Abstract
Background:
Autologous stem cell transplantation and the development of new agents with potent anti-tumor activity have considerably improved the survival of multiple myeloma (MM) patients. However, there is still a high risk of relapse mainly due to the inability of these agents to cure and eliminate definitively the MM cells. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment particularly for patients with high risk factors but its use is still controversial. We recently demonstrated in a prospective study that long-term outcome in MM patients was better with auto/RIC-allo as compared with auto-HSCT alone (Gahrton et al. , Blood 2013). The debate is still ongoing concerning the best time to propose allogeneic transplantation for MM patients; while there is agreement in the scientific community to perform it preferentially within a clinical trial, the majority of patients in Europe are still treated outside of a clinical trial.
The objective of this study is to describe the context of use of allo-HSCT for MM in Europe within EBMT centers over more than two decades with the evaluation of different outcomes.
Material and methods:
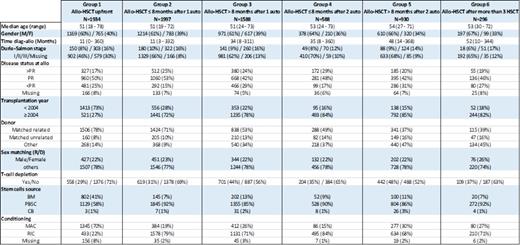
We included in this study a total of 7333 patients who received allo-HSCT between January 1990 and December 2012, 4539 (62%) males and 2794 (38%) females with a median age at allo-HSCT of 51 years (range: 18-78), of which 4726 (64%) have been transplanted after year 2004. We identified 6 groups in this population, patients who received allo-HSCT upfront after induction therapy (Group 1, N=1934), patients who received allo-HSCT maximum 8 months after single auto-HSCT (Group 2, N=1997), patients who received allo-HSCT later than 8 months after single auto-HSCT (Group 3, N=1588), patients who received allo-HSCT maximum 8 months after double auto-HSCT (Group 4, N=588), patients who received allo-HSCT later than 8 months after double auto-HSCT (Group 5, N=930), and finally patients who received allo-HSCT after having received at least 3 different HSCT (Group 6, N=296). The different patient, disease and transplantation characteristics according to each group are detailed in the table.
Results:
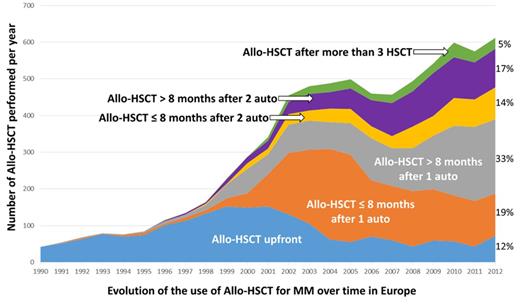
The upfront use of allo-HSCT was seen to decrease after year 2000 to represent 12% of allo-HSCT performed in 2012 while the peak of allo-HSCT use directly after 1 auto-HSCT (within 8 months) was around year 2004 to attend 19% of usage in 2012. Remarkably, allo-HSCT was moreover used at the highest rate during the last years later than 8 months after single auto-HSCT which could be translated in a context of relapse post- first auto-HSCT to reach 33% of usage in 2012. The usage according to groups 4, 5 and 6 was 14%, 17% and 5% in 2012 respectively (Figure).
The median overall survival (OS) for groups 1, 2, 3, 4, 5 and 6 was 33, 69, 25, 25, 23 and 15 months respectively with a 5 years OS probability of 39%, 53%, 33%, 31%, 29% and 23% respectively; the median progression-free survival (PFS) was 45, 39, 21, 22, 21 and 13 months respectively with a 5 years PFS probability of 43%, 42%, 26%, 28%, 24%, and 15% respectively. The cumulative incidence of transplant-related mortality for the 6 groups at 3 years was 36%, 20%, 32%, 33%, 36% and 35% respectively. The use of reduced intensity conditioning was associated with a significantly better OS only in group 2 compared to myeloablative conditioning with a median OS of 76 months versus 45 months (p=0.002) respectively. Year of transplantation before or after 2004 did not influence on outcomes.
Conclusion:
This large retrospective study shows different ways of using allo-HSCT for MM in Europe over a defined time period, it describes the different expected outcomes in each case. Allo-HSCT has not found its place yet in the treatment course of MM patients and the debate should continue based on advantages and disadvantages showed for each group of patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal