Abstract
Background: The salivary gland is one of the most common sites of involvement by non-gastric extranodal marginal zone lymphomas of mucosa-associated lymphoid tissue (MALT). This multicenter, international trial sought to characterize the clinical course, treatment and outcome of patients with salivary gland MALT lymphoma.
Methods:
Patients were identified from multiple international sites including the Mayo Clinic Lymphoma Database (n=89), three International Extranodal Lymphoma Study Group (IELSG) cohorts (n=138), and the University of Athens (n=21). All patients had biopsy-confirmed MALT lymphoma of the salivary gland by WHO criteria. Clinical characteristics, treatment, and outcomes were obtained from the respective patient databases. Survival probability was estimated using the Kaplan-Meier method and compared between groups with the Wilcoxon or log-rank. The impact of variables on survival was assessed by the Cox proportional hazards model.
Results:
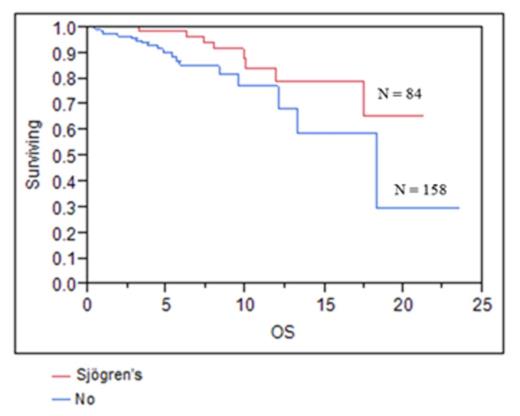
Patients and clinical features: From 1983 to 2012, 247 patients with MALT lymphoma were included. Clinical characteristics are shown in Table 1. The median age at diagnosis was 59, and the male-to-female ratio was 1:3. The majority of patients (76%) presented with limited stage disease, and the parotid gland was the most common site of involvement (78%). There was a history of antecedent autoimmune disease in 41% of patients, and Sjögren’s disease was the most common (83%).
Treatment: Information on initial treatment was available on 242 patients. 137/242 patients (57%) initially received local therapy with either surgery (n=81), radiation (n=26), or both (n=30). Of these, 25 received adjuvant systemic therapy. 90/242 patients (37%) were treated initially with systemic therapy, of whom 54% had localized and 46% had stage IV disease. Rituximab was used alone (n=16) or in combination with chemotherapy (n=26) in 42 of the 90. 15/242 patients (6%) were initially observed.
Survival: The median OS for all patients was 18.3 years. PFS following primary therapy was 9.3 years. There was no difference in the outcomes between patients receiving local or systemic therapy in first line for all patients, or for those with stage I or II disease. On univariate analysis age <60, stage, IPI, normal LDH, the presence of Sjögren’s disease, and treatment with rituximab were associated with improved overall survival. Age <60, low to intermediate IPI and normal LDH were associated with improved PFS. On multivariate analysis, age<60, and IPI were associated with improved OS and PFS and Sjögren’s disease was associated with improved OS.
Relapse/progression: The site of progression was known in 51 of 76 patients who progressed. 15 (29%) patients progressed in the same salivary gland or regional nodes; in 16 (21%) recurrence involved the contralateral salivary gland; 20 (31%) had distant progression. Relapse histology was identified in 30. The most common pathology at relapse was MALT (n=28), with one patient progressing to diffuse large B-cell lymphoma. A second patient developed a mantle cell lymphoma.
Conclusions:
Salivary gland MALT lymphoma commonly presents at an early stage, and has a favorable prognosis with a median OS of 18 years. Surgical or radiation therapy does not appear to have benefit over systemic therapy. Patients with Sjögren’s disease appear to have a better survival. The role of rituximab and optimal first-line therapy should be evaluated further in prospective trials.
Characteristics of 247 patients with salivary MALT
| Characteristic . | N (%) . |
|---|---|
| Age, median; range | 59; 18-93 |
| Sex, Male: Female | 62: 185 (25: 75) |
| Primary site of involvement | |
| Parotid | 193 (78) |
| Submandibular | 12 (5) |
| Other/not specified | 42 (17) |
| Extent (unilateral: bilateral) | 89:21 |
| Missing data | 137 |
| Autoimmune disorder* | 101(41) |
| Sjögren’s | 84 (33) |
| Rheumatoid arthritis | 9 (4) |
| Raynaud’s phenomenon | 8 (3) |
| Scleroderma | 5 (2) |
| Other | 19 (8) |
| Stage | |
| IE | 147 (59) |
| IIE | 42 (17) |
| IV | 56 (23) |
| Missing data | 2 (1) |
| IPI Score | |
| Low (0-1) | 126 (51) |
| Low-Intermediate (2) | 20 (8) |
| High-intermediate (3) | 21 (8) |
| High (4-5) | 5 (2) |
| Missing data | 75 (31) |
| Elevated LDH | 17 (7) |
| ECOG PS | |
| 0-1 | 246 |
| 2 | 1 |
| Extranodal sites | |
| 1 | 58 (23) |
| 2 | 187 (76) |
| Missing data | 2 (1) |
| B symptoms | 9 (4) |
| * Percents may not equal 100 as some patients had more than one disorder. | |
| Characteristic . | N (%) . |
|---|---|
| Age, median; range | 59; 18-93 |
| Sex, Male: Female | 62: 185 (25: 75) |
| Primary site of involvement | |
| Parotid | 193 (78) |
| Submandibular | 12 (5) |
| Other/not specified | 42 (17) |
| Extent (unilateral: bilateral) | 89:21 |
| Missing data | 137 |
| Autoimmune disorder* | 101(41) |
| Sjögren’s | 84 (33) |
| Rheumatoid arthritis | 9 (4) |
| Raynaud’s phenomenon | 8 (3) |
| Scleroderma | 5 (2) |
| Other | 19 (8) |
| Stage | |
| IE | 147 (59) |
| IIE | 42 (17) |
| IV | 56 (23) |
| Missing data | 2 (1) |
| IPI Score | |
| Low (0-1) | 126 (51) |
| Low-Intermediate (2) | 20 (8) |
| High-intermediate (3) | 21 (8) |
| High (4-5) | 5 (2) |
| Missing data | 75 (31) |
| Elevated LDH | 17 (7) |
| ECOG PS | |
| 0-1 | 246 |
| 2 | 1 |
| Extranodal sites | |
| 1 | 58 (23) |
| 2 | 187 (76) |
| Missing data | 2 (1) |
| B symptoms | 9 (4) |
| * Percents may not equal 100 as some patients had more than one disorder. | |
Nowakowski:Celgene, Morphosis: Consultancy. Zucca:Roche, Mundipharma, Novartis, Jannsen, Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal