Abstract
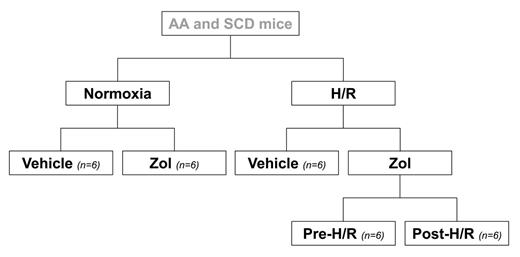
Sickle cell disease (SCD) is a worldwide distributed hereditary red cell disorder, characterized by the synthesis of pathological hemoglobin S (HbS). Up to now, limited studies in SCD patients have proposed a bone loss related to recurrent acute vaso-occlusive events (VOCs). Here, we designed a study in a humanized mouse model for SCD to evaluate (i) bone structure turnover and micro-architecture by histomorphometric approach; (ii) osteoblastic differentiation by RT-PCR analysis of bone runx2, sparc and alp gene expression. We used humanized healthy control (Hbatm1(HBA)Tow Hbbtm3(HBG1,HBB)Tow) and SCD (Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow) mice. Mice were divided into different groups as detailed in the enclosed flow-chart. In steady state, we first evaluated bone-marrow erythropoiesis by FACS analysis based on CD44-TER119 strategy. No signs of ineffective erythropoiesis were detectable in SCD mice compared to controls. Bone analysis was performed by histomorphometric approach after double labelling with demeclocycline followed by calcein to evaluate the dynamic parameters of bone turnover and analyzed by a specific software (bone3.1, Explora Nova, Larochelle, France). The histomorphometric bone analysis revealed (i) reduced bone volume with associated decreased trabecular number and increased trabecular separation; (ii) increased bone-turnover measured as bone formation rate (BFR) and activation frequency (AcF); (iii) decreased number of nodes (NdN) and node termini ratio; (iv) increased marrow star volume (MSV) and fractal dimension (D) compared to healthy mice. The bone molecular analysis revealed a significant down-regulation of runx2, sparc and alp gene expression in SCD mice compared to healthy ones. These data indicate a severe osteoporosis with bone fragility associated with alteration of osteogenic differentiation in SCD mice. Zoledronic acid (Zol) is the most powerful available bisphosphonate used to reduce bone turnover and bone fragility. Since VOCs are life-threatening complication of SCD and mainly involved skeletal system, requiring multidiscliplinary approach, we exposed SCD and control mice to hypoxia/reoxygenation (H/R) stress to mimic acute VOCs (see flow-chart). Mice were exposed to hypoxia (8% oxygen, 10 hours) followed by reoxygenation (21% oxygen) for either 18 hours to carry out molecular analysis or 10 days to carry out histomorphometric analysis. In H/R exposed vehicle treated SCD mice, we found (i) a significant increase in AcF (bone turnover), without differences in bone structure and microarchitecture compared to SCD mice under normoxia; (ii) a further reduction in osteogenic differentiation molecular markers. Zol (100 μg/ Kg in a single ip injection) was administrated either 7 days before (Zol-pre) or immediately after (Zol-post) H/R stress. In SCD mice, both Zol-pre and Zol-post significantly prevented the H/R induced increased bone turnover, while no differences were detectable in bone structure and microarchitecture compared to vehicle treated H/R SCD mice. The molecular analysis showed that Zol increased the osteogenic related gene expression, indicating a positive induction of osteogenic differentiation. Our data suggest that murine SCD is characterized by a significant bone impairment related to the unbalance between osteoblastic/osteoclastic activity in favor of osteoclastic one in presence of a down-regulation of osteogenic differentiation. In SCD, Zol plays a pivotal role decreasing osteoclastic activity and promoting osteogenic differentiation, representing a powerful new therapeutic strategy to limit bone disease in SCD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal