Abstract
Background: High dose melphalan with autologous stem cell transplant (SCT) is an effective therapy for patients with light chain (AL) amyloidosis. With appropriate patient selection, risk-adapted melphalan dosing (RA-SCT), and post transplant consolidation in patients with <CR, treatment-related mortality is low and overall median survival (OS) in a large series is beyond 6 years and more than a decade for patients who achieve a complete hematologic remission (CR).1,2 However, some patients who achieve CR relapse within 2 years and have inferior OS. A recent study suggested that bone marrow plasmacytosis (BMPC) exceeding 10% at diagnosis is associated with an inferior survival, similar to patients considered to have AL with concurrent symptomatic multiple myeloma (MM) based on the presence of hypercalcemia, renal dysfunction, anemia and bone lesions (CRAB criteria).3 We aimed to confirm the prognostic significance of BMPC at diagnosis in AL patients who underwent RA-SCT +/- consolidation on OS and evaluate the impact on event-free survival (EFS).
Methods: We assessed the outcomes of all AL patients who underwent RA-SCT at Memorial Sloan Kettering Cancer Center. Patients with >2 major organs involved, NYHA class III/IV CHF, critical arrhythmias, cardiac syncope or concurrent symptomatic MM based on CRAB criteria were ineligible. Response was assessed at 3 and 12 months after RA-SCT; patients with < CR at 3 months were offered consolidation. EFS and OS were calculated from transplant to next treatment, death or last follow-up and were estimated by Kaplan-Meier methods. A one-year landmark was used to compare survival by treatment response (CR vs <CR). OS and EFS were compared across covariates of interest using the log-rank test. The patients were divided based upon percentage of BMPCs on initial diagnostic bone marrow using the highest estimate from the aspirate or biopsy. Cox proportional hazards model was used to examine the effect of BMPC on OS after adjusting for response at 1 year and cardiac stage.
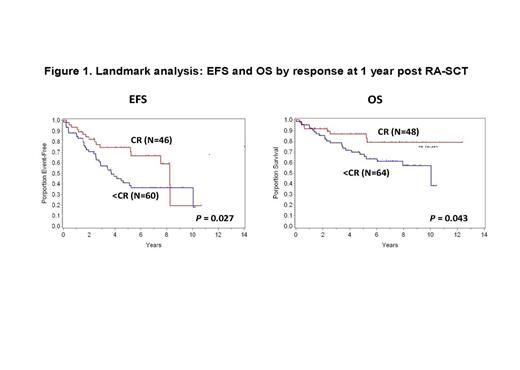
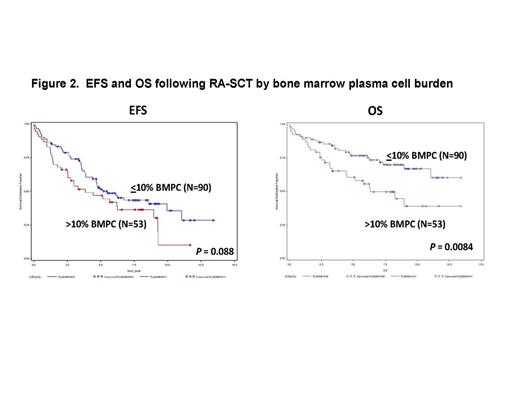
Results: Between 2/2000 and 6/2011, 148 patients with AL amyloidosis underwent RA-SCT and were followed. Five patients without diagnostic bone marrow results available were excluded. Of those included, 63% (N=90) of patients had ≤ to 10% BMPC at diagnosis and 37% (N=53) had more than 10%. With a median follow up among survivors of 7.73 years, the median EFS is 4.82 years, 95% CI (3.84 – 6.23), and the median OS has not been reached. By univariate analysis, melphalan dose (200, 140, or 100mg/m2) (EFS P = 0.002, OS P = 0.002), organ involvement (≤ 1 vs >1) (EFS P = <0.01, OS P = 0.003), and Mayo cardiac stage (I, II, or III) (EFS P = 0.003, OS P = <0.01) were all significantly associated with both EFS and OS. Patients with ≤ 10% BMPCs at diagnosis had a trend toward better EFS (P = 0.08) and statistically significant longer OS (P = 0.008) compared to patients with a higher burden of plasma cell disease at baseline (> 10% BMPCs) (Figure 2). Achieving a CR at 1 year following RA-SCT was associated with superior EFS (P = 0.027) and OS (P = 0.043) (Figure 1) but was independent of whether or not CR was achieved with or without consolidation (EFS P = 0.14; OS P = 0.24). In multivariate analysis, higher burden of plasma cell disease at baseline remained an independent risk factor for OS [HR: 4.7 (95%CI: 1.7-12.9, p<0.01)], after adjusting for treatment response at 1 year and cardiac stage.
Conclusion: AL amyloidosis patients who are eligible for RA-SCT have excellent outcomes with OS at 10 years of 58% (95%CI: 47%-66%). Patients who achieve a CR with RA-SCT alone or with RA-SCT plus consolidation do equally well overall. Patients with greater burden of organ disease as indicated by number of organs involved, the requirement for dose reduction, or Mayo cardiac stage have inferior outcomes. This is unlikely to change without earlier diagnosis. The finding that greater plasma cell burden at diagnosis also impacts OS in patients who received RA-SCT independent of cardiac disease, treatment response and/or consolidation suggests that a more proliferative clone may require more MM-like therapy, and that these patients may benefit from more prolonged maintenance therapy. A clinical trial addressing this question in patients with AL amyloidosis is planned.
1. Gertz et al. BMT 2013; 48: 557.
2. Cibieira MT, et al. Blood 2011; 118: 4346.
3. Kourelis TV et al. JCO 2013; 31: 4319.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal