Abstract
Introduction
Many drug discovery efforts and pharmacogenomic studies are based on testing established cancer cell lines for their sensitivity to a given drug or a panel of drugs. This approach has been criticized due to high selectivity and fast proliferation rate of cancer cell lines. To explore new therapeutic avenues for acute myeloid leukemia (AML) and to compare experimental model systems, we applied high-throughput Drug Sensitivity and Resistance Testing (DSRT) platform with 305 approved and investigational drugs for 28 established AML cell lines and compared their drug responses with our earlier study of 28 ex vivo AML patient samples (Pemovska et al., 2013). We then correlated drug sensitivities with genomic and molecular profiles of the samples.
Methods
DSRT was carried out with 305 clinical, emerging and experimental drugs and small molecule chemical inhibitors. The drugs were tested at five different concentrations over a 10,000-fold concentration range. Cell viability was measured after 72 hours using Cell Titre Glow assay. IC50 values were calculated with Dotmatics software and drug sensitivity scores (DSS, a modified area under the curve metric) were derived for each drug (Yadav et al., 2014). Nimblegen's SeqCap EZ Designs Comprehensive Cancer Design kit was used to identify mutations from 578 oncogenes in cell lines.
Results
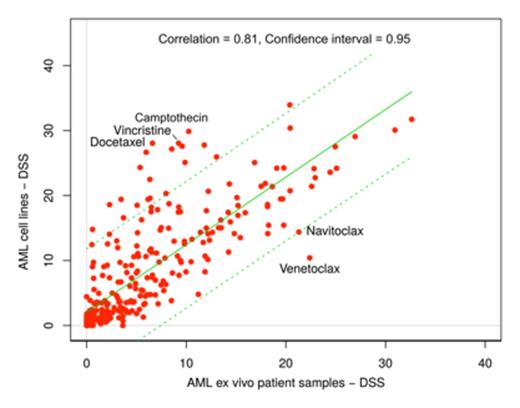
The 28 established AML cell lines were in general more sensitive to the drugs as compared to the 28 ex vivo patient samples, with some important exceptions. Sensitivity towards many targeted drugs was observed in both AML cell lines and in patient samples. These included inhibitors of MEK (e.g. trametinib in 56% of cell lines and 36% of ex vivo samples), mTOR (e.g. temsirolimus in 42% and 32%) and FLT3 (quizartinib in 28% and 18%). Overall, drug responses between cell lines and ex vivo patient cells in AML showed an overall correlation coefficient of r=0.81. BCL2 inhibitors (venetoclax and navitoclax) showed more sensitivity in ex vivo patient cells than in AML cancer cell lines, whereas responses to anti-mitotic agents (docetaxel, camptothecin, vincristine) showed stronger responses in cell lines (Figure). Only 7% of AML cell lines exhibited responses to a broad-spectrum tyrosine kinase inhibitor dasatinib, in contrast to 36% patient samples.
AML cell lines that carried FLT3 mutations showed high sensitivity to FLT3 inhibitors. Similarly, cell lines harbouring mutations in RAS or RAF were strongly sensitive to MEK inhibitors. MEK and FLT3 inhibitor responses were mutually exclusive, indicating alternative pathway dependencies in cell lines. However, these pharmacogenomics correlations were not as clearly seen in the clinical samples.
Summary
These data revealed a few important differences as well as many similarities between established AML cell lines and primary AML patient samples in terms of their response to a panel of cancer drugs. The hope is that patient-derived primary cells in ex vivo testing predict clinical response better as compared to the established cancer cell lines, which indeed seem to overestimate the likelihood of responses to many drugs. On the other hand, cancer cell line studies may also underestimate the potential of dasatinib and BCL2 inhibitors as emerging AML therapeutics.
References
1. Pemovska T, Kontro M, Yadav B, Edgren H, Eldfors S, Szwajda A, et al. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discovery. 2013 Dec;3(12):1416-29
2. Yadav B, Pemovska T, Szwajda A, Kulesskiy E, Kontro M, Karjalainen R, et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Scientific reports. 2014;4:5193.
Correlation of average drug responses (n=305) between 28 AML cell lines and 28 AML ex vivo patient samples
Correlation of average drug responses (n=305) between 28 AML cell lines and 28 AML ex vivo patient samples
Heckman:Celgene: Research Funding. Porkka:BMS: Honoraria; BMS: Research Funding; Novartis: Honoraria; Novartis: Research Funding; Pfizer: Research Funding. Kallioniemi:Medisapiens: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal