Abstract
Introduction: Older (age ≥60 years) patients with acute myeloid leukemia (AML) have poor outcomes and allogeneic hematopoietic cell transplantation (allo-HCT) remains the most curative option for this population. Pre-transplant remission status strongly influences leukemia free survival in AML patients with most studies showing similar survival outcomes in patients in first versus second complete remission (CR1 vs. CR2). A recent study has shown poor outcomes in CR2 compared to CR1 in older patients (Gupta et al, BBMT 2014). However, studies comparing disease status in older AML patients at the time of transplantation remain scarce. We retrospectively evaluated the outcomes of older AML patients who undergoing allo-HCT at our institution.
Patients and methods: A total of 114 older AML patients (median age 64 years, range 60-73 years, 73 males and 41 females) between July 2009 and December 2013 underwent allo-HCT at Washington University School of Medicine in St Louis. Thirty-four percent were HLA-matched related, 54% were HLA-matched unrelated and 12% were HLA-mismatched unrelated transplants. Conditioning regimen was myeloablative in 47%, radiation was utilized as part of conditioning regimen in 43% and anti-thymocyte globulin was part of the immunosuppression regimen in 17%. A higher percentage of patients in CP and active disease had MA regimen while more patients in CR1 and CR2 had RIC/NMA regimen (p=.04). Based on cytogenetics, 2% had favorable, 60% intermediate and 51% poor prognosis, whereas 1% had unknown risk disease. The entire cohort was stratified into 4 groups: CR1 (n=52), CR2 (n=24), active disease (n=21) and cytogenetic persistence [CP; (n=17)] based on disease status at time of allo-HCT. CP was defined as the persistence of cytogenetic abnormalities identified through karyotyping or FISH but otherwise in morphologic remission. Primary endpoint was overall survival (OS) and secondary endpoints were leukemia free survival (LFS) and non-relapse mortality (NRM).
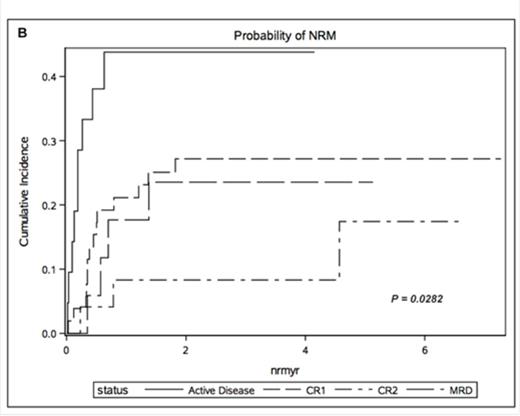
Results: The median follow up for survivors was 47months (9.7 months for the entire cohort). Three year OS was 35.0% (95% CI 22-48) in CR1, 36.4% (95% CI 17-55) in CR2, 29.4% (95% CI 10-51) in CP and 11.4% (95% CI 1-35) in patients with active disease. The cumulative incidence of relapse at 1 year was 26.9% for CR1 (95% CI 16-40), 45.8% for CR2 (95% CI 25-64), 46.9% for CP (95% CI 29-63) and 48.6% for active disease (95% CI 35-61). We found 1-year LFS of CR1 24% (95% CI 8-26), CR2 15% (95% CI 12-25), CP 11% (95% CI 8-16) and active disease cohort 10% (95% CI 7-18). Furthermore, Cox multivariate analysis revealed active disease at the time of allo-HCT to be a significant variable associated with poor OS (p <.0004) (HR 3.648; CI 1.980-6.720) (Figure 1A). Nevertheless, CR2 status, CP and conditioning regimen were not identified as independent risk factors for poor OS. NRM however differed significantly in these cohorts with 21.1% (95% CI 11-33) in CR1, 8.3% (95% CI 1-24) in CR2 and 17.6% (95% CI 4-39) in CP (p=.0282) (Figure 1B). There was no difference in the cumulative incidence of aGVHD and cGVHD between these four groups.
Conclusions: Based on our experience, allo-HCT provides a viable treatment option with acceptable long term survival in older AML patients in CR1, CR2 and CP, in contrast to a recent study suggesting extremely poor outcomes in CR2. Tailoring intensity of conditioning regimen may favorably impact NRM in these cohorts. Nevertheless, survival of older AML patients with active disease continues to be grim and myeloablative regimens, though associated with less relapse, come with a cost of unacceptable NRM. Based on the poor outcomes in our active disease cohort, we encourage treating these patients on clinical trials incorporating less toxic regimens or with alternative non-transplant regimens.
Overall Survival (A) and cumulative incidence of non-relapse mortality (NRM) (B) stratified by disease status at the time of transplantation
Overall Survival (A) and cumulative incidence of non-relapse mortality (NRM) (B) stratified by disease status at the time of transplantation
Vij:celgene: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal