Abstract
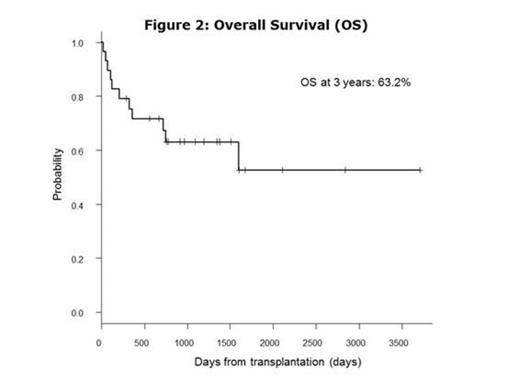
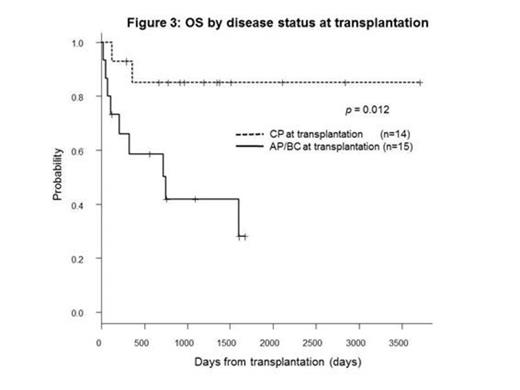
BACKGROUND: The prognosis of chronic myeloid leukemia (CML) in advanced stages (accelerated phase, AP or blast crisis, BC) is still extremely poor even with tyrosine kinase inhibitors (TKIs) and allogeneic hematopoietic cell transplantation (allo-HCT) is the only curative treatment for them. METHOD: Using our database, we retrospectively collected CML patients transplanted at Toranomon Hospital between June 2004 and March 2014, after the introduction of TKIs in Japan. RESULT: Twenty-nine consecutive patients were extracted. The median age was 52 years (range; 16-70). The disease status at diagnosis was chronic phase (CP, n=11), accelerated phase (AP, n=5) and blast crisis (BC, n=13). All the patients were treated with TKIs before transplantation, including imatinib (n=15), nilotinib (n=1), dasatinib (n=6), imatinib/dasatinib (n=4), nilotinib/dasatinib (n=1) and imatinib/nilotinib/dasatinib (n=2). All the 11 patients in CP at diagnosis progressed into AP/BC in their course and only 3 patients achieved second CP (MinorCyR, n=1; PCyR, n=1; MMR, n=1) at transplantation. On the other hand, 11 of 18 patients in AP/BC at diagnosis achieved CP (MinorCyR, n=1; PCyR, n=4; CCyR, n=3; MMR, n=3) at transplantation and the remaining 7 patients did not achieve CHR (Fig. 1). The median HCT-CI and EBMT score at transplantation was 2 (range, 0-5) and 5 (range, 0-7), respectively. Additional cytogenetic abnormalities developed until transplantation in 8 of 11 patients (73%) in CP at diagnosis and in 11 of 18 (61%) in AP/BC at diagnosis. Point mutations in ABL gene were detected in 9 of 20 patients (45%) in their course. Four of 7 patients (57%) in CP at diagnosis had ABL mutations, including T315I (n=1), E255K (n=2) and L359C (n=1). Five of 13 (38%) in AP/BC at diagnosis had ABL mutations, including T315I (n=4) and V299L (n=1). Overall, 14 of 29 (48%) patients underwent transplantation in CP stage (MinorCyR, n=2; PCyR, n=5; CCyR, n=3; MMR, n=4). The donors were related PBSC (n=6), unrelated BM (n=4) or unrelated CB (n=19). The conditioning regimens were myeloablative in 20 patients and reduced-intensity in 9. Twenty-seven patients achieved neutrophil engraftment at a median day of 19 (range, 10-34). The cumulative incidence of neutrophil engraftment was 93.1% at day 42 (patients engrafted, n=27; dead before day 19, n=2). At 3 years, the cumulative incidence of relapse and non-relapse mortality was 32.3% and 14.0%, respectively. In 15 patients who did not achieve CP before transplantation, 11 patients (73.3%) achieved CR after transplantation. With a median follow-up of survivors of 1144 days (range, 127-3705), overall survival (OS) and event free survival (EFS) at 3 years was 63.2% and 56.3%, respectively. In univariate analysis, age (<53 vs. ≥53, p=0.19), EBMT score (<5 vs. ≥5, p=0.32) and donor selection (rPB/uBM vs. uCB, p=0.17) had no impact on OS at 3 years. The variables that influenced on OS were disease status at transplantation (CP vs. AP/BC, 85% vs. 42%, p=0.012), karyotype (sole Ph-chromosome vs. additional cytogenetic abnormalities, 90% vs. 47.5%, p=0.042) and conditioning regimen (MAC vs. RIC, 72.7% vs 41.7%, p=0.039). In multivariate analysis, the only variable that influenced on OS was disease status at transplantation (HR=0.16, 95% CI 0.04-0.70, p=0.015). No patients received maintenance therapy with TKIs. All 9 patients who relapsed after transplantation were re-treated with TKIs. Three patients achieved MMR and the remaining 6 patients who failed received 2nd transplantation. CONCLUSION: We concluded that allo-HCT from any cell sources is a curative treatment option even for the CML patients who had AP/BC stage during their course of the disease. Remarkably good OS can be expected for those who achieved CP status before transplantation, suggesting better disease control before allo-HCT could be one of the critical factors to improve the final outcome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal