Abstract
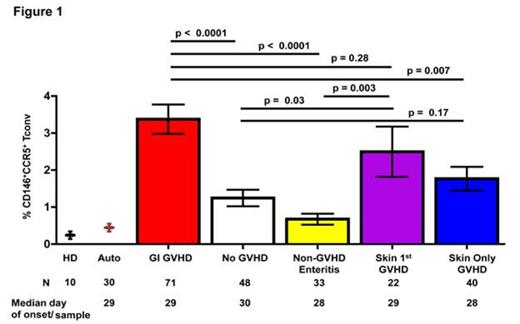
GVHD of the gastrointestinal tract (GI) is associated with high mortality. We have identified systemic, skin- and GI-specific plasma biomarkers present at clinical GVHD onset, and more recently biomarkers for treatment responsiveness. However, in order to identify early GI-specific biomarkers prior to GVHD onset, we performed state-of-the-art proteomics on plasma samples taken 14 days prior to clinical manifestation of GI GVHD. We selected candidates that were increased at least 1.5 fold in plasma from GI GVHD patients compared to HSCT patients without GVHD at matched time points. We identified two lead proteins: CD146, a cell adhesion and trafficking molecule expressed on a subset of CD4+ T cells and endothelial cells, and the chemokine (C-C motif) ligand 14 that binds to the chemokine receptor CCR5 on T cells. As these proteins have not been previously identified in proteomics experiments and antibodies for their corresponding receptors on T cells were available, we analyzed their expression profiles on PB cells from 214 HSCT patients (71 GI GVHD, 48 no GVHD, 33 non-GVHD enteritis, 22 skin first GVHD, 40 isolated skin GVHD) at the onset of symptoms. The frequency of CD146+CCR5+ T cells was significantly increased in GI GVHD patients compared to patients without GVHD, non-GVHD enteritis, or with isolated skin GVHD, as well as increased in patients who first experienced skin and then GI GVHD (Fig. 1). When using the median % of CD146+CCR5+ T cells detected in GI GVHD patients to classify patients into low and high-risk groups, patients in the high-risk group had higher 6-month non-relapse mortality (42 vs. 20%, p = 0.02). Importantly, CD146+CCR5+ T cells at onset of clinical symptoms were not correlated with GI histologic severity, suggesting that these cells are not mucosa damage products but rather systemic effectors. Thus, we measured their frequencies in samples taken at a median of 19 days post-transplant and with a median interval of 14 days prior to clinical symptoms and found that CD146+CCR5+ T cells circulate in patients before GI GVHD clinical onset.
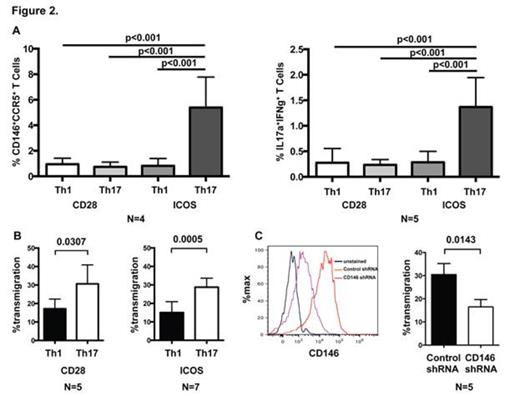
In GI GVHD patients, these cells expressed a Th1 and Th17 phenotype and expressed high levels of the activation marker ICOS known to be critical for the development of human Th17 cells. To test the hypothesis that CD146+ T cells are Th17-prone, we next investigated whether in vitro polarization of CD4 T cells with defined stimulation conditions will increase the CD146+CCR5+ expression as well as the production of Th1 and Th17 cytokines. Fig. 2A demonstrates that CD4 T cells differentiated with both Th17-inducing cytokines and ICOS co-stimulation had a significantly higher percentage of CD146+CCR5+ T cells and co-expressed more IL-17A+IFNγ+ than T-cells stimulated with Th1-inducing cytokines or by CD28.
We then analyzed colonic mucosa biopsies from patients with GI GVHD (N = 18) and non-GVHD enteritis (N = 10) for the expression of CD146 by immunohistochemistry. CD146 expression was detected on CD3 lymphoid cells and was strongly present on the endothelium. The CD146+ vessel count in GI GVHD tissues was significantly higher than in non-GVHD enteritis tissues (p < 0.001).
Th17 cells migrated more efficiently through endothelial cell monolayers than their Th1 counterparts (Fig. 2B). Lentivirus-mediated shRNA knock-down of CD146 in either cell type demonstrated that reduced CD146 expression on CD4+ T cells, but not on endothelial cells significantly reduced the T-cell transendothelial migration (Fig. 2C), suggesting that CD146 on T cells is paramount for promoting infiltration of pathogenic T cells into GVHD target organs. As proof of principle for this hypothesis, we tested donor CD146-/- T cells in an allogeneic murine GVHD model and did not find any difference in GVHD severity when compared to wild type CD146+/+ T cells. Finally, we used a xenogeneic GVHD mouse model with injection of human CD4+ T cells lentivirally transduced with CD146 or control shRNA. In comparison to the vector control group, mice transplanted with CD146 shRNA transduced T cells did not lose weight (Fig. 3A), had similar human T cells engraftment (B), had less splenic CD146+CCR5+ T cells (C), and expressed less TBET 53 days after transplant (D).
In conclusion, early quantification of a novel CD146+CCR5+ Th17-prone and ICOS-induced population may allow identification of patients at risk for GI GVHD development and subsequent mortality. Targeting CD146 may represent a new avenue to treat GVHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal