Abstract
Background: The molecular diagnosis in ALL allows by the research for rearrangements V(D)J on lymphoblast DNA , to find markers of clonality in 95% of the cases. These markers also are used to quantify the minimal residual disease by real time Q-PCR to adapt treatments. This strategy fails in some cases : Absence of initial marker, failure of sequencing or emergence at relapse time of a clone not observed at diagnosis time or in very minority. Several studies have asserted the usefulness of high-throughput sequencing (HTS). It enables deep sequencing of a lymphoid population, bypassing some of these problems. However, the huge amount of data raises two challenges. First, hospitals must be able to store and process terabytes of data per year. Second, the data must be nicely synthesized to ease clinician interpretation.
Here, we report the use of HTS, in a hematology lab, for diagnosis and follow-up of ALL combined with a bioinformatic analysis and visualization with the new dedicated Vidjil software (Giraud, Salson, et al, BMC Genomics 2014, http://www.vidjil.org).
Patients and methods: We studied the clonality of 8 pediatric patients (5 B-ALL and 3 T-ALL, 2w/6m, 2-14 years) at diagnosis and follow-up (37 follow-up time points). The sensitivity was estimated by a range of dilution of DNA tumoral in DNA of PBL from healthy donors (10-2 to 10-5). For every sample, 500ng of bone marrow DNA are extracted on Qiagen® Kit, measured on NanoDrop system® and amplified by a classical (not fluorescent) PCR system for TCRg and IgH target. These systems are described or derived from the BIOMED-2 works. The sequencing libraries are done from the PCR products, verified by electrophoresis on agarose gel then bar-coded with Ion Fragment Plus® kit and sequenced with an Ion Torrent® 318 Chip system.
The obtained sequences are classified on the basis of their V(D)J rearrangements. The dedicated Vidjil browser enables to explore the lymphocyte population and to track the clones along the time. We can inspect the sequences and send them directly to IMGT/V-QUEST or IgBlast for further analysis. It is possible to tag, rename or filter out some clones, and export the resulting graphs to a printable file. Due to sequencing errors, there may be several clones corresponding to a real clone. The browser enables to align such sequences, and we can choose to merge them. The browser can also be used to compare several runs on the same sample, for example with different PCR conditions.
Results: We identified several clones in the diagnosis sample and observed their evolution at different follow-up time points. Clones that were detected by classic methods were also found by Vidjil. Moreover the software allows us to look more in-depth at other clones appearing at lower concentrations. Relapses were detected, and for one patient, two emerging clones were observed.
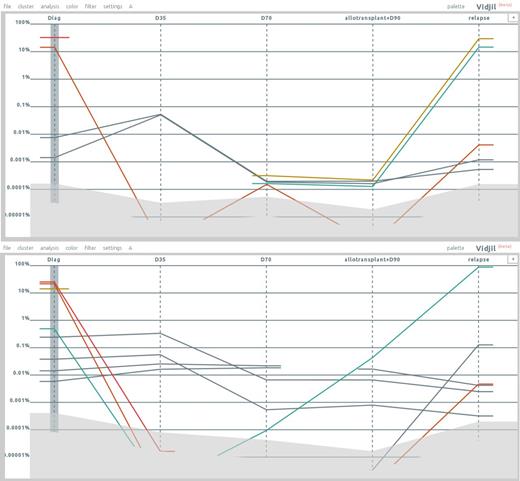
Figure 1 shows plots of the concentration for a patient with B common-ALL. The first point is the diagnosis; the four other points are respectively D35, D70, and D90 after bone marrow transplantation, and relapse. The patient was followed both on IgH (upper plot) and TCRg (lower plot). In both systems, there is the emergence of a new clone at relapse while some of the main clones at diagnosis were not detectable anymore or at a very low concentration. Those clones were also confirmed by conventional methods.
Using high-throughput sequencing together with bioinformatic analysis and visualization with Vidjil allows identifying very easily the emergence of new clones that were not detected at diagnosis.
Conclusion: The HTS prefigures new steps both for the knowledge of the lymphoid and auto-immune pathologies and for the ALL MRD follow-up. Coupled with a bioinformatic analysis, it gives a more complete insight of the blastic population at diagnosis and allows observing the evolution of this population. The whole analysis including the preparation, the sequencing, the software analysis and the clinician validation seems faster than the current protocols.
Our protocol has been designed and tested for two years in Lille and is now being tested in other French hospitals involved in ALL-MRD. We believe that such integrated approaches, where clinicians maintain control over patient data, have their role to play. This raises the need for people having experience with high-throughput sequencing in hospitals. With the advent of this technology and its biomedical applications, that should not be a great issue.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal