Abstract
Introduction: Acute GVHD (aGVHD) is a leading cause of morbidity and mortality following allo-HSCT. The statin class of cholesterol lowering medications can inhibit T-helper 1(Th-1) driven responses, reduce pro-inflammatory cytokines, increase anti-inflammatory cytokines, and stimulate T-regulatory and T-helper-2 cells (Th-2), thus holding promise for prevention of aGVHD. A recent single institution phase II study showed that the addition of atorvastatin to matched sibling donors prior to stem cell collection and to recipients (30 patients) as aGVHD prophylaxis resulted in day 100 and 180 aGVHD incidence of only 3.3% and 11.1% respectively.(Hamadani et al, J Clin Oncol, 2013). We present the results of our phase II clinical prospective trial evaluating essentially the same atorvastatin-based strategy for the prophylaxis of aGVHD in HLA-matched related donor allo-HSCT. The inclusion/exclusion criteria and treatment plan were identical to the above study. The results of this study were compared to historical matched controls at Ohio State where neither related donor nor patients were exposed to any cholesterol lowering medication.
Methods: Forty patients (statin gp) were enrolled (NCT01491958) between March 2012 and January 2014. Atorvastatin at 40mg/day orally was administered to sibling donors, starting 14-28 days before the anticipated 1st day of stem cell collection. In allo-HSCT recipients atorvastatin (40mg/day) was administered from day -14 to day +180 (or until stopping immunosuppression, toxicity, development of grade [Gr] II-IV aGVHD, or severe chronic GVHD (cGVHD). In addition all recipients received standard GVHD prophylaxis with tacrolimus and methotrexate. Primary outcomes were rate of Gr II-IV aGVHD at day +100 and safety of atorvastatin administration to allo HSCT donor/recipient pairs. The study was designed to test the null hypothesis H0: p≥35%, vs. the alternate H1: p≤15%, where p is probability of Gr II-IV aGVHD at day 100. Controls were 96 matched patients and donors not on any cholesterol lowering medications (non-statin gp).
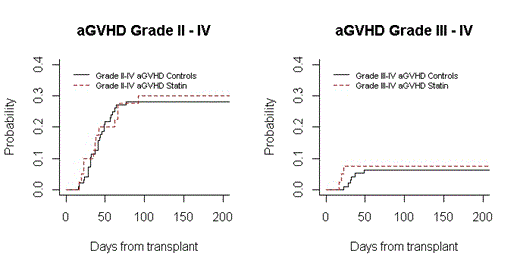
Results: Median donor age was 50 (range 25-68) in statin and 55yrs (18-73) in non-statin gps. Median duration of atorvastatin prophylaxis in statin donors was 14 days (range 13-26) with no atorvastatin related Gr 3-4 toxicities seen. Baseline patient’s characteristics were well matched between the groups with respect to age, sex, donor/recipient sex, intensity of regimen, disease and disease status at transplant and number of stem cells infused. Median duration of atorvastatin in statin gp was 132 days (range 32-400) and was well tolerated with no Gr 2-4 adverse events attributable to atorvastatin. All pts engrafted. The respective median time to ANC ≥500/μL was 18 days (range 13-26) vs 15(8-34) (p=0.0004) and to platelets ≥20k/μL 14 days (9-29) vs 19 (11-141) (p<0.0001) for the statin and non-statin gps, respectively. Day 100 and 180 chimerism and infectious complications were similar between the two gps. Incidences of aGVHD, cGVHD, NRM, relapse, PFS and OS were not statistically different between the two gps (Table 1, Figure1). Four independent reviewers who were blinded to patient’s outcome reviewed the charts for aGVHD and cGVHD outcomes for the statin pts. Their GVHD analysis and grading were similar to that obtained by the GVHD grading team, mitigating any biases.
Conclusions: The administration of atorvastatin to allo-HSCT sibling donors/recipients appears to be feasible, safe and tolerable. However, we did not find any reduction in the incidence of aGVHD and cGVHD compared to historical patients (and their related donors) not exposed to statins. In addition, our findings support the notion that trials evaluating novel strategies to prevent GVHD should be adequately controlled and involve multiple institutions as early as possible..
| Outcome . | Statin n=40 (%) . | Non-statin n=96(%) . | p-value . |
|---|---|---|---|
| Median follow up | 13.04 mos (2.20-28.49) | 24.03 mos (0.30-92.25) | 0.024 |
| D 100 aGVHD Grade II-IV Grade III-IV | 12(30) 3(8) | 27 (28) 6 (6) | 0.830 0.755 |
| Late aGVHD (>day 100) II-IV III-IV | 5(13%) 0 | ||
| cGVHD | 17(43) | 46(48) | 0.915 |
| Non-relapse mortality | 3(8) | 21(22) | 0.286 |
| Cuminc Relapse at 12 mos | 0.32 (0.18-0.47) | 0.29 (0.21-0.39) | 0.431 |
| Median PFS (range) | NR (5.78-NR) | 9.46 (5.06-23.43) | 0.286 |
| Median OS (range) | NR (17.28 - NR) | 24.03 (10.58-57.17) | 0.0978 |
| Outcome . | Statin n=40 (%) . | Non-statin n=96(%) . | p-value . |
|---|---|---|---|
| Median follow up | 13.04 mos (2.20-28.49) | 24.03 mos (0.30-92.25) | 0.024 |
| D 100 aGVHD Grade II-IV Grade III-IV | 12(30) 3(8) | 27 (28) 6 (6) | 0.830 0.755 |
| Late aGVHD (>day 100) II-IV III-IV | 5(13%) 0 | ||
| cGVHD | 17(43) | 46(48) | 0.915 |
| Non-relapse mortality | 3(8) | 21(22) | 0.286 |
| Cuminc Relapse at 12 mos | 0.32 (0.18-0.47) | 0.29 (0.21-0.39) | 0.431 |
| Median PFS (range) | NR (5.78-NR) | 9.46 (5.06-23.43) | 0.286 |
| Median OS (range) | NR (17.28 - NR) | 24.03 (10.58-57.17) | 0.0978 |
Blum:Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal