Abstract
Background. Conceptually mRNA processing and ribosomal regulation should interact as both affect mRNA translation and protein production. We studied protein expression and functional relationships between proteins in AML using a custom made reverse phase protein array (RPPA), probed with 231 strictly validated antibodies. We found a relationship between expression of Ribosomal Protein S6 (HUGO name R6SP, a.k.a. S6RP) and Eukaryotic Translation Initiation Factor 4EBinding Protein 1, (HUGO name EIF4EBP1). R6SP, a 40S ribosomal subunit component, activated by phosphorylation, regulates cell growth via selective mRNA translation. EIF4EBP1 interacts with eIF4E to recruit the 40S ribosomal subunit, thereby affecting ribosomal assembly. When phosphorylated, in response to cellular signaling, it releases eIF4E allowing transcription.
Methods. Our RPPA has protein from leukemia enriched cells from 511 newly diagnosed AML patients and was probed with 231 strictly validated antibodies, including antibodies against total RPS6 and forms phosphorylated on S235-236 and S240-244, and against total EIF4EBP1 and forms phosphorylated on T37 & 46, T70 and S65. Expression was compared to normal bone marrow derived CD34+ cells. Interaction networks with the other 224 proteins were generated from the RPPA data using glasso and supplemented by the literature of known interactions.
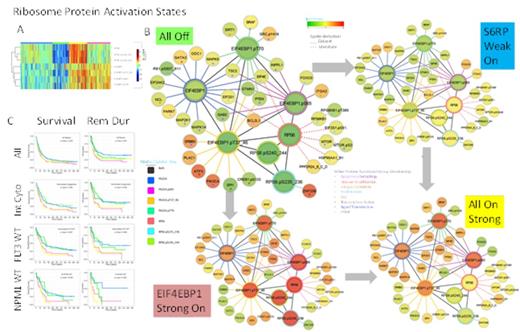
Results. A heatmap of expression of the 3 R6SP and 4 PA2 forms was generated and hierarchical k-and means clustering performed (Fig A). Using the Prototype Clustering method an optimal division into four clusters (Fig B) was determined. This includes an All-Off state (18%), a state characterized by weak activation of RPS6 alone (RP-Only, 36%) activation of only EIF4EBP1 (EIF4EBP1-Only, 26%) and a group where both were on simultaneously (Both-On). The RPS6 interactome (Fig B) showed the expected positive correlation with mTOR, and P70 (Hugo RPS6KB1) and a previously unknown, but very strong, negative correlation with transcription factor ZNF296. The EIF4EBP1 interactome showed the expected strong positive correlation with many signal transduction pathways (MAP2K1, MAPK14) and proliferation related proteins (pRB, EIF2AK, EIF2S1, FOXO3) and negative correlation with several transcription factors (GATA3, SPI1, CREB). Cluster membership was unassociated with most clinical features including cytogenetics, FLT3 , RAS and NPM1 mutation, excluding gender (more F in All-Off, more M in Both-On, p=0.01). EIF4EBP1 and Both-On had higher WBC (p=0.0001) and % marrow (p=0.0001) and blood blasts (0.0007) and lower platelet counts (p=0.025). Response rates did not differ, although fewer All-Off were primary refractory. Relapse was more common in EIF4EBP1-Only and Both-On clusters. Overall survival (OS) and remission duration (RemDur) (Fig C) of the EIF4EBP1-Only and Both-On clusters was inferior to that of the All-Off and RP-Only clusters (OS median 41 & 45 vs. 52 &63,p=0.06, RemDur 39 & 27 weeks vs. 63 & 53, p=0.008) but this was restricted to Intermediate cytogenetics cases (Fig C IntCyto OS 49 & 55 weeks vs. 107& 79 p=0.01, RemDur 37 & 35 weeks vs. 89 & 53 , p = 0.005) that were FLT3 mutation ((Fig C FLT3-WT OS p=0.006, RemDur p0.007) and NPM1 mutation negative (Fig C NPM1-WT, OS p=0.006, RemDur p=0.001).
Conclusions. Activation of EIF4EBP1, with or without RPS6 activation is prognostically adverse in AML, particularly in intermediate cytogenetic cases with wildtype FLT3 and NPM1. This is associated with increased proliferation. Therapy directed against EIF4EBP1 activity, e.g. that block it's phosphorylation, may have utility in the ~46% of cases of AML that demonstrate high levels of EIF4EBP1 phosphorylation, especially in FLT3/NPM1 wildtype cases. Many agents that inhibit signal transduction pathways are in clinical development, analyzing them for the ability to inhibition the activation of EIF4EBP1 might identify clinically useful molecules.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal