Key Points
Major CALR-mutated clones may be observed in polycythemia vera negative for JAK2 mutations.
Abstract
Calreticulin (CALR) mutations have been reported in Janus kinase 2 (JAK2)– and myeloproliferative leukemia (MPL)–negative essential thrombocythemia and primary myelofibrosis. In contrast, no CALR mutations have ever been reported in the context of polycythemia vera (PV). Here, we describe 2 JAK2V617F-JAK2exon12–negative PV patients who presented with a CALR mutation in peripheral granulocytes at the time of diagnosis. In both cases, the CALR mutation was a 52-bp deletion. Single burst-forming units–erythroid (BFU-E) from 1 patient were grown in vitro and genotyped: the same CALR del 52-bp mutation was noted in 31 of the 37 colonies examined; 30 of 31 BFU-E were heterozygous for CALR del 52 bp, and 1 of 31 BFU-E was homozygous for CALR del 52 bp. In summary, although unknown mutations leading to PV cannot be ruled out, our results suggest that CALR mutations can be associated with JAK2-negative PV.
Introduction
Calreticulin (CALR) mutations have recently been reported in Janus kinase 2 (JAK2)– and myeloproliferative leukemia (MPL)–negative myeloproliferative neoplasms (MPNs), particularly essential thrombocythemia (ET) and primary myelofibrosis (PMF).1,2 The clinical course of sporadic CALR-mutated patients seems to be more indolent than that of JAK2-mutated patients.1,3 In contrast, no CALR mutations were reported in 647 published cases of polycythemia vera (PV). Consequently, CALR mutations were considered exclusive to JAK2 and MPL mutations and absent in PV. Because 96% to 99% of PV patients harbor a JAK2 mutation (mostly the V617F mutation in exon 14 and, more rarely, insertion/deletion in exon 12),4 it seemed logical to assume that CALR mutations would be rare or absent. However, we and others have demonstrated that JAK2V617F and CALR mutations can coexist in rare cases of refractory anemia with ring sideroblasts and thrombocytosis,5 PMF,6 or ET.7,8
Here, we describe 2 JAK2V617F-negative PV patients who presented with a CALR mutation at the time of diagnosis; for 1 patient the CALR-mutated clone represented >50% of cells, and homozygous cells for the CALR mutation were detected.
Study design
The procedures followed were in accordance with the Declaration of Helsinki, and samples were obtained with patients’ written informed consent. Two JAK2 (exons 12, 13, and 14)– and MPL (exon 10)–negative PV patients were identified in our database and tested for a CALR mutation. Purification of granulocytes and peripheral blood mononuclear cells (PBMCs) and extraction of DNA were performed as previously described.9 The JAK2V617F mutation was analyzed by allele-specific real-time quantitative polymerase chain reaction (PCR) to estimate the JAK2V617F-mutated allele burden according to the method published by Lippert et al with a sensitivity <1%.9 Analyses of JAK2 exons 12, 13, and 14 were performed according to the method described by Carillo et al10 and the MPL mutations were analyzed by high-resolution melting (HRM) curve analyses followed by Sanger sequencing if positive, as reported by Boyd et al.11 The CALR exon 9 mutations were screened by either HRM (S.C., unpublished data) or product sizing analysis and Sanger sequencing according to the methods of Klampfl et al.1 Single burst-forming unit–erythroid (BFU-E) colony assays were performed using PBMCs from patient 1 with methylcellulose-based (no. 5112) or collagen-based (no. 5411) media containing erythropoietin from Stem Alpha (Saint Denis l’Argentière, France). To ensure that an optimal colony density was obtained so that single colonies could be picked without contamination by cells from neighboring colonies, PBMCs were plated at 4 different concentrations (20 000, 40 000, 100 000, and 200 000 per mL).
Results and discussion
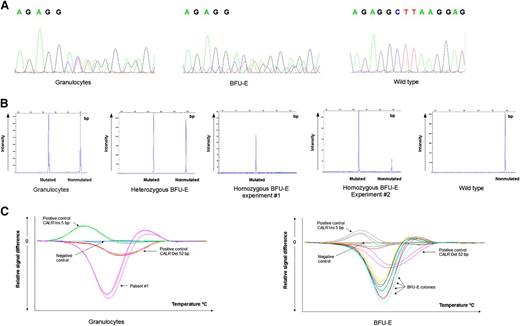
Patient 1 had hemoglobin at 168 g/L, hematocrit at 51.3%, and increased red cell mass (RCM) at 128% associated with a normal erythropoietin level. The bone marrow biopsy showed hypercellularity for age, panmyelosis associated with normal megakaryocytes, and rare isolated abnormal enlarged forms. Using reticulin staining, no myelofibrosis was noted. Patient 2 had hemoglobin at 194 g/L, hematocrit at 53%, and a low erythropoietin level without dehydration (Table 1). Both had moderately elevated platelet counts (658 and 575 × 109/L, respectively) with normal leukocyte counts. Both patients were negative for breakpoint cluster region–Abelson (BCR-ABL). No mutation was found in JAK2 exons 12, 13, and 14 using HRM and allele-specific real-time quantitative PCR,9,10 nor in MPL exon 10. Following HRM analysis, CALR mutations were suspected in both patients and confirmed using Sanger sequencing and product sizing analysis: in both patients, the CALR mutations were type 1 deletions (52-bp deletion; c.1092_1143del; Figure 1). For patient 1, the CALR del 52-bp allele burden assessed using product sizing analysis was found to be 64% at the time of diagnosis and 50% after 26 months of treatment with hydroxyurea. A recent report by Cassinat et al described complete molecular response in 2 CALR-mutated ET patients under treatment with pegylated interferon α12 ; here, no significant change in the CALR-mutated allele burden was noted after 26 months of treatment with hydroxyurea.
Characteristics of the 2 patients at the time of diagnosis
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Age, y | 69 | 83 |
| Sex, M/F | M | M |
| Hemoglobin level, g/L | 168 | 194 |
| Hematocrit, % | 51.3 | 53 |
| Platelet count, 109/L | 658 | 575 |
| White blood cells, 109/L | 7.6 | 7.5 |
| Erythropoietin level, mIU/mL | 5.9 | 2.8 |
| Bone marrow biopsy | PV | N/A |
| RCM | Increased | N/A |
| JAK2V617F mutation | Negative | Negative |
| JAK2 exon 12 mutation | Negative | Negative |
| JAK2 exon 13 mutation | Negative | N/A |
| JAK2 exon 14 mutation | Negative | N/A |
| MPL mutation | Negative | Negative |
| BCR-ABL1 mutation | Negative | Negative |
| CALR mutation | Positive | Positive |
| . | Patient 1 . | Patient 2 . |
|---|---|---|
| Age, y | 69 | 83 |
| Sex, M/F | M | M |
| Hemoglobin level, g/L | 168 | 194 |
| Hematocrit, % | 51.3 | 53 |
| Platelet count, 109/L | 658 | 575 |
| White blood cells, 109/L | 7.6 | 7.5 |
| Erythropoietin level, mIU/mL | 5.9 | 2.8 |
| Bone marrow biopsy | PV | N/A |
| RCM | Increased | N/A |
| JAK2V617F mutation | Negative | Negative |
| JAK2 exon 12 mutation | Negative | Negative |
| JAK2 exon 13 mutation | Negative | N/A |
| JAK2 exon 14 mutation | Negative | N/A |
| MPL mutation | Negative | Negative |
| BCR-ABL1 mutation | Negative | Negative |
| CALR mutation | Positive | Positive |
F, female; M, male; mIU/ml, milli International Unit per millimeter; N/A, not available.
CALR mutations.CALR mutations determined using (A) sequencing, (B) product sizing analysis, and (C) HRM curve analysis in peripheral blood granulocytes and in BFU-E from patient 1. The homozygous BFU-E was tested twice, showing 100% and 83% allele burden. In the later experiment, the WT peak was probably due to contaminating monocytes from the media. WT, wild-type.
CALR mutations.CALR mutations determined using (A) sequencing, (B) product sizing analysis, and (C) HRM curve analysis in peripheral blood granulocytes and in BFU-E from patient 1. The homozygous BFU-E was tested twice, showing 100% and 83% allele burden. In the later experiment, the WT peak was probably due to contaminating monocytes from the media. WT, wild-type.
To complete the first mutation analysis in peripheral blood granulocytes, we performed colony assays in methylcellulose- and in collagen-based media, picked single BFU-E colonies grown for 14 days in the presence of erythropoietin, and genotyped each colony individually for CALR mutations. Among the 37 colonies thus examined, 3 had no PCR amplification, 3 were wild-type, and 31 harbored the same CALR mutation observed in peripheral blood granulocytes, that is, the 52-bp deletion c.1092_1143del. Of the 31 BFU-E, 30 were heterozygous for CALR del 52 bp and 1 was homozygous. To our knowledge, these patients are the first cases of CALR-mutated PV to be reported. The presence of a CALR mutation in peripheral granulocytes and in BFU-E (with homozygous status for some colonies) suggests that the CALR mutation played a role in the polycythemia phenotype of these patients. However, because cases of biclonal JAK2V617F- and CALR-mutated MPNs were recently reported,7 we cannot rule out the possibility that an additional, unknown mutation occurred prior to or in parallel with the CALR mutation. In any case, our observations highlight the fact that in the absence of a JAK2 mutation, CALR mutations can be associated with a PV phenotype.
Of note, it has been reported that when RCM was measured in patients initially diagnosed with ET, it could reveal masked PV with normal hematocrit and hemoglobin levels in as many as 46.5% of cases.13 Iron deficiency and/or splenomegaly may explain, in part, artificially normal hemoglobin levels in PV patients. Johansson et al demonstrated that the hemoglobin level is not a good surrogate marker for absolute erythrocytosis attested by an RCM >125% because it presents a hemoglobin level below 18.5 g/dL in 65% of male patients and below 16.5 g/dL in 37% of female patients.14 This observation was confirmed by Alvarez-Larrán et al who studied a large cohort of 179 PV and ET patients. In this cohort, 53 of the 114 (46%) PV patients presented with hemoglobin values below those defined by the World Health Organization classification for the diagnosis of PV. Of these patients, 75% presented with thrombocytosis and would have been misdiagnosed with ET in the absence of RCM measurement.15 It is therefore possible that the large series of published cases of CALR-positive ET may have included a few PV patients with undetected increased RCM, leading to an erroneous diagnosis of ET.
In conclusion, testing JAK2-negative PV patients for CALR mutations may be useful.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr Philip Bastable (University Hospital, Dijon, France) for revising the manuscript, and Mrs Martine Courtois, Dominique Bouchot, and Mathilde Bas (University Hospital, Dijon, France) for excellent technical assistance.
This work was supported by a grant from the Association “Tulipes Contre le Cancer” (Châlon s/Saône, Burgundy, France).
J.B., S.C., S.H., and F.G. are members of the French Intergroup of Myeloproliferative Disorders.
Authorship
Contribution: J.B., S.H., and F.G. analyzed the data and wrote the paper; J.-H.P. performed the analyses; F.G. designed research; and S.C. performed research and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: François Girodon, Laboratoire d'Hématologie, PTB 2 rue Angelique, Ducoudray, CHU Dijon, 21079 Dijon, France; e-mail: francois.girodon@chu-dijon.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal