To the editor:
Sézary syndrome (SS) is an aggressive leukemic and erythrodermic variant of cutaneous T-cell lymphoma characterized by the presence of a clonal T-lymphocyte population in the skin, lymph nodes, and peripheral blood. We previously reported that KIR3DL2 represents a specific cell surface marker for the evaluation of the circulating tumoral burden and the follow-up of patients with SS.1,2 KIR3DL2 is a member of the killer cell immunoglobulin (Ig)-like receptor (KIR) family for which ligand specificity was assigned to HLA-A3 and HLA-A11 through a peptide-specific interaction,3 was assigned to HLA-B27 homodimer through a peptide-independent recognition,4 and more recently was assigned to CpG oligodeoxynucleotides.5 Although the molecular mechanisms governing inhibitory KIR (KIR-L) functions in natural killer or cytotoxic T lymphocytes are well documented,6,7 almost no data are available regarding their functions in CD4+ T cells. We previously established that KIR2DL1/L2 could provide coinhibitory signals on the CD3-mediated activation and proliferation processes of circulating CD4+ T cells from healthy individuals.8 Given the specific expression of KIR3DL2 by SS patients’ malignant cells, we investigated its function in Sézary cells with a focus on its possible influence on mechanisms resulting in previously described impaired activation and resistance to apoptosis.9 To this aim, we selected untreated SS patients (n = 6) presenting a high blood tumor burden (more than 90% of malignant cells within their CD4+ T-cell population) and identifiable TCR-Vβ rearrangement of their tumoral T-cell clone by immunolabeling.
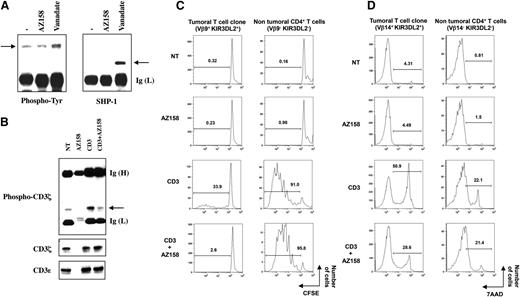
KIR-L proteins deliver negative signals through the phosphorylation of their intracellular immunoreceptor tyrosine-based inhibition motif and their subsequent interaction with SH2-domain-containing phosphatase 1 (SHP-1).10 In sorted CD4+ cells of SS patients, we observed that KIR3DL2 engagement (achieved by using the anti-KIR3DL2 mAb AZ158) is not by itself sufficient to elicit receptor phosphorylation and association to SHP-1, ruling out a fully independent receptor function for KIR3DL2 in this cellular context (Figure 1A). Nevertheless, vanadate treatment led to an increased tyrosine phosphorylation of KIR3DL2 and its consecutive interaction with SHP-1 (Figure 1A), showing its structural and functional integrity in Sézary cells. We therefore investigated its potential function as a T-cell coreceptor by testing its influence on CD3-mediated intracellular events. Under coligation conditions, KIR3DL2 engagement on Sézary cells resulted in a strong inhibition of the CD3-induced phosphorylation of CD3ζ (Figure 1B). This lower level of phosphorylation was not the result of a less efficient immunoprecipitation of CD3ζ or a lower targeting of CD3ε in the presence of AZ158 mAb, as both CD3 molecules were equally recovered in all immunoprecipitates (Figure 1B).
KIR3DL2 inhibitory function on SS patients’ malignant T-cell clone. (A) Sorted CD4+ T cells from SS patients were left untreated (−) or incubated with anti-KIR3DL2 monoclonal antibody (mAb) AZ158 plus goat anti-mouse IgG antibodies or with the phosphatase inhibitor vanadate. Cell lysates were prepared and subjected to immunoprecipitation using the anti-KIR3DL2 mAb AZ158. The immunoprecipitates were separated by SDS-8% PAGE, transferred onto a nitrocellulose membrane, and then probed in series with anti-phosphotyrosine (Tyr) and anti-SHP-1 antibodies. Arrows indicate the position of phosphorylated KIR3DL2 and SHP-1. Results shown (patient 2) are representative of all patients tested (n = 3). (B) Sorted CD4+ T-cells from SS patients were incubated with anti-CD3ε or/and AZ158 mAb, as indicated. An isotype-matched control mAb was used to equalize the amount of antibodies used in each condition. Cross-linking was induced by the addition of goat anti-mouse Igs except for resting condition (NT). After lysis, the antibody-targeted molecules were collected and the resulting immunoprecipitates subjected to electrophoresis and western blotting procedures. The immunoblot was revealed with an anti-phospho-CD3ζ mAb (upper panel) and was reprobed after dehybridization using an anti-CD3ζ mAb (middle panel). CD3ε immunoblotting was performed to assess efficient cell targeting and immunoprecipitation (lower panel). Arrow indicates the position of phospho-CD3ζ. Results shown (patient 3) are representative of all patients tested (n = 3). (C) Peripheral blood mononuclear cells from SS patients were preloaded with carboxyfluorescein diacetate succinimidyl ester (CFSE) and left untreated (NT) or stimulated with platebound anti-CD3ε and AZ158 mAb alone or in combination, as indicated. After 4 days of culture, cells were collected and immunolabelings performed using anti-TCRVβ-PE, anti-CD3-PE-Cy5, and anti-CD4-PE-Cy7 mAb. The percentages of dividing cells among the malignant (left) and the nonmalignant (right) CD4+ T-cell populations are presented. Results shown corresponded to patient 3, whose tumoral clone was identified as TCR-Vβ14+, and are representative of experiments performed on 3 SS patients. (D) Peripheral blood mononuclear cells of SS patients were activated as described for panel C for 6 days. After immunostaining with anti-CD3-FITC, -TCRVβ-PE, and -CD4-PE-Cy7 mAbs, and 7-AAD, cells were analyzed by flow cytometry. The percentage of 7-AAD+ apoptotic cells within the malignant (left) and the nonmalignant (right) CD4+ T cells is indicated for each condition of incubation. Results shown (patient 5, with a TCR-Vβ9+ malignant clone) are representative of experiments performed on 3 SS patients. Ig (H) and (L), Ig heavy and light chain; NT, nontreated.
KIR3DL2 inhibitory function on SS patients’ malignant T-cell clone. (A) Sorted CD4+ T cells from SS patients were left untreated (−) or incubated with anti-KIR3DL2 monoclonal antibody (mAb) AZ158 plus goat anti-mouse IgG antibodies or with the phosphatase inhibitor vanadate. Cell lysates were prepared and subjected to immunoprecipitation using the anti-KIR3DL2 mAb AZ158. The immunoprecipitates were separated by SDS-8% PAGE, transferred onto a nitrocellulose membrane, and then probed in series with anti-phosphotyrosine (Tyr) and anti-SHP-1 antibodies. Arrows indicate the position of phosphorylated KIR3DL2 and SHP-1. Results shown (patient 2) are representative of all patients tested (n = 3). (B) Sorted CD4+ T-cells from SS patients were incubated with anti-CD3ε or/and AZ158 mAb, as indicated. An isotype-matched control mAb was used to equalize the amount of antibodies used in each condition. Cross-linking was induced by the addition of goat anti-mouse Igs except for resting condition (NT). After lysis, the antibody-targeted molecules were collected and the resulting immunoprecipitates subjected to electrophoresis and western blotting procedures. The immunoblot was revealed with an anti-phospho-CD3ζ mAb (upper panel) and was reprobed after dehybridization using an anti-CD3ζ mAb (middle panel). CD3ε immunoblotting was performed to assess efficient cell targeting and immunoprecipitation (lower panel). Arrow indicates the position of phospho-CD3ζ. Results shown (patient 3) are representative of all patients tested (n = 3). (C) Peripheral blood mononuclear cells from SS patients were preloaded with carboxyfluorescein diacetate succinimidyl ester (CFSE) and left untreated (NT) or stimulated with platebound anti-CD3ε and AZ158 mAb alone or in combination, as indicated. After 4 days of culture, cells were collected and immunolabelings performed using anti-TCRVβ-PE, anti-CD3-PE-Cy5, and anti-CD4-PE-Cy7 mAb. The percentages of dividing cells among the malignant (left) and the nonmalignant (right) CD4+ T-cell populations are presented. Results shown corresponded to patient 3, whose tumoral clone was identified as TCR-Vβ14+, and are representative of experiments performed on 3 SS patients. (D) Peripheral blood mononuclear cells of SS patients were activated as described for panel C for 6 days. After immunostaining with anti-CD3-FITC, -TCRVβ-PE, and -CD4-PE-Cy7 mAbs, and 7-AAD, cells were analyzed by flow cytometry. The percentage of 7-AAD+ apoptotic cells within the malignant (left) and the nonmalignant (right) CD4+ T cells is indicated for each condition of incubation. Results shown (patient 5, with a TCR-Vβ9+ malignant clone) are representative of experiments performed on 3 SS patients. Ig (H) and (L), Ig heavy and light chain; NT, nontreated.
Previous studies established that the accumulation of Sézary cells was not a result of increased proliferation but rather reflected a resistance to apoptosis and particularly to activation-induced cell death (AICD).9 This prompted us to analyze further the consequences of KIR3DL2 triggering on the process of Sézary cells’ CD3-dependent proliferation and cell death. Time-course experiments were performed to ensure detection of maximal levels of cell growth or AICD (reached after 4 and 6 days of treatment, respectively). In our experimental settings, Sézary tumor cells were defined as CD3+CD4+Vβ+KIR3DL2+ cells, whereas the nontumoral CD4+ T cells were identified as CD3+CD4+Vβ−KIR3DL2− cells. As expected from previous reports, the malignant T-cell clone displayed a lower proliferation level than the nonmalignant T cells on CD3 targeting (Figure 1C). However, a nearly complete inhibition of the tumor cells’ proliferation was observed on coligation of KIR3DL2, whereas at the same time the nontumoral cells’ growth was not affected (Figure 1C). Furthermore, the CD3-mediated apoptosis of SS patients’ tumoral T-cell clone was strongly inhibited on coengagement of KIR3DL2 with AZ158 mAb (Figure 1D left panels). In contrast, the CD3-dependent AICD of the nonmalignant CD4+ T cells was unmodified in the presence of AZ158 mAb (Figure 1D right panels). Note that similar results were obtained at all tested anti-CD3 mAb concentrations leading to proliferation, and that KIR3DL2-dependent inhibition specificity was confirmed by dose-response experiments using increasing amounts of anti-KIR3DL2 mAb (data not shown). Altogether our data demonstrated that KIR3DL2 acts as an inhibitory coreceptor in Sézary cells given its ability to downmodulate CD3-dependent early signaling events. These results agreed with our previous studies on KIR2DL1/L2 function in normal T cells, suggesting that, in Sézary cells, KIR-L-derived pathways rely on a regulation process distinct from the one described in natural killer lymphocytes8 and require the generation of T-cell receptor (TCR)/CD3-mediated early activation signals. In addition, our data provide evidence for a possible role of KIR3DL2 in the maintenance of a high circulating malignant-cell burden by preventing AICD.
Authorship
Acknowledgments: This work was supported by grants from the French Society for Dermatology (SFD) and Institut National de la Santé et de la Recherche Médicale.
Contribution: N.T., A.C., I.L., and A.M.-C. performed the experiments; A.M.-C. designed the research and analyzed the data; and A.M.-C., M.B., and A.B. wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Anne Marie-Cardine, INSERM U976, Hôpital Saint Louis, Pavillon Bazin, 1 avenue Claude Vellefaux, 75010 Paris, France; e-mail: anne.marie-cardine@inserm.fr.