Abstract
The evolution of acute myeloid leukemia (AML) has been previously described either in studies of large patient cohorts with focus on only a restricted number of AML-associated genes or in smaller series of relapsed patients studied by genome-wide techniques. We set out to comprehensively characterize the genetic evolution in a large AML cohort in order to understand molecular mechanisms of relapse and therapy-resistance.
We performed exome-sequencing of matched bone marrow or peripheral blood samples taken at diagnosis, complete remission and relapse from 47 patients with cytogenetically normal AML (CN-AML). Samples were collected within the German Cancer Consortium (DKTK) at the partner sites in Berlin and Munich. The median age at diagnosis was 65y (range: 21-89y). FLT3 internal tandem duplication (ITD) and NPM1 mutation status at diagnosis was available for all but one patient (FLT3-ITD-/NPM1-, n=5; FLT3-ITD+/NPM1-, n=9; FLT3-ITD-/NPM1+, n=16; FLT3-ITD+/NPM1+, n=16).
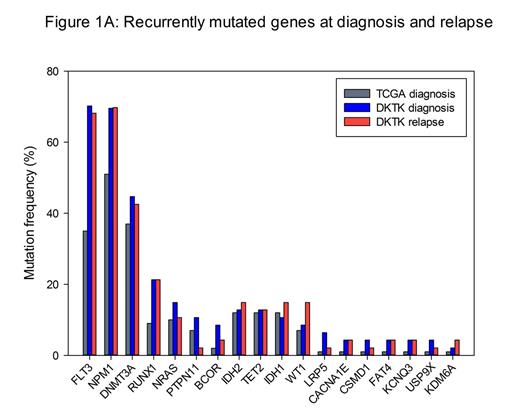
On average, 96% of the target sequence was covered at least 10-fold (minimum coverage defined for variant calling). The following criteria were applied for identification of somatic mutations: Variant allele frequency (VAF) ³20% either at diagnosis or at relapse and VAF<5% at remission. We filtered for mutations with translational consequences, excluded known error-prone genes and dismissed common germline polymorphisms (dbSNP 138; MAF³1%). Thereby, we identified a total of 777 genes to be somatically mutated, of which 104 were recurrently affected. Mutation frequencies of 18 genes found mutated both in our cohort and in 86 CN-AML patients reported by The Cancer Genome Atlas (TCGA, NEJM 2013) are shown in Figure 1 A. Seven genes were recurrently altered only at diagnosis (e.g. CBL) and 16 genes were recurrently altered only at relapse in our cohort (e.g. KDM6A, SF3B1 and SRSF2). At diagnosis, the number of somatic mutations per patient varied between 5 and 34 (median: 17). At relapse, the number of mutations ranged from 2 to 57 (median: 17). Mutations in several AML-associated genes (e.g. DNMT3A, RUNX1, IDH1 and IDH2) showed similar VAFs at diagnosis and relapse in the vast majority of cases. In contrast, WT1 mutations were gained at relapse in 4/6 (67%) patients and FLT3 point mutations were below 5% VAF at relapse in 7/12 (58%) patients initially positive for these variants. In total, 92 mutations present at diagnosis were lost at relapse (VAF <5%) while 116 mutations were acquired during disease progression.
Based on cytogenetics and copy number alteration (CNA) analysis of exome data, we detected partial or complete gain/loss of chromosomes. Five patients (11%) acquired chromosomal alterations during disease progression. Trisomy 8 was the only recurrent chromosomal abnormality gained in 3 patients (6%) at relapse.
To detect pre-leukemic lesions, we evaluated our exome data for the persistence of mutations in 40 AML-associated driver genes during remission. We limited our analysis to mutations previously reported as confirmed somatic (COSMIC annotation) to avoid confounding with private germline variants. Strikingly, 25/47 (53%) of patients carried non-silent mutations in these genes with VAF>5% (median: 31%, range: 9-75%) at remission (30 mutations in total). In contrast, other mutations (e.g. in FLT3 or NRAS) found in these patients could not be detected at remission, consistent with therapy response. Based on VAF, 23/30 (77%) persistent mutations showed a dynamic pattern over the course of disease with a relative change of >20%, likely due to partial eradication/expansion of leukemic or pre-leukemic clones. Persistent mutations in DNMT3A, TET2, RUNX1 and IDH2 were observed in 28%, 11%, 6% and 4% of patients in our cohort, respectively (Figure 1 B). Among patients with DNMT3A mutation at diagnosis, those with persistent mutations tended to relapse earlier (n=13; median time to relapse 270 days; range: 81-586) than patients without detectable DNMT3A mutations at remission (n=7; median time to relapse 508 days; range: 235-1697; p=0.111).
Our findings provide insights into the genetic evolution during the course of disease in a large cohort of relapsed CN-AML. Information about the dynamics of genetic lesions (e.g. persistent or relapse-specific mutations) may have prognostic significance and allow for tailored approaches to treat or to prevent relapse of AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal