 68% females), 310 PV (median age 58 years; 46% females), and 161 PMF (median age, 64 years; 32% females) (supplemental Table 1, see supplemental Data available at the Blood Web site). All 157 patients from Bergamo had PV and the 445 Italian patients with ET or PMF were all recruited from the University of Florence.
Presenting clinical and laboratory features of 826 Mayo Clinic patients with ET vs PV vs PMF
| Variables . | ET, n = 292 . | PV, n = 267 . | PMF, n = 267 . | P . | ||
|---|---|---|---|---|---|---|
| ET vs PV . | ET vs PMF . | PV vs PMF . | ||||
| Age, median (range), y | 55 (15-91) | 64 (19-95) | 63 (14-87) | <.0001 | <.0001 | .6 |
| Age ≥60 y, n (%) | 123 (42.1) | 157 (58.8) | 163 (61) | <.0001 | <.0001 | .6 |
| Females, n (%) | 173 (59.2) | 137 (51.3) | 102 (38.2) | .06 | <.0001 | .002 |
| Hemoglobin, median (range), g/dL | 13.9 (6.9-17.9) | 18.4 (15.1-24.5) | 10.6 (5.8-16.1) | <.0001 | <.0001 | <.0001 |
| Leukocytes, median (range), ×109/L | 9.6 (2.8-53.4) | 11.8 (3.8-171.6) | 8.6 (0.8-146.6) | <.0001 | .1 | <.0001 |
| Platelets, median (range), ×109/L | 1000 (454-3460) | 467 (37-1720) | 253 (12-2466) | <.0001 | <.0001 | <.0001 |
| Risk stratification,*% | ||||||
| Low | 37 | 25 | 17 | |||
| Intermediate | 40 | 32 | ||||
| Intermediate-1 | 22 | |||||
| Intermediate-2 | 33 | |||||
| High | 23 | 43 | 28 | |||
| Leukocytes, ≥11 × 109/L, % | 33.1 | 56.5 | 36.3 | <.0001 | .4 | <.0001 |
| Platelets, >1000 × 109/L, % | 52.4 | 7.1 | 2.2 | <.0001 | <.0001 | .008 |
| Abnormal karyotype, “N” evaluable = 610, n (%) | 16 (7.7) | 26 (18.3) | 87 (33.4) | .003 | <.0001 | .001 |
| Palpable splenomegaly, % | 23.6 | 37.3 | 72.6 | .0005 | <.0001 | <.0001 |
| Microcirculatory symptoms, “N” evaluable = 510, n (%) | 52 (17.8) | 89 (40.8) | NA | <.0001 | NA | NA |
| Mutational status, n (%) | NA | .02 | NA | |||
| JAK2 | 157 (53.8) | 149 (55.8) | ||||
| CALR | 89 (30.5) | 64 (24) | ||||
| MPL | 8 (2.7) | 21 (7.9) | ||||
| Triple negative | 38 (13) | 33 (12.3) | ||||
| Variables . | ET, n = 292 . | PV, n = 267 . | PMF, n = 267 . | P . | ||
|---|---|---|---|---|---|---|
| ET vs PV . | ET vs PMF . | PV vs PMF . | ||||
| Age, median (range), y | 55 (15-91) | 64 (19-95) | 63 (14-87) | <.0001 | <.0001 | .6 |
| Age ≥60 y, n (%) | 123 (42.1) | 157 (58.8) | 163 (61) | <.0001 | <.0001 | .6 |
| Females, n (%) | 173 (59.2) | 137 (51.3) | 102 (38.2) | .06 | <.0001 | .002 |
| Hemoglobin, median (range), g/dL | 13.9 (6.9-17.9) | 18.4 (15.1-24.5) | 10.6 (5.8-16.1) | <.0001 | <.0001 | <.0001 |
| Leukocytes, median (range), ×109/L | 9.6 (2.8-53.4) | 11.8 (3.8-171.6) | 8.6 (0.8-146.6) | <.0001 | .1 | <.0001 |
| Platelets, median (range), ×109/L | 1000 (454-3460) | 467 (37-1720) | 253 (12-2466) | <.0001 | <.0001 | <.0001 |
| Risk stratification,*% | ||||||
| Low | 37 | 25 | 17 | |||
| Intermediate | 40 | 32 | ||||
| Intermediate-1 | 22 | |||||
| Intermediate-2 | 33 | |||||
| High | 23 | 43 | 28 | |||
| Leukocytes, ≥11 × 109/L, % | 33.1 | 56.5 | 36.3 | <.0001 | .4 | <.0001 |
| Platelets, >1000 × 109/L, % | 52.4 | 7.1 | 2.2 | <.0001 | <.0001 | .008 |
| Abnormal karyotype, “N” evaluable = 610, n (%) | 16 (7.7) | 26 (18.3) | 87 (33.4) | .003 | <.0001 | .001 |
| Palpable splenomegaly, % | 23.6 | 37.3 | 72.6 | .0005 | <.0001 | <.0001 |
| Microcirculatory symptoms, “N” evaluable = 510, n (%) | 52 (17.8) | 89 (40.8) | NA | <.0001 | NA | NA |
| Mutational status, n (%) | NA | .02 | NA | |||
| JAK2 | 157 (53.8) | 149 (55.8) | ||||
| CALR | 89 (30.5) | 64 (24) | ||||
| MPL | 8 (2.7) | 21 (7.9) | ||||
| Triple negative | 38 (13) | 33 (12.3) | ||||
NA, not available.
Risk stratification in PMF was according to the Dynamic International Prognostic Scoring System plus; in ET, according to the International Prognostic Scoring System; and in PV, according to the International Working Group for Myeloproliferative Neoplasms Research and Treatment criteria. References for these prognostic criteria are included in the main text.
Presenting features: ET vs PV vs PMF
Table 1 outlines clinical and laboratory features at diagnosis (or within 1 year of diagnosis) for the 826 patients from the Mayo Clinic, stratified by MPN subtype. In addition to the expected differences in hemoglobin level and platelet count, other significant differences between ET, PV, and PMF included younger age in ET, preponderance of male sex in PMF, and higher leukocyte count in PV. Similar analysis restricted to JAK2-mutated cases revealed mostly similar results (supplemental Table 2). Supplemental Table 1 outlines comparative results for the patients from Italy, which were mostly similar, with few exceptions.
Presenting features: JAK2 vs CALR vs MPL mutated vs triple-negative ET
Table 2 lists the presenting features in the combined Mayo-Italian cohort of patients with ET (n = 576). Compared with JAK2-mutated cases, CALR-mutated ET patients displayed younger age, male sex, higher platelet count, lower hemoglobin level, lower leukocyte count, and lower incidence of thrombosis. Triple-negative ET patients also displayed younger age, lower hemoglobin level, lower leukocyte count, and lower incidence of thrombosis, compared with JAK2-mutated cases. Comparison of triple-negative and CALR-mutated ET patients showed the latter to be associated with male sex and higher platelet count. MPL-mutated cases were similar to JAK2-mutated cases in terms of age and gender distribution and thrombosis risk but displayed lower hemoglobin level.
Presenting features of 1004 patients with ET or PMF, stratified by their mutational status
| . | JAK2 mutated (A) . | CALR mutated (B) . | Triple negative (C) . | MPL mutated (D) . | P . | |||
|---|---|---|---|---|---|---|---|---|
| A vs B . | A vs C . | B vs C . | A vs D . | |||||
| ET, N = 576 | ||||||||
| No. of patients | 336 | 134 | 84 | 22 | ||||
| Age, median (range), y | 58 (16-88) | 49 (13-91) | 47 (16-81) | 57 (22-85) | <.0001 | .0004 | .8 | 1.0 |
| Age ≥60 y, n (%) | 157 (47) | 36 (27) | 29 (34) | 8 (36) | <.0001 | .04 | .2 | .3 |
| Females, n (%) | 227 (68) | 66 (49) | 61 (73) | 13 (59) | .0002 | .4 | .0007 | .4 |
| Hemoglobin, median (range), g/dL | 14.3 (9.8-17.9) | 13.5 (6.9-16.4) | 13.2 (8.4-16.7) | 13.8 (9.0-16.0) | <.0001 | <.0001 | .7 | .02 |
| Leukocytes, median (range), ×109/L | 9.6 (3.5-53.4) | 8.5 (2.6-32.6) | 8.3 (2.8-15.4) | 7.4 (4.0-17.7) | .0007 | .0006 | .6 | .07 |
| Platelets, median (range), ×109/L | 841 (469-3000) | 1000 (454-3460) | 854 (500-3300) | 900 (685-2249) | <.0001 | .4 | .003 | .1 |
| Leukocytes ≥11 × 109/L, % | 31 | 24 | 20 | 26 | .1 | .04 | .5 | .6 |
| Platelets >1000 × 109/L, % | 29 | 54 | 38 | 41 | <.0001 | .1 | .02 | .2 |
| Thrombosis history, % | 37 | 27 | 20 | 45 | .04 | .004 | .3 | .4 |
| PMF, N = 428 | ||||||||
| No. of patients | 258 | 92 | 52 | 26 | ||||
| Age, median (range), y | 65 (28-90) | 54 (18-83) | 67 (14-88) | 60 (29-80) | <.0001 | .3 | <.0001 | .046 |
| Age ≥60 y, n (%) | 173 (67%) | 35 (38%) | 39 (75%) | 15 (58%) | <.0001 | .3 | <.0001 | .3 |
| Females, n (%) | 88 (34%) | 39 (42%) | 18 (35%) | 9 (35%) | .1 | .9 | .4 | 1.0 |
| Hemoglobin, median (range), g/dL | 11.0 (5.4-17.5) | 11.3 (8.0-15.5) | 9.6 (5.2-13.5) | 10.2 (6-14) | .3 | <.0001 | <.0001 | .08 |
| Leukocytes, median (range), ×109/L | 9.9 (0.8-106.1) | 8.0 (1.8-40.0) | 6.7 (1.4-146.6) | 5.8 (2.5-42.0) | .003 | .1 | .7 | .03 |
| Platelets, median (range), ×109/L | 260 (12-2466) | 387 (15-1563) | 147 (14-900) | 179 (31-925) | <.0001 | .001 | <.0001 | .06 |
| Leukocytes ≥11 × 109/L, % | 46 | 23 | 41 | 33 | .0002 | .5 | .03 | .2 |
| Platelets >1000 × 109/L, % | 3 | 9 | 0 | 0 | .02 | .2 | .03 | .4 |
| . | JAK2 mutated (A) . | CALR mutated (B) . | Triple negative (C) . | MPL mutated (D) . | P . | |||
|---|---|---|---|---|---|---|---|---|
| A vs B . | A vs C . | B vs C . | A vs D . | |||||
| ET, N = 576 | ||||||||
| No. of patients | 336 | 134 | 84 | 22 | ||||
| Age, median (range), y | 58 (16-88) | 49 (13-91) | 47 (16-81) | 57 (22-85) | <.0001 | .0004 | .8 | 1.0 |
| Age ≥60 y, n (%) | 157 (47) | 36 (27) | 29 (34) | 8 (36) | <.0001 | .04 | .2 | .3 |
| Females, n (%) | 227 (68) | 66 (49) | 61 (73) | 13 (59) | .0002 | .4 | .0007 | .4 |
| Hemoglobin, median (range), g/dL | 14.3 (9.8-17.9) | 13.5 (6.9-16.4) | 13.2 (8.4-16.7) | 13.8 (9.0-16.0) | <.0001 | <.0001 | .7 | .02 |
| Leukocytes, median (range), ×109/L | 9.6 (3.5-53.4) | 8.5 (2.6-32.6) | 8.3 (2.8-15.4) | 7.4 (4.0-17.7) | .0007 | .0006 | .6 | .07 |
| Platelets, median (range), ×109/L | 841 (469-3000) | 1000 (454-3460) | 854 (500-3300) | 900 (685-2249) | <.0001 | .4 | .003 | .1 |
| Leukocytes ≥11 × 109/L, % | 31 | 24 | 20 | 26 | .1 | .04 | .5 | .6 |
| Platelets >1000 × 109/L, % | 29 | 54 | 38 | 41 | <.0001 | .1 | .02 | .2 |
| Thrombosis history, % | 37 | 27 | 20 | 45 | .04 | .004 | .3 | .4 |
| PMF, N = 428 | ||||||||
| No. of patients | 258 | 92 | 52 | 26 | ||||
| Age, median (range), y | 65 (28-90) | 54 (18-83) | 67 (14-88) | 60 (29-80) | <.0001 | .3 | <.0001 | .046 |
| Age ≥60 y, n (%) | 173 (67%) | 35 (38%) | 39 (75%) | 15 (58%) | <.0001 | .3 | <.0001 | .3 |
| Females, n (%) | 88 (34%) | 39 (42%) | 18 (35%) | 9 (35%) | .1 | .9 | .4 | 1.0 |
| Hemoglobin, median (range), g/dL | 11.0 (5.4-17.5) | 11.3 (8.0-15.5) | 9.6 (5.2-13.5) | 10.2 (6-14) | .3 | <.0001 | <.0001 | .08 |
| Leukocytes, median (range), ×109/L | 9.9 (0.8-106.1) | 8.0 (1.8-40.0) | 6.7 (1.4-146.6) | 5.8 (2.5-42.0) | .003 | .1 | .7 | .03 |
| Platelets, median (range), ×109/L | 260 (12-2466) | 387 (15-1563) | 147 (14-900) | 179 (31-925) | <.0001 | .001 | <.0001 | .06 |
| Leukocytes ≥11 × 109/L, % | 46 | 23 | 41 | 33 | .0002 | .5 | .03 | .2 |
| Platelets >1000 × 109/L, % | 3 | 9 | 0 | 0 | .02 | .2 | .03 | .4 |
Percentages are rounded up to the nearest whole percent.
Presenting features: JAK2 vs CALR vs MPL mutated vs triple-negative PMF
Table 2 lists the presenting features in the combined Mayo-Italian cohort of patients with PMF (n = 428). Compared with JAK2-mutated cases, CALR-mutated PMF patients displayed younger age, higher platelet count, and lower leukocyte count. Triple-negative PMF patients displayed lower hemoglobin level and lower platelet count, compared with JAK2-mutated cases. Comparison of CALR and triple-negative PMF patients showed the former to be younger and display higher hemoglobin and platelet counts. Compared with JAK2-mutated cases, MPL-mutated PMF patients were younger and displayed lower leukocyte count.
Cytogenetic information was available for the Mayo Clinic PMF patients and showed an abnormal karyotype in 33% with no difference between the JAK2/CALR/MPL mutational categories (P = .43); there was also no difference in the distribution of normal vs favorable vs unfavorable cytogenetic abnormalities (P = .46).
Clinical course
Among the 826 Mayo patients, 475 (58%) were followed until death, including 139 (48%) with ET, 164 (61%) with PV, and 172 (64%) with PMF; the median follow-up time for living patients was 17.3 years for ET (range, 6.8-43.6), 11.8 years for PV (range, 8.3-39.3) and 7.7 years for PMF (range 3.3-26). BT was reported in 64 Mayo patients, including 34 (12.7%) with PMF, 18 (6.7%) with PV, and 12 (4.1%) with ET; fibrotic transformations were reported in 63 patients including 34 (12.7%) with PV and 29 (9.9%) with ET.
Among the 755 Italian patients, 168 (22%) were followed until death, including 24 (9%) with ET, 84 (27%) with PV, and 60 (37%) with PMF. The median follow-up time for living patients in the Italian cohort was 10.7 years for ET, 11.1 years for PV, and 4.8 years for PMF; BT was reported in 33 patients, including 19 (11.8%) with PMF, 10 (3.2%) with PV, and 4 (1.4%) with ET. Fibrotic transformations were reported in 91 Italian patients including 65 (21%) with PV and 26 (9.2%) with ET. Polycythemic conversions were infrequent in both the Mayo (n = 9; 3%) and Italian (n = 2; 1%) cohorts.
Considering the fact that the study population was selected based on diagnosis dates of prior to 2006 for ET and PV and 2011 for PMF, there was not much difference in treatment approaches between the Mayo Clinic and the 2 Italian centers. In all instances, hydroxyurea was the primary cytoreductive agent used for high-risk PV or ET and PMF. Other frequently used drugs included aspirin for PV and ET and a spectrum of agents for PMF-associated anemia, including androgen preparations, erythropoiesis-stimulating agents, prednisone, danazol, and thalidomide.
Survival: ET vs PV vs PMF
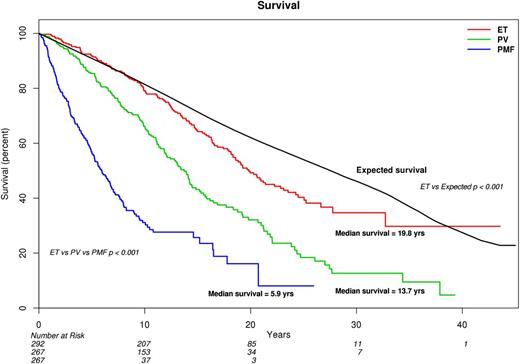
Primary analysis of survival data, for comparison of ET vs PV vs PMF, was performed using the Mayo Clinic cohort of 826 patients, where the majority (58%) was followed until death, thus ensuring mature survival data (Figure 1). Median survivals were 19.8 years for ET, 13.5 for PV (hazard ratio [HR], 1.8; 95% confidence interval [CI], 1.4-2.2), and 5.9 for PMF (HR, 4.5; 95% CI, 3.5-5.7). The corresponding median survivals for patients younger than age 60 years were 32.7 years for ET, 23.8 years for PV, and 14.6 years for PMF (P < .001; supplemental Figure 1). Despite the very long survival estimates in patients with ET, their life expectancy remained inferior to the age- and sex-matched US population (Figure 1; P < .001).
Comparison of survival in 826 Mayo Clinic patients with ET vs PV vs PMF. Survival in ET was also compared with the age- and sex-matched US population.
Comparison of survival in 826 Mayo Clinic patients with ET vs PV vs PMF. Survival in ET was also compared with the age- and sex-matched US population.
The survival advantage of ET over PV was not affected by age (HR, 1.5; 95% CI, 1.2-1.9), sex (HR, 1.8; 95% CI, 1.4-2.3), or mutational status (HR, 1.7; 95% CI, 1.3-2.2) and was similarly demonstrated by a separate analysis of the Italian patient cohort (n = 755; supplemental Figure 2). The HRs (95% CI) for the Italian cohort, compared with ET, were 12.3 (7.6-20.0) for PMF and 3.1 (2.0-4.9) for PV; the corresponding values for analysis restricted to the 598 patients from Florence were 10.9 (6.7-17.8) and 2.3 (1.3-3.8).
BT and fibrotic progression: ET vs PV vs PMF
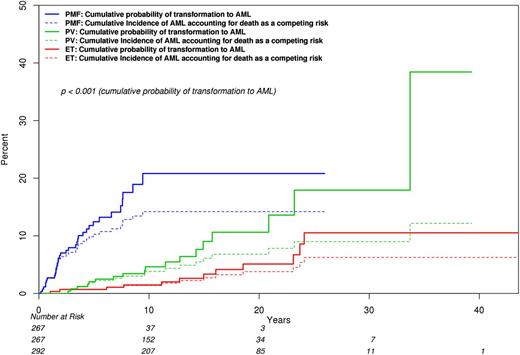
In the Mayo cohort, the cumulative incidence of BT, with death as a competing risk, was 3.8% for ET (95% CI, 1.3-6.2), 6.8% for PV (95% CI, 3.3-10.2), and 14.2% for PMF (95% CI, 9.5-18.6) (Figure 2). The difference was significant for ET vs PMF (P = .002) and for PV vs PMF (P = .03). The difference between ET and PV was of borderline significance (0.16). Calculation of LFS without competing risk adjustment showed worse outcome in both PMF (HR, 9.4; 95% CI, 4.6-19.3) and in PV (HR, 2.3; 95% CI, 1.1-4.7), compared with ET. The corresponding HRs (95% CI), during separate analysis of JAK2-mutated cases, were 18.8 (6.4-55.4) and 2.9 (1.1-7.9). A similar analysis of LFS in the Italian patient cohort also showed a significant difference between PMF and ET (HR, 19.1; 95% CI, 6.3-57.7) but the difference between PV and ET did not reach statistical significance (HR, 2.3; 95% CI, 0.7-7.3).
Comparison of BT rates among 826 Mayo Clinic patients with ET vs PV vs PMF, which includes accounting for death as a competing risk. Cumulative incidences of BT (95% CI) were 0.038 (0.013-0.062) for ET, 0.068 (0.033-0.102) for PV, and 0.142 (0.095-0.186) for PMF; P values were .03 for PV vs PMF at 20 years, .002 for ET vs PMF at 20 years, and .16 for ET vs PV at 20 years.
Comparison of BT rates among 826 Mayo Clinic patients with ET vs PV vs PMF, which includes accounting for death as a competing risk. Cumulative incidences of BT (95% CI) were 0.038 (0.013-0.062) for ET, 0.068 (0.033-0.102) for PV, and 0.142 (0.095-0.186) for PMF; P values were .03 for PV vs PMF at 20 years, .002 for ET vs PMF at 20 years, and .16 for ET vs PV at 20 years.
Among the Italian cohort of 594 patients with PV (n = 310) and ET (n = 284), 91 fibrotic transformations were reported, including 65 (21%) in PV and 26 (9.2%) in ET (P < .0001). In contrast, comparison of fibrotic progression rates in the Mayo cohort was not significantly different between ET (10.3%) and PV (12.5%).
Mutation-specific survival: ET vs PV vs PMF
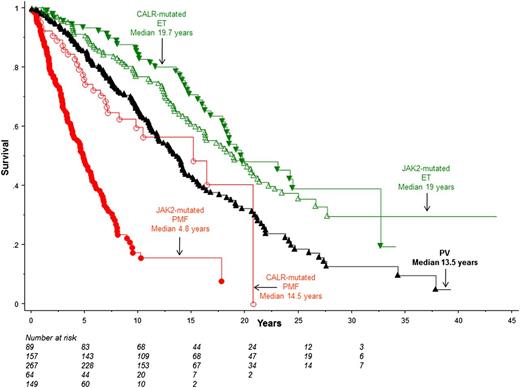
Figure 3 depicts survival data comparisons, using the Mayo patient cohort, between PV and patients with ET and PMF stratified by the 2 most frequent mutations: JAK2 and CALR. The figure illustrates the inferior survival in both PV (HR 1.6, 95% CI 1.2-2.1) and JAK2-mutated PMF (HR, 6.2; 95% CI, 4.5-8.5), compared with JAK2-mutated ET. This difference in survival between PV and JAK2-mutated ET remained significant during multivariable analysis that included both age and sex (P = .007; HR, 1.5; 95% CI, 1.1-1.9).
Survival data comparisons in Mayo Clinic patients with PV vs JAK2- or CALR-mutated ET or PMF.P values were <.01 for (1) JAK2-mutated PMF vs CALR-mutated PMF, PV, JAK2-mutated ET or CALR-mutated ET, (2) CALR-mutated PMF vs CALR-mutated ET or JAK2-mutated ET, and (3) PV vs JAK2-mutated or CALR-mutated ET. Univariate analysis did not show a difference between JAK2 and CALR-mutated ET (P = .28) or CALR-mutated PMF and PV (P = .54).
Survival data comparisons in Mayo Clinic patients with PV vs JAK2- or CALR-mutated ET or PMF.P values were <.01 for (1) JAK2-mutated PMF vs CALR-mutated PMF, PV, JAK2-mutated ET or CALR-mutated ET, (2) CALR-mutated PMF vs CALR-mutated ET or JAK2-mutated ET, and (3) PV vs JAK2-mutated or CALR-mutated ET. Univariate analysis did not show a difference between JAK2 and CALR-mutated ET (P = .28) or CALR-mutated PMF and PV (P = .54).
In univariate analysis, there was no significant difference in survival between PV and CALR-mutated PMF (P = .54; Figure 3); however, survival was shown to be worse in CALR-mutated PMF (median age, 54 years), compared with PV (median age, 64 years; P < .001), when the analysis was adjusted for age (HR, 1.6; 95% CI, 1.1-2.4). The JAK2 mutation-specific survival difference between ET, PV, and PMF was similarly demonstrated in the Italian patient cohort (supplemental Figure 3); HRs (95% CI), compared with JAK2-mutated ET, were 15.1 (8.0-28.5) for JAK2-mutated PMF and 2.7 (1.6-4.6) for PV.
Mutational status and disease outcome in PMF
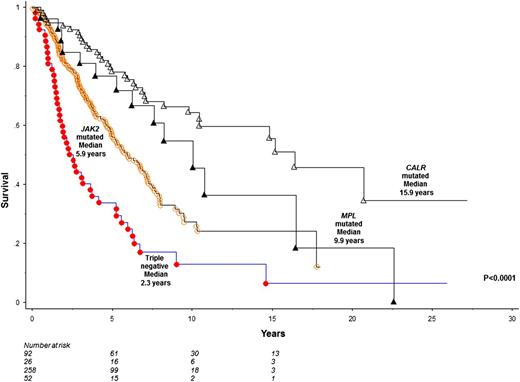
To optimize sample size for comparison of survival among specific mutational groups in PMF, we analyzed the Mayo and Italian cohorts together. This resulted in a total number of 428 patients with JAK2 (n = 258; 60%), CALR (n = 92; 22%), MPL (n = 26; 6%) mutated or triple-negative (n = 52; 12%) mutational status. Triple-negative patients displayed significantly worse survival (median, 2.3 years), compared with CALR (median, 15.9 years; HR, 0.2; 95% CI, 0.1-0.3), JAK2 (median, 5.9 years; HR, 0.5; 95% CI, 0.4-0.7), or MPL (median, 9.9 years; HR, 0.3, 95% CI, 0.2-0.6) mutated cases (Figure 4); these differences remained significant when analysis was adjusted for age and sex. Conversely, survival was significantly better in CALR-mutated patients, compared with triple-negative (HR, 5.1; 95% CI, 3.2-8.0) and JAK2 (HR, 2.5; 95% CI, 1.7-3.7) mutated cases; the difference in survival between CALR- and MPL-mutated PMF did not reach significance level (HR, 1.6; 95% CI, 0.9-3.0).
Comparison of survival among 428 patients with PMF stratified by their mutational status.
Comparison of survival among 428 patients with PMF stratified by their mutational status.
Among the 92 CALR mutated PMF patients recruited from the Mayo Clinic and University of Florence, 72 harbored type 1, 10 type 2, and 10 other CALR variants. Survival was significantly better in type 1, compared with both type 2 (P = .03) CALR variant and mutant JAK2 (P < .0001). Survival was not different between type 2 CALR and JAK2 mutations (P = .3) or mutant JAK2 and other CALR variants (P = .7).
LFS in PMF was significantly worse in the presence of triple-negative mutational status, compared with CALR (HR, 0.1; 95% CI, 0.05-0.35), JAK2 (HR, 0.4; 95% CI, 0.2-0.7) and MPL (HR, 0.3; 95% CI, 0.1-0.9) mutated status. Conversely, CALR-mutated patients were at lesser risk of leukemic transformation, compared with triple-negative (HR, 7.6; 95% CI, 2.8-20.2) and JAK2 (HR, 2.7; 95% CI, 1.1-6.6) mutated cases, but not when compared with MPL-mutated cases (HR, 1.9; 95% CI, 0.5-7.7).
Mutational status and disease outcome in ET
The 576 patients with ET included JAK2 (n = 336; 58%), CALR (n = 134; 23%), MPL (n = 22; 4%), mutated or triple-negative (n = 84; 15%) cases. Survival differences between mutational categories, noted during univariate analyses (P values of .04 and .02 in favor of triple-negative vs JAK2- and MPL-mutated cases, respectively), were fully accounted for by differences in age distribution (age-adjusted P values were .38 and .21, respectively). Similarly, a near-significant (P = .06) difference in age-adjusted survival between triple-negative and CALR-mutated cases (P = .06) became insignificant when adjusted for gender (P = .31). LFS in ET was not affected by mutational status.
A total of 55 (9.5%) fibrotic transformations were documented among the 576 ET patients from Mayo and Italy. These included 27 (8%) of 336 JAK2-mutated, 18 (13.4%) of 134 CALR-mutated, 6 (27%) of 22 MPL-mutated, and 4 (4.8%) of 84 triple-negative cases (P = .004); the apparently significant difference was attributed to the higher transformation rate in MPL-mutated ET and the P value between the other 3 mutational categories was .06.
Discussion
Among the BCR-ABL1–negative MPN, ET is generally considered to have the best prognosis and PMF the worst.18 In this regard, recent reports have underscored the prognostic relevance of strict adherence to WHO-defined morphologic criteria, in distinguishing ET from both prefibrotic PMF and masked PV.19,20 The recent discovery of CALR mutations in JAK2 and MPL unmutated MPN,2,3 their remarkable specificity to ET and PMF, and their potential relevance to disease outcome warranted a revisit on the prognostic interrelationship between morphologically defined PV, ET, and PMF.
The current study, which is distinguished by the consideration of strictly WHO-defined MPN that is fully annotated for JAK2/CALR/MPL mutational status, establishes the superiority of morphologically defined ET to PV, regardless of mutational status. We also show reduced life expectancy in ET, which was suggested in a previous population-based study.18 Mature survival data in patients younger than age 60 years revealed very long median survivals of ∼33 years for ET, 24 years for PV, and 15 years for PMF. The particular observation underscores the need to respect the generally stable clonal biology in ET and PV and avoid subjecting patients to investigational drug therapy whose long-term ill effects are unknown.
The second major point from the current study is the demonstration of similar overall and LFS data between JAK2- and CALR-mutated ET, both of which were significantly better than those of PV. Also, patients with JAK2-mutated ET who converted to bona fide PV were rare in both the Mayo and Italian cohorts. These observations do not support the concept of a disease continuum between JAK2-mutated ET and PV and, instead, suggest 2 separate disease entities that are primarily distinguished by their morphologic traits. In other words, PV and ET are not distinguished by their genetic profile but by their phenotypic characteristics and clinical course.
The respectively favorable and unfavorable prognostic impact of CALR-mutated and triple-negative mutational status in PMF is therapeutically relevant. Obviously, treatment decisions should also account for the Dynamic International Prognostic Scoring System–plus risk stratification21 and additional prognostically detrimental mutations.10,11,22 The apparently higher incidence of fibrotic transformations in MPL-mutated ET warrants further investigation.
In conclusion, currently known mutations have yet to overshadow the need for morphologic distinction of ET from PV, in terms of not only diagnostic but also prognostic relevance. In other words, determination of JAK2/CALR mutational status alone, without morphologic examination, is not sufficient to differentiate PV from JAK2 mutant ET. On the other hand, distinguishing morphologically defined ET into JAK2 and CALR subtypes carries limited prognostic relevance, in terms of survival and BT. In contrast, JAK2/CALR/MPL mutational status provides significant prognostic information in PMF, which should be further clarified in view of the possible difference in prognostic contribution from distinct CALR variants.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study from the Mayo Clinic was supported by the Mayo Clinic Harvey-Yulman Charitable Foundation for Myelofibrosis Tissue Bank and Clinical Database of Molecular and Biological Abnormalities. The study in Florence was supported by a special grant from Associazione Italiana per la Ricerca sul Cancro (AIRC 5 per Mille) to AIRC-Gruppo Italiano Malattie Mieloproliferative (AGIMM) (#1005; for a description of the AGIMM project and list of investigators, see www.progettoagimm.it). This work was also supported also by Fondo per gli Investimenti della Ricerca di Base (FIRB; RBAP11CZLK) and Progetti di ricerca di interesse nazionale (PRIN; 2010NYKNS7) (A.M.V.).
Authorship
Contribution: A.T. designed the study, contributed patients, extracted data, performed statistical analysis, wrote the paper, and approved the final draft of the manuscript; A.M.V., P.G., and T.B. designed the study, contributed patients, extracted data, and approved the final draft of the manuscript; L.P., N.G., R.F., A.R., G.F., and A.P. contributed patients, extracted data, and approved the final draft of the manuscript; C.F., E.A.W., A.A.B., and T.L.L. performed mutation analysis, extracted data, and approved final draft of manuscript; D.R.L. performed statistical analysis; R.P.K. provided resources for cytogenetic analysis and expertise in result interpretation; and C.A.H. and J.T. reviewed bone marrow pathology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ayalew Tefferi, Division of Hematology, Department of Medicine, Mayo Clinic, Rochester MN 55905; e-mail: tefferi.ayalew@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal