Abstract
ARG (ABL2) is a member of ABL family kinases and highly homologous to ABL (ABL1) except the C-terminal domain adjacent to the kinase domain. TEL/ARG that consists of ARG fused to TEL (ETV6) has been found in AML M3, M4 or T-ALL patients, with additional chromosomal abnormalities of t(15;17)(q12;q21), inv(16)(p13;q12) or t(1;10;12)(q25;q23;p13) translocation, respectively. The structure of TEL/ARG is similar to that of TEL/ABL, which has been found in patients with T-ALL, B-ALL, AML and CML. TEL mediates homo-oligomerization of these fusion proteins, TEL/ABL and TEL/ARG, resulting in constitutive activation of the tyrosine kinases. Although ABL fusion proteins such as BCR/ABL and TEL/ABL have been intensively investigated, the involvement of TEL/ARG in leukemogenesis is not fully elucidated yet.
We have recently reported that in vitro transforming activity of TEL/ARG was significantly lower than that of TEL/ABL although their kinase activities were almost identical. Interestingly, the in vitro transforming activities of C-terminus-swapped mutants, TEL/ABL with C-terminal domain of ARG [TEL-ABL (ARG-C)] or TEL/ARG with C-terminal domain of ABL [TEL/ARG (ABL-C)], were comparable to those of TEL/ARG or TEL/ABL, respectively, while kinase activities in the swapped mutants were not altered. These results suggest that C-termini of ABL family kinases contain some functional domain that defines their distinct transforming activities. The purpose of this study is to compare the in vivo leukemogenic activities of TEL/ABL and TEL/ARG, and evaluate the impact of the C-terminal domains.
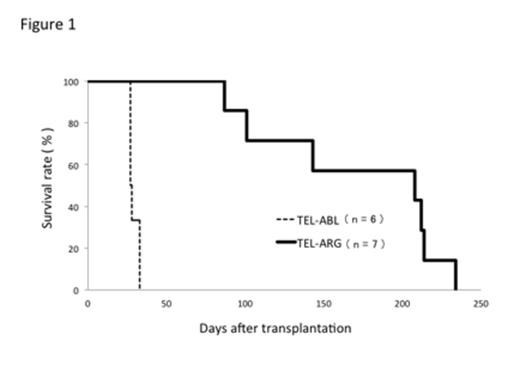
First, we investigated whether TEL/ABL or TEL/ARG caused leukemia in mice. Each fusion gene together with GFP gene was retrovirally transduced into the bone marrow cells harvested from C57BL/6 mice treated with 5-fluorouracil, and the transduced cells were transplanted into lethally irradiated mice. Similar to BCR/ABL, transplantation of TEL/ABL-transduced cells induced rapid myeloproliferative status accompanied by hepatomegaly and/or splenomegaly, and all the recipient mice died within 33 days after transplantation, indicating the development of myeloid leukemia. In contrast, the recipient mice transplanted with TEL/ARG-transduced cells did not develop myeloid leukemia but infiltrative mastocytosis, and died around 200 days after transplantation (Figure 1). Hemophagocytic mast cells accumulating in the bone marrow, and mast cells circulating in the peripheral blood were also observed in these mice.
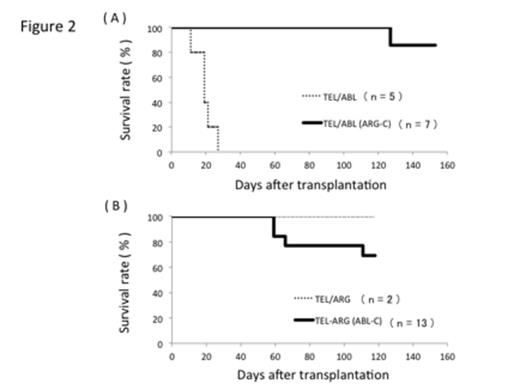
Next we investigated the roles of C-terminal domains of ABL and ARG in their in vivo leukemogenic activities. C-terminus-swapped mutants, TEL/ABL (ARG-C) and TEL/ARG (ABL-C) were retrovirally transduced into bone marrow cells and the transduced cells were transplanted as described above. Intriguingly, TEL/ABL (ARG-C) mutant failed to cause myeloproliferative status or leukemia at day 153 (Figure 2A). On the other hand, TEL/ARG (ABL-C) induced lethal myeloid leukemia in 4 out of 13 mice (30.8%) within 111 days after transplantation (Figure 2B). Collectively, the in vivo phenotypes induced by TEL/ABL (ARG-C) or TEL/ARG (ABL-C) resembled those induced by TEL/ARG or TEL/ABL, respectively. Mastocytosis, a characteristic of TEL-ARG-induced phenotype, has not been observed so far in any of the recipients of TEL/ABL (ARG-C) or TEL/ARG (ABL-C).
In conclusion, these results indicate that C-terminal domain of ABL family kinases defines their distinct leukemogenic activities in vivo through modulating both proliferation and differentiation. Notably, C-terminus of ARG strongly suppressed the in vivo leukemogenic activity of TEL/ABL without impairing the tyrosine kinase activity. Further clarification of the molecular mechanisms underlying the suppressive activity of C-terminus of ARG will lead to development of a novel therapeutic strategy, especially for patients with CML harboring mutations, which are resistant to tyrosine kinase inhibitors.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal