Key Points
DIAPH1 (mDia1) is involved in both Rho-mediated actin polymerization and microtubule assembly and stability during proplatelet formation.
Abstract
Megakaryocytes are highly specialized precursor cells that produce platelets via cytoplasmic extensions called proplatelets. Proplatelet formation (PPF) requires profound changes in microtubule and actin organization. In this work, we demonstrated that DIAPH1 (mDia1), a mammalian homolog of Drosophila diaphanous that works as an effector of the small GTPase Rho, negatively regulates PPF by controlling the dynamics of the actin and microtubule cytoskeletons. Moreover, we showed that inhibition of both DIAPH1 and the Rho-associated protein kinase (Rock)/myosin pathway increased PPF via coordination of both cytoskeletons. We provide evidence that 2 major effectors of the Rho GTPase pathway (DIAPH1 and Rock/myosin II) are involved not only in Rho-mediated stress fibers assembly, but also in the regulation of microtubule stability and dynamics during PPF.
Introduction
Formins are highly conserved, eukaryotic, multidomain proteins that assemble the actin microfilament and microtubule cytoskeletons into architectures that physically support cell adhesion, migration during development, and responses to injury and infection.1-3 The mammalian Diaphanous-related formins are encoded by the DIAPH genes. Three DIAPH isoforms have been described in mammals: DIAPH1 (mDia1), DIAPH2 (mDia3), and DIAPH3 (mDia2). DIAPH1 (mDia1)4 is encoded by the DIAPH1 gene localized on human chromosome 5q31.3. Diap1-deficient mice develop age-dependent myeloproliferative/myelodysplastic phenotypes, suggesting that DIAPH1 may act as a tumor suppressor.5 DIAPH1 may be involved in the myeloid malignancies associated with the 5q deletion, because interstitial loss of a variable part of the long arm of chromosome 5 (5q- syndrome) is a frequent clonal chromosomal abnormality in human myelodysplastic syndrome and acute myeloid leukemia.6,7
DIAPH1 serves as an effector of Rho small guanosine triphosphate-binding proteins.4 Guanosine triphosphate-bound Rho activates DIAPH1 by interaction with the GTPase-binding domain in its N-terminus.8,9 Rho binding sterically disrupts autoinhibitory interactions between the diaphanous inhibitory domain within the N-terminal armadillo repeat region and the C-terminal Dia-autoregulatory domain.10,11 Released from autoinhibition, the free formin homology (FH)-2 domain collaborates with the FH-1 domain bound to profilin-actin complexes to add monomers to the growing (plus) ends of actin filaments.12
Rho-activated DIAPH1 also induces the selective stabilization of polarized microtubules in serum-deprived NIH 3T3 fibroblasts by binding microtubules directly to its FH2 domain.13 This affects not only actin and microtubule dynamics, but also other cellular processes.14 Thus, DIAPH1 is an ideal candidate for coordinating actin and microtubules during proplatelet formation (PPF).
At the end of their maturation, megakaryocytes (MKs) extend their demarcation membranes to form proplatelets.15 Changes in cytoskeletal structures cause proplatelets to extend further and to relocalize organelles such as mitochondria in the proplatelet. Cytoskeletal changes are also involved in the final abscission and cleavage in platelet formation in the blood stream of marrow sinusoids. Actin undergoes continuous remodeling during PPF and serves as the motor machinery for MK migration in the bone marrow. Previous data from in vitro and in vivo models have underscored the key role of the Rho/Rock/Myosin II pathway in PPF.16,17 MYH9 mutations in humans are known to lead to macrothrombocytopenia.18 Microtubules also play a crucial role in PPF by facilitating the motor forces that elongate the shaft and the tips of proplatelets.19-23 Because of its dual function on actin and microtubule cytoskeletons, we hypothesized that DIAPH1 (mDia1) is an essential Rho effector in governing actin and microtubule dynamics in PPF in cooperation with Rock kinase.
To pursue this hypothesis, we studied the precise role of DIAPH1 and its cooperation with other Rho effectors such as Rock and myosin II in MK maturation and PPF. Our results show a crucial role for DIAPH1 in generating F-actin structures and assisting the microtubule stabilization underlying PPF and platelet production.
Methods
In vitro culture of MKs derived from human CD34+ cells
In agreement with our Institute Ethic Committee (Assistance Publique des Hôpitaux de Paris), CD34+ cells were obtained from leukapheresis samples after mobilization performed on patients, from the bone marrow of healthy patients undergoing hip surgery, or from umbilical cord blood. CD34+ cells were isolated and cultured in serum-free liquid medium with recombinant human thrombopoietin (TPO) (10 ng/mL; Kirin Brewery) and stem cell factor (SCF) (25 ng/mL, Biovitrum AB) for inducing MK differentiation, as previously described.16,24
The shRNA cloning in lentiviral vector
Two short hairpin RNAs (shRNAs) against human DIAPH1 (1 shRNA against human MYH9 and a control scrambled sequence [SCR]) were cloned separately into a lentiviral vector (pRRL-PGK-GFP or pRRL-PGK-Cherry) as previously described.25
The mDia1ΔN3 cloning in lentiviral vector
The mDia1ΔN3 sequence lacking autoregulatory components was provided by Shuh Narumiya.4,14 It was verified by DNA sequencing and amplified by polymerase chain reaction (PCR) using oligonucleotide adaptors containing Kazak and Stop sequences. The PCR product was digested at MluI sites in the adaptor sequence and then introduced into an HIV-derived lentiviral vector pRRL-EF1α-PGK-GFP.
Lentivirus production and cell transduction
Lentivirus particles were prepared and stoked as previously described.25 Purified CD34+ cells were cultured for 4 to 5 days with TPO and SCF. Lentivirus particles were added at a concentration of 107 infectious particles/105 cells for 12 hours, followed by a second transduction exactly done as the first. CD41+GFP+, CD41+Cherry+, or CD41+GFP+Cherry+ cells were purified by cell sorting 48 hours after infection (Influx flow cytometer; Becton Dickinson, San Jose, CA).
Immunofluorescence
The cells were plated on poly-l-lysine-coated slides (O. Kindler GmbH & Co., Freiburg, Germany) or Horm type I collagen-coated slides (Nycomed, Austria) for 1 hour (37°C, 5% CO2, and 100% humidity). Immunofluorescence was performed as described previously.16 The detailed information about first antibodies and fixation are presented in the supplemental Methods, available on the Blood Web site. Cells were examined under a Zeiss laser scanning microscope (LSM 510; Carl Zeiss, Oberkochen, Germany) with a 63 × 1.4 numerical aperture oil objective.
Western blot
Western blot analysis was performed with 8% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel as previously described with the primary antibodies in the supplemental Methods.16 The cells were lysed in radio-immunoprecipitation assay buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% Triton X-100) with a cocktail of proteinase and phosphatase inhibitors (Sigma-Aldrich; Lyon, France).
Stress fiber formation assay
MKs derived from CD34+ cells were infected with lentivirus and sorted by flow cytometry. The CD41+GFP+ cells were then recultured for 1 additional day before layered on collagen-coated Super Frost slides (Menzel-Glaser, Braunschweig, Germany) for 1 hour (37°C, 5% CO2, and 100% humidity) to induce stress fiber formation. There were 300 MKs were counted for each independent experiment and the percentage of MKs showing stress fibers was averaged for 3 independent experiments.
Tubulin polymerization assay
As described for the stress fiber assay, CD41+GFP+ MKs were plated on collagen-coated slides for 1 hour, then incubated with serum-free medium containing 5 μM nocodazole (Sigma, France) for 1 hour. Slides were carefully washed 3 times with pre-warmed serum-free medium and put back in the incubator (37°C, 5% CO2, and 100% humidity) to induce tubulin polymerization. After 10 minutes of incubation, pre-warmed extract solution (100 mM PIPES pH 6.9, 1 mM MgCl2, 5 mM EGTA, 0.5% Triton X-100) was added to the slides for 30 seconds to stop the reaction. Cells were fixed with 4% paraformaldehyde for 10 minutes at 37°C, washed once by Tris-buffered saline (50 mM Tris, 150 mM NaCl, pH 7.6), and blocked with antibody diluting solution (Tris-buffered saline, 0.1% Triton, 2% bovine serum albumin) for 10 minutes before starting immunofluorescence staining as previously described.16 The ratio of the α-tubulin or β-tubulin fluorescence area compared with total cell area of 30 to 36 cells was quantified with Image J in 3 independent experiments. Error bars represent standard error of the mean of all the analyzed cells.
Quantitative RT-PCR
Quantitative real-time (RT) PCR was performed with CD41+GFP+, CD41+Cherry+, or CD41+GFP+Cherry+ MKs as previously described.26 All gene-expression levels were normalized to that of the housekeeping HPRT gene.
Statistics
Statistical significance was determined by a Student t test. P < .05 was considered statistically significant. Data are presented as means (± standard deviation), except those noted as means (± standard error of the mean).
Results
DIAPH1 expression increases during MK differentiation
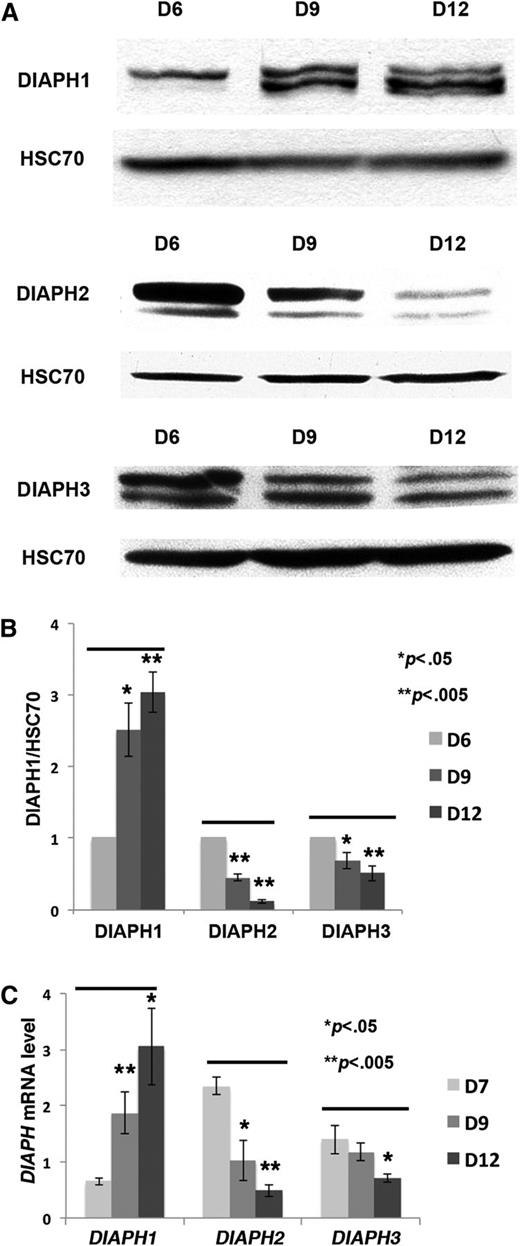
To measure DIAPH1 expression during MK differentiation, western blots and quantitative RT-PCR analyses were performed on cultured CD41+ MKs from day 6 to day 12. Compared with the protein loading control (HSC70) and relative to HPRT transcripts, DIAPH1 protein and RNA level increased during MK differentiation, particularly during the late days of culture; on the other hand, DIAPH2 and DIAPH3 protein, and RNA expression levels, particularly those of DIAPH2, decreased (Figure 1A-C).
DIAPH1, DIAPH2 and DIAPH3 expression during MK differentiation. (A) CD41+ MKs were sorted on day 6 of culture and recultured for various periods according to the experiments. In the western blots, HSC70 was used as the protein loading control; an 8% SDS-PAGE gel was used for protein separation. (B) The ratio between DIAPH (DIAPH1, DIAPH2, or DIAPH3) and HSC70 protein was analyzed by Image J in 3 independent experiments. DIAPH1 expression was increased, and DIAPH2 and DIAPH3 were decreased during MK differentiation. (C) RT-PCR (normalized to HPRT) was performed on CD41+ MKs collected at days (D)7, D9, and D12 of culture (3 independent experiments). DIAPH1 mRNA expression was increased during MK differentiation. *P < .05; **P < .005.
DIAPH1, DIAPH2 and DIAPH3 expression during MK differentiation. (A) CD41+ MKs were sorted on day 6 of culture and recultured for various periods according to the experiments. In the western blots, HSC70 was used as the protein loading control; an 8% SDS-PAGE gel was used for protein separation. (B) The ratio between DIAPH (DIAPH1, DIAPH2, or DIAPH3) and HSC70 protein was analyzed by Image J in 3 independent experiments. DIAPH1 expression was increased, and DIAPH2 and DIAPH3 were decreased during MK differentiation. (C) RT-PCR (normalized to HPRT) was performed on CD41+ MKs collected at days (D)7, D9, and D12 of culture (3 independent experiments). DIAPH1 mRNA expression was increased during MK differentiation. *P < .05; **P < .005.
DIAPH1 knockdown by shRNA increases MK PPF
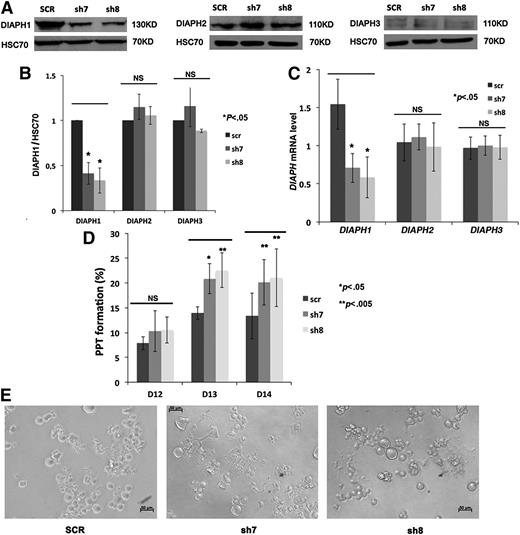
To determine if DIAPH1 is implicated in MK PPF, human CD34+ cells were induced in vitro toward MK differentiation and transduced at days 4 and 5 of culture with lentiviruses carrying GFP and either a control shRNA (SCR) or 2 different shRNAs targeting DIAPH1 (sh7 and sh8). The CD41+GFP+ cell population was sorted 48 hours after infection, and western blot and RT-PCR analyses were performed on day 10 or 11 of culture. DIAPH1 protein level was reduced 50% to 60% by two shRNAs (sh7 or sh8) treatment, while DIAPH2 (mDia3) and DIAPH3 (mDia2) protein levels remained unchanged (Figure 2A-B). Quantitative RT-PCR showed a 50% to 60% reduction in DIAPH1 messenger RNA (mRNA) without significant changes in DIAPH2 and DIAPH3 messenger RNA (Figure 2C).
DIAPH1 knockdown by shRNA increases MK PPF. (A) Western blots revealed that shRNA (sh7 and sh8) targeting of DIAPH1 decreased DIAPH1 protein level (left), but not DIAPH2 (middle) and DIAPH3 protein (right) expression, relative to control shRNA (SCR). A 10% SDS-PAGE gel was used for protein separation. (B) DIAPH1 (left), DIAPH2 (middle), or DIAPH3 (right) protein levels (relative to HSC70) were analyzed by Image J in 3 independent experiments. Only DIAPH1 expression was decreased by sh7 and sh8. (C) RT-PCR (normalized to HPRT) results showed that only the DIAPH1 mRNA level was decreased by sh7 and sh8 (4 independent experiments). (D) DIAPH1 knockdown resulted in a marked increase in MK PPF relative to the control (scr) (5 independent experiments). (E) Phase-contrast microscopy images (lens ×20) of day 13 culture of control (SCR), sh7-, or sh8-infected MKs. There was more PPF in sh7- or sh8-infected cells. NS, no significant difference. *P < .05; **P < .005.
DIAPH1 knockdown by shRNA increases MK PPF. (A) Western blots revealed that shRNA (sh7 and sh8) targeting of DIAPH1 decreased DIAPH1 protein level (left), but not DIAPH2 (middle) and DIAPH3 protein (right) expression, relative to control shRNA (SCR). A 10% SDS-PAGE gel was used for protein separation. (B) DIAPH1 (left), DIAPH2 (middle), or DIAPH3 (right) protein levels (relative to HSC70) were analyzed by Image J in 3 independent experiments. Only DIAPH1 expression was decreased by sh7 and sh8. (C) RT-PCR (normalized to HPRT) results showed that only the DIAPH1 mRNA level was decreased by sh7 and sh8 (4 independent experiments). (D) DIAPH1 knockdown resulted in a marked increase in MK PPF relative to the control (scr) (5 independent experiments). (E) Phase-contrast microscopy images (lens ×20) of day 13 culture of control (SCR), sh7-, or sh8-infected MKs. There was more PPF in sh7- or sh8-infected cells. NS, no significant difference. *P < .05; **P < .005.
The percentage of MKs bearing proplatelets was quantified daily from day 12 to day 14 of culture. The presence of at least 1 pseudopodial extension was considered a PPF. Five independent experiments were performed (Figure 2D-E). DIAPH1 knockdown resulted in a marked increase in the number of MKs bearing PPF relative to the control (SCR) at days 13 and 14 of culture.
DIAPH1 knockdown decreases F-actin content, but increases tubulin polymerization and stability
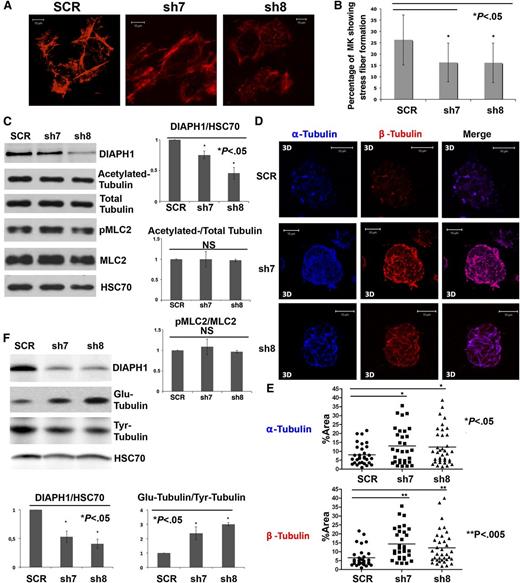
The adhesion of primary human MKs onto type I collagen substrate induces stress fiber formation, which depends on actin polymerization and myosin activation.27 To evaluate the role of DIAPH1 on actin cytoskeleton reorganization, MKs were infected with sh7 or sh8 and made to adhere onto type I collagen–coated slides for a stress fiber assay on day 8 of culture (Figure 3A). In each independent experiment, we counted the number of MKs forming stress fibers in at least 300 MKs. A lower percentage of MKs transduced with sh7 and sh8 showed stress fiber formation: 26.3% of MKs infected with the control (SCR) vs 16.3% for sh7 and 16.1% for sh8 (Figure 3B). In addition, sh7- or sh8-infected MKs showed thinner stress fibers relative to the control (Figure 3A). DIAPH1 knockdown did not change the pMLC2 level, suggesting that the reduction of stress-fiber formation results from a reduction of actin polymerization, not from inhibition of myosin II activation (Figure 3C). This result suggests DIAPH1 knockdown could reduce contractile forces during MK differentiation.
DIAPH1 knockdown decreases stress fiber formation, but increases tubulin polymerization and stability. (A) MKs transduced with different shRNAs (SCR, sh7, or sh8) after 1 hour of adhesion onto a fibrillar collagen I substrate for a stress fiber formation assay. MKs infected with sh7 (middle) and sh8 (right) showed fewer stress fibers than did control (SCR) (left). (B) MKs transduced with sh7 and sh8 showed 16.3% and 16.1% stress fiber formation, respectively, relative to 26.3% for the control (SCR) in 3 independent experiments. (C) DIAPH1 knockdown did not modify the ratio of pMLC2/MLC2 or of acetylated-tubulin/total tubulin. A 10% SDS-PAGE gels was used for protein separation (left). Three independent experiments were quantified with Image J (right). (D) CD41+ MKs infected with SCR, sh7, or sh8 were plated on collagen-coated slides for 1 hour and then treated for another hour with nocodazole. Tubulin polymerization assays were performed 10 minutes after washing. MKs infected with sh7 (middle) or sh8 (bottom) show increased tubulin polymerization relative to the control (top). The α-Tubulin is labeled in blue and β-tubulin in red. (E) MKs infected with sh7 or sh8 showed a greater α-tubulin or β-tubulin fluorescence area relative to total cell area (%Area). We quantified 30 to 40 cells for each condition in 3 independent experiments using Image J. The data are presented as means ± standard error of the mean. Top: α-tubulin (SCR: 8.04 ± 1.02; sh7: 12.96 ± 1.83; sh8: 12.37 ± 1.69). Bottom: β-Tubulin (SCR: 6.60 ± 0.96; sh7: 14.32 ± 1.61; sh8: 12.15 ± 1.58). (F) Western blots revealed that MKs infected with sh7 or sh8 presented a higher proportion of Glu-tubulin and a lower proportion of Tyr-tubulin relative to SCR controls. A 10% SDS-PAGE gel was used for protein separation (top). The expression ratios between DIAPH1 and HSC70 and between Glu-tubulin and Tyr-tubulin were quantified in 3 independent experiments using Image J (bottom). 3D, 3-dimensional. *P < .05; **P < .005.
DIAPH1 knockdown decreases stress fiber formation, but increases tubulin polymerization and stability. (A) MKs transduced with different shRNAs (SCR, sh7, or sh8) after 1 hour of adhesion onto a fibrillar collagen I substrate for a stress fiber formation assay. MKs infected with sh7 (middle) and sh8 (right) showed fewer stress fibers than did control (SCR) (left). (B) MKs transduced with sh7 and sh8 showed 16.3% and 16.1% stress fiber formation, respectively, relative to 26.3% for the control (SCR) in 3 independent experiments. (C) DIAPH1 knockdown did not modify the ratio of pMLC2/MLC2 or of acetylated-tubulin/total tubulin. A 10% SDS-PAGE gels was used for protein separation (left). Three independent experiments were quantified with Image J (right). (D) CD41+ MKs infected with SCR, sh7, or sh8 were plated on collagen-coated slides for 1 hour and then treated for another hour with nocodazole. Tubulin polymerization assays were performed 10 minutes after washing. MKs infected with sh7 (middle) or sh8 (bottom) show increased tubulin polymerization relative to the control (top). The α-Tubulin is labeled in blue and β-tubulin in red. (E) MKs infected with sh7 or sh8 showed a greater α-tubulin or β-tubulin fluorescence area relative to total cell area (%Area). We quantified 30 to 40 cells for each condition in 3 independent experiments using Image J. The data are presented as means ± standard error of the mean. Top: α-tubulin (SCR: 8.04 ± 1.02; sh7: 12.96 ± 1.83; sh8: 12.37 ± 1.69). Bottom: β-Tubulin (SCR: 6.60 ± 0.96; sh7: 14.32 ± 1.61; sh8: 12.15 ± 1.58). (F) Western blots revealed that MKs infected with sh7 or sh8 presented a higher proportion of Glu-tubulin and a lower proportion of Tyr-tubulin relative to SCR controls. A 10% SDS-PAGE gel was used for protein separation (top). The expression ratios between DIAPH1 and HSC70 and between Glu-tubulin and Tyr-tubulin were quantified in 3 independent experiments using Image J (bottom). 3D, 3-dimensional. *P < .05; **P < .005.
The microtubule cytoskeleton plays an essential role in PPF, and DIAPH1 is known to stabilize and orientate microtubules.28 To determine whether DIAPH1 could also affect microtubule dynamics during MK differentiation, tubulin polymerization assays were performed on sorted CD41+GFP+ MKs infected with various shRNAs. Quite surprisingly, sh7- and sh8-infected MKs showed more obvious microtubule elongation after nocodazole inhibition compared with the SCR control (Figure 3D-E; supplemental Figure 1). It is known that stable microtubules accumulate a posttranslationally modified form of tubulin recognized as detyrosinated tubulin (Glu-tubulin), whereas dynamic microtubules contain predominantly tyrosinated tubulin (Tyr-tubulin).29,30 We found that the ratio between Glu-tubulin and Tyr-tubulin was increased in MKs infected by DIAPH1 shRNA (Figure 3F), indicating that DIAPH1 knockdown increases microtubule stability.
Expression of a constitutively active DIAPH1 inhibits PPF
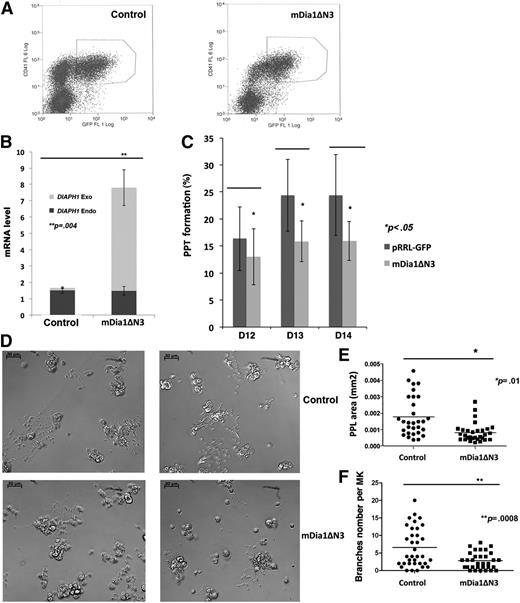
To confirm the role of DIAPH1 revealed by the shRNA strategy, a constitutively active form of murine DIAPH1 (mDia1ΔN3, an active mDia1 containing the FH1 and FH2 regions without the Rho-binding domain) was expressed in human MKs (Figure 4A). Two sets of RT-PCR primers were designed, 1 set specific for the constitutively active form of mDia1ΔN3 (DIAPH1 Exo) and the other for endogenous DIAPH1 (DIAPH1 Endo). Expression of the endogenous and exogenous forms was analyzed in sorted CD41+GFP+ MKs by quantitative RT-PCR (Figure 4B). PPF was counted from days 12 to 14 of culture. As shown in Figure 4B, total DIAPH1 mRNA (DIAPH1 Endo + DIAPH1 Exo) increased about fourfold to sixfold in mDia1ΔN3-infected MKs relative to the empty vector infected MK controls. In contrast, PPF was decreased in MKs expressing mDia1ΔN3 (Figure 4C). Remarkably, proplatelets formed by MKs expressing mDia1ΔN3 often presented a simpler structure with less branching and shorter extensions, which lead to a clear decrease in proplatelet area (Figure 4D-F). Furthermore, DIAPH1 knockdown only slightly increased platelet production in vitro in spite of the marked increase in PPF, but overexpression of mDia1ΔN3 induced a decrease in platelet production parallel to the decrease in PPF, suggesting that DIAPH1 contributes to the platelet release process (supplemental Figure 2A-B). Together, these data suggest that DIAPH1 activation inhibits platelet production.
Expression of a constitutively active DIAPH1 (mDia1ΔN3) inhibits PPF. (A) CD41+GFP+ MKs were sorted by flow cytometry after infection with mDia1ΔN3 (right) or with the empty lentiviral vector (left) (pRRL-EF1α-PGKGFP, control) at day 7 of MK culture. (B) Quantitative RT-PCR for endogenous and exogenous mRNA expression in sorted CD41+GFP+ MKs. Total DIAPH1 mRNA expression was threefold to fourfold higher in MKs transduced with mDia1ΔN3 relative to controls (cells transduced with pRRL-EF1α-PGKGFP) in 3 independent experiments. (C) PPF measured from day 12 to day 14 in MK culture was lower in MKs expressing mDia1ΔN3 than in controls (cells transduced with pRRL-EF1α-PGKGFP) in 4 independent experiments. (D) mDia1ΔN3-expressing MKs (bottom) often presented proplatelets with shorter extensions and less branching than controls (top); phase contrast images with a ×20 lens. (E) Proplatelet area was measured by Image J in 3 independent experiments. The data are presented as mean ± standard error of the mean. The mDia1ΔN3-infected MKs (0.0018 ± 0.0002 mm2; n = 32) had a lower proplatelet area than controls (0.0008 ± 0.0001 mm2; n = 30). (F) Proplatelet branch numbers were counted in 3 independent experiments. The mDia1ΔN3-infected MK (6.57 ± 0.94; n = 32) showed less branching than the controls (cells transduced with pRRL-EF1α-PGKGFP) (2.81 ± 0.42; n = 35). *P < .01; **P < .0008.
Expression of a constitutively active DIAPH1 (mDia1ΔN3) inhibits PPF. (A) CD41+GFP+ MKs were sorted by flow cytometry after infection with mDia1ΔN3 (right) or with the empty lentiviral vector (left) (pRRL-EF1α-PGKGFP, control) at day 7 of MK culture. (B) Quantitative RT-PCR for endogenous and exogenous mRNA expression in sorted CD41+GFP+ MKs. Total DIAPH1 mRNA expression was threefold to fourfold higher in MKs transduced with mDia1ΔN3 relative to controls (cells transduced with pRRL-EF1α-PGKGFP) in 3 independent experiments. (C) PPF measured from day 12 to day 14 in MK culture was lower in MKs expressing mDia1ΔN3 than in controls (cells transduced with pRRL-EF1α-PGKGFP) in 4 independent experiments. (D) mDia1ΔN3-expressing MKs (bottom) often presented proplatelets with shorter extensions and less branching than controls (top); phase contrast images with a ×20 lens. (E) Proplatelet area was measured by Image J in 3 independent experiments. The data are presented as mean ± standard error of the mean. The mDia1ΔN3-infected MKs (0.0018 ± 0.0002 mm2; n = 32) had a lower proplatelet area than controls (0.0008 ± 0.0001 mm2; n = 30). (F) Proplatelet branch numbers were counted in 3 independent experiments. The mDia1ΔN3-infected MK (6.57 ± 0.94; n = 32) showed less branching than the controls (cells transduced with pRRL-EF1α-PGKGFP) (2.81 ± 0.42; n = 35). *P < .01; **P < .0008.
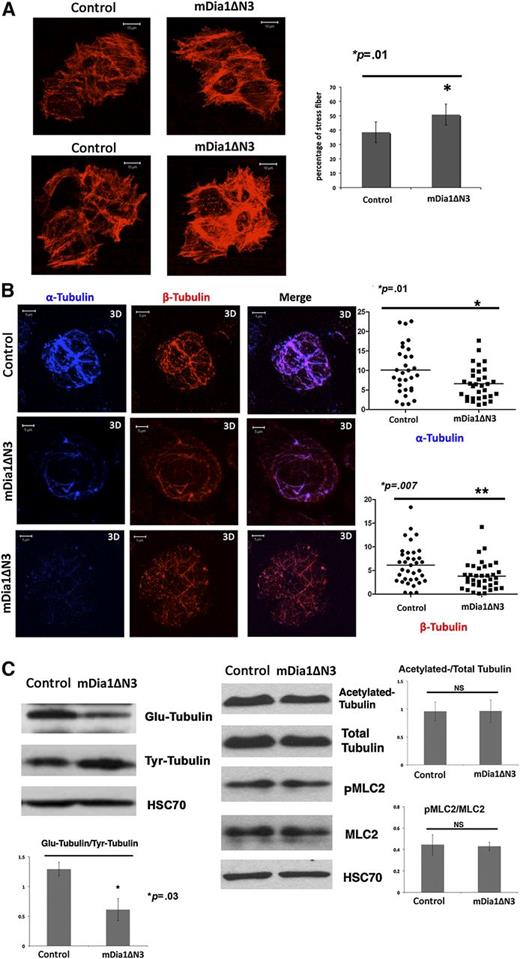
Expression of mDia1ΔN3 increases stress fiber formation, but decreases tubulin polymerization and stability
We performed assays to evaluate the effects of mDia1ΔN3 on actin and tubulin cytoskeleton, stress fiber formation, and tubulin polymerization on day 8 of MK culture. Stress fiber formation increased in MKs expressing mDia1ΔN3 (50.7%) relative to empty vector controls (38.5%) (Figure 5A), but tubulin polymerization after nocodazole treatment was lower in MKs expressing mDia1ΔN3 relative to the control (Figure 5B). In agreement with the immunofluorescence results, western blots showed that mDia1ΔN3 expression decreased the ratio between Glu- and Tyr-tubulin, indicating a loss in microtubule stability (Figure 5C).
Expression of a constitutively active DIAPH1 (mDia1ΔN3) increases stress fiber formation and decreases tubulin polymerization and stability. (A) Left: A stress fiber formation assay was performed in sorted CD41+GFP+ MKs transduced with mDia1ΔN3 or with an empty vector as described in “Methods.” Right: A higher percentage (50.7%) of mDia1ΔN3 MKs showed stress fiber formation than did controls (38.5%) in 3 independent experiments. *P < .01. (B) Left: CD41+ MKs infected by mDia1ΔN3 or the control vector were plated on collagen-coated slides and treated for 1 hour with nocodazole. Tubulin polymerization assays were performed 10 minutes after recovery. The mDia1ΔN3-infected MKs showed lower tubulin polymerization relative to controls. Right: MKs infected by mDia1ΔN3 showed lower α-tubulin (top) or β-tubulin fluorescence area (bottom) relative to total cell area (%Area). We quantified 37 cells in each condition in 3 independent experiments using Image J. The data are presented as mean ± standard error of the mean. α-Tubulin: control: 10.10 ± 1.14; mDia1ΔN3: 6.64 ± 0.73. β-Tubulin: control: 6.14 ± 0.69; mDia1ΔN3: 3.82 ± 0.48. *P < .01; *P < .007. (C) Western blot analysis and quantification were performed in sorted CD41+GFP+ MKs transduced with mDia1ΔN3 or with an empty vector in 3 independent experiments showing that mDia1ΔN3 expression decreased the ratio between Glu-tubulin and Tyr-tubulin, but did not change the ratio between PMLC2 and MLC2. A 10% SDS-PAGE gel was used for protein separation. *P < .03.
Expression of a constitutively active DIAPH1 (mDia1ΔN3) increases stress fiber formation and decreases tubulin polymerization and stability. (A) Left: A stress fiber formation assay was performed in sorted CD41+GFP+ MKs transduced with mDia1ΔN3 or with an empty vector as described in “Methods.” Right: A higher percentage (50.7%) of mDia1ΔN3 MKs showed stress fiber formation than did controls (38.5%) in 3 independent experiments. *P < .01. (B) Left: CD41+ MKs infected by mDia1ΔN3 or the control vector were plated on collagen-coated slides and treated for 1 hour with nocodazole. Tubulin polymerization assays were performed 10 minutes after recovery. The mDia1ΔN3-infected MKs showed lower tubulin polymerization relative to controls. Right: MKs infected by mDia1ΔN3 showed lower α-tubulin (top) or β-tubulin fluorescence area (bottom) relative to total cell area (%Area). We quantified 37 cells in each condition in 3 independent experiments using Image J. The data are presented as mean ± standard error of the mean. α-Tubulin: control: 10.10 ± 1.14; mDia1ΔN3: 6.64 ± 0.73. β-Tubulin: control: 6.14 ± 0.69; mDia1ΔN3: 3.82 ± 0.48. *P < .01; *P < .007. (C) Western blot analysis and quantification were performed in sorted CD41+GFP+ MKs transduced with mDia1ΔN3 or with an empty vector in 3 independent experiments showing that mDia1ΔN3 expression decreased the ratio between Glu-tubulin and Tyr-tubulin, but did not change the ratio between PMLC2 and MLC2. A 10% SDS-PAGE gel was used for protein separation. *P < .03.
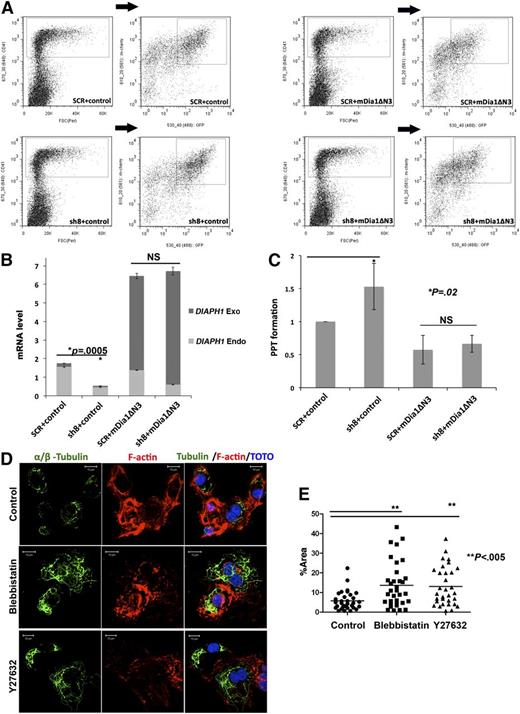
Expression of mDia1ΔN3 rescues the effects induced by DIAPH1 shRNA on PPF
To demonstrate that the increase in PPF observed after DIAPH1 knockdown was not an off-target effect, we performed rescue experiments using mDia1ΔN3. For these experiments, we used sh8 because it targets the endogenous DIAPH1, but not mDia1ΔN3 (data not shown). To perform a double infection, sh8 was cloned in a lentiviral vector containing Cherry (a nonrelevant shRNA SCR was used as a control) and mDia1ΔN3 was cloned in a lentivirus vector expressing GFP (an empty vector was the control). Expression of these constructs was analyzed both by RT-PCR (with primer sets allowing distinction between DIAPH1 Endo and mDia1ΔN3 mRNA) or by flow cytometry. The sh8-cloned in the Cherry vector was as efficient as the previous vector in decreasing endogenous DIAPH1 in CD41+ MKs (supplemental Figure 3A). Similarly, mDia1ΔN3 was well expressed as revealed by both mRNA and GFP levels (supplemental Figure 3B). Double infections were performed in different combinations (shSCR plus control, sh8 plus control, mDia1ΔN3 plus shSCR, and mDia1ΔN3 plus sh8) and the double-infected cells (Cherry+GFP+CD41+) were then sorted (Figure 6A). As shown in Figure 6B, sh8 decreased the level of endogenous DIAPH1 without an effect on mDia1ΔN3. The increase in PPF induced by sh8 was lessened by mDia1ΔN3 expression (Figure 6C). Together, the rescue of DIAPH1 expression abolished the increase in PPF induced by DIAPH1 knockdown, demonstrating the sh8 specificity for DIAPH1 and underscoring the role of DIAPH1 in PPF.
The constitutively active DIAPH1 (mDia1ΔN3) rescues the effects of shRNA-induced knockdown of DIAPH1. (A) CD41+GFP+Cherry+ MK populations double-infected with 2 lentiviral vectors containing GFP or Cherry were purified by flow cytometry. In each combination (top left: SCR plus control; bottom left: sh8+control; top right: SCR+mDia1ΔN3; bottom right: sh8+mDia1ΔN3), the CD41+ population was first gated and then the double-labeled population was obtained by gating the GFP+ Cherry+ population in the previously gated CD41+ population. (B) Quantitative RT-PCR showing the levels of endogenous and exogenous DIAPH1 mRNA in the double-infected populations purified by flow cytometry (3 independent experiments). (C) PPF was counted in the double-transduced MK population CD41+GFP+Cherry+. The increase of PPF caused by the shRNA targeting DIAPH1 was abolished by mDia1ΔN3 expression (4 independent experiments; PPF in SCR plus control MKs was used as a normalized baseline). (D) Tubulin polymerization assays in CD41+ MKs show that Rock or myosin II inhibition increased tubulin polymerization, but disturbed stress fiber formation. Top: Control; middle: Blebbistatin; bottom: Y27632. MKs were stained for tubulin (α and β, green) and phalloidin-TRITC (red); DNA was stained with TOTO (blue). (E) Blebbistatin or Y27632 incubation increased α-tubulin or β-tubulin fluorescence area relative to total cell area (%Area). There were 30 to 40 cells in each condition were quantified in 3 independent experiments using Image J. The data are presented as mean ± standard error of the mean: control (5.77 ± 0.85); blebbistatin (13.63 ± 1.99); Y27632 (13.07 ± 1.75). *P < .02; **P < .005.
The constitutively active DIAPH1 (mDia1ΔN3) rescues the effects of shRNA-induced knockdown of DIAPH1. (A) CD41+GFP+Cherry+ MK populations double-infected with 2 lentiviral vectors containing GFP or Cherry were purified by flow cytometry. In each combination (top left: SCR plus control; bottom left: sh8+control; top right: SCR+mDia1ΔN3; bottom right: sh8+mDia1ΔN3), the CD41+ population was first gated and then the double-labeled population was obtained by gating the GFP+ Cherry+ population in the previously gated CD41+ population. (B) Quantitative RT-PCR showing the levels of endogenous and exogenous DIAPH1 mRNA in the double-infected populations purified by flow cytometry (3 independent experiments). (C) PPF was counted in the double-transduced MK population CD41+GFP+Cherry+. The increase of PPF caused by the shRNA targeting DIAPH1 was abolished by mDia1ΔN3 expression (4 independent experiments; PPF in SCR plus control MKs was used as a normalized baseline). (D) Tubulin polymerization assays in CD41+ MKs show that Rock or myosin II inhibition increased tubulin polymerization, but disturbed stress fiber formation. Top: Control; middle: Blebbistatin; bottom: Y27632. MKs were stained for tubulin (α and β, green) and phalloidin-TRITC (red); DNA was stained with TOTO (blue). (E) Blebbistatin or Y27632 incubation increased α-tubulin or β-tubulin fluorescence area relative to total cell area (%Area). There were 30 to 40 cells in each condition were quantified in 3 independent experiments using Image J. The data are presented as mean ± standard error of the mean: control (5.77 ± 0.85); blebbistatin (13.63 ± 1.99); Y27632 (13.07 ± 1.75). *P < .02; **P < .005.
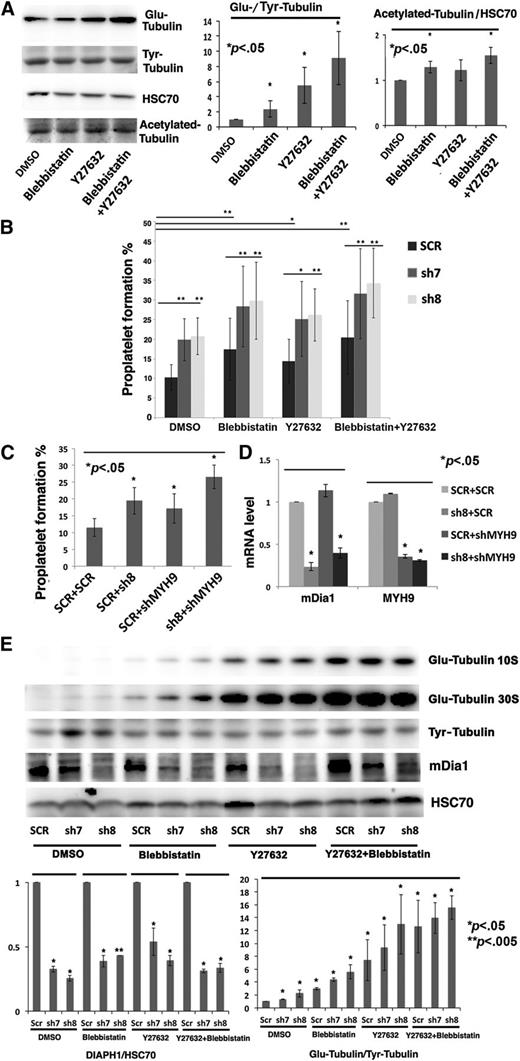
Effects of double inhibition of DIAPH1 and Rock/myosin II on PPF
The Rho pathway could also play a negative role in PPF by inducing myosin II activation.16,31 Thus, we checked double inhibition of myosin IIA and DIAPH1 in cytoskeleton dynamics regulation during PPF.
First, tubulin polymerization assays showed that inhibition of Rock by Y27632 (10 μM) or myosin II by blebbistatin (25 μM) decreased CD41+ MK stress fiber formation, but increased tubulin polymerization (Figure 6D-E). Western blots revealed that Rock or myosin II inhibition increased Glu-/Tyr-tubulin ratio and slightly increased acetylated-tubulin (Figure 7A).
Effects of the double inhibition of DIAPH1 and Rock/myosin II on PPF. (A) Left: Western blot performed on CD41+ MKs at day 8 of culture revealed Glu-tubulin, Tyr-tubulin, and acetylated-tubulin expression after ROCK (Y27632, 10 μM) or myosin II inhibition (blebbistatin 25 μM) with overnight incubation. Right: The ratio of Glu-tubulin to Tyr-tubulin and quantification of acetylated-tubulin expression relative to HSC70 showed a clear increase of stable Glu-tubulin, but only a slight increase of acetylated-tubulin. (B) The sh7-, sh8- or SCR-transduced MKs were incubated overnight just before PPF (at day 10 of culture) with inhibitors of Rock (Y27632, 10 μM) or myosin II (blebbistatin 25 μM). Inhibition of Rock and of myosin II combined with DIAPH1 knockdown by shRNA increased PPF even more in 3 independent experiments. (C) Knockdown of both MYH9 and DIAPH1 by shRNA increased PPF more the knockdown of either alone in 3 independent experiments. MKs were double-infected with 2 lentiviral vectors containing GFP (shMYH9 or SCR control) or Cherry (sh8 or SCR control). CD41+GFP+Cherry+ populations (SCR plus SCR, SCR plus sh8, SCR plus shMYH9, or sh8 plus shMYH9) were purified by flow cytometry. (D) Quantitative RT-PCR showing DIAPH1 and MYH9 mRNA levels in double-infected MK populations (SCR plus SCR, SCR plus sh8, SCR plus shMYH9, or sh8 plus shMYH9). Cells were isolated by flow cytometry in 3 independent experiments. (E) Top: Western blots of sh7-, sh8-, or SCR-transduced MKs incubated overnight with inhibitors of ROCK (Y27632, 10 μM) or myosin II (blebbistatin, 25 μM) at day 8 of culture. Glu-tubulin levels increased even more after DIAPH1 knockdown combined with Rock/myosin inhibition. A 10% SDS-PAGE gel was used for protein separation. Bottom: The ratios of DIAPH1/HSC70 and Glu-tubulin/Tyr-tubulin showed a clear decrease of DIAPH1 expression and an increase of stable Glu-tubulin after DIAPH1 knockdown combined with inhibitor. DMSO, dimethylsulfoxide. *P < .05; **P < .005.
Effects of the double inhibition of DIAPH1 and Rock/myosin II on PPF. (A) Left: Western blot performed on CD41+ MKs at day 8 of culture revealed Glu-tubulin, Tyr-tubulin, and acetylated-tubulin expression after ROCK (Y27632, 10 μM) or myosin II inhibition (blebbistatin 25 μM) with overnight incubation. Right: The ratio of Glu-tubulin to Tyr-tubulin and quantification of acetylated-tubulin expression relative to HSC70 showed a clear increase of stable Glu-tubulin, but only a slight increase of acetylated-tubulin. (B) The sh7-, sh8- or SCR-transduced MKs were incubated overnight just before PPF (at day 10 of culture) with inhibitors of Rock (Y27632, 10 μM) or myosin II (blebbistatin 25 μM). Inhibition of Rock and of myosin II combined with DIAPH1 knockdown by shRNA increased PPF even more in 3 independent experiments. (C) Knockdown of both MYH9 and DIAPH1 by shRNA increased PPF more the knockdown of either alone in 3 independent experiments. MKs were double-infected with 2 lentiviral vectors containing GFP (shMYH9 or SCR control) or Cherry (sh8 or SCR control). CD41+GFP+Cherry+ populations (SCR plus SCR, SCR plus sh8, SCR plus shMYH9, or sh8 plus shMYH9) were purified by flow cytometry. (D) Quantitative RT-PCR showing DIAPH1 and MYH9 mRNA levels in double-infected MK populations (SCR plus SCR, SCR plus sh8, SCR plus shMYH9, or sh8 plus shMYH9). Cells were isolated by flow cytometry in 3 independent experiments. (E) Top: Western blots of sh7-, sh8-, or SCR-transduced MKs incubated overnight with inhibitors of ROCK (Y27632, 10 μM) or myosin II (blebbistatin, 25 μM) at day 8 of culture. Glu-tubulin levels increased even more after DIAPH1 knockdown combined with Rock/myosin inhibition. A 10% SDS-PAGE gel was used for protein separation. Bottom: The ratios of DIAPH1/HSC70 and Glu-tubulin/Tyr-tubulin showed a clear decrease of DIAPH1 expression and an increase of stable Glu-tubulin after DIAPH1 knockdown combined with inhibitor. DMSO, dimethylsulfoxide. *P < .05; **P < .005.
To verify the effect of a double inhibition of DIAPH1 and the Rock/myosin II pathway on PPF, MKs transduced with sh7, sh8, or an SCR control were incubated overnight just before PPF (about day 10 of culture) with Y27632 (10 μM) or blebbistatin (25 μM) alone or in combination. The increase in PPF observed in sh7- or sh8-transduced MKs was augmented when Rock or myosin II inhibitors were added alone or together (Figure 7B). Moreover, MKs double-transduced with an shRNA against DIAPH1 cloned in a lentiviral vector containing Cherry (sh8) plus an shRNA against MYH9 cloned in a lentiviral vector containing GFP (shMYH9) showed an increased PPF relative to a single knockdown (Figure 7C-D).
Finally, sh7-, sh8- or SCR-transduced MKs were incubated overnight with Rock or myosin II inhibitors at day 8 of culture and analyzed by western blots. In SCR-transduced MKs, Rock/myosin II inhibition increased Glu-/Tyr-tubulin ratio, which was further augmented in sh7- or sh8-transduced MKs. This suggests that the inhibition of both DIAPH1 and the Rock/myosin II pathway increases microtubule stability more than DIAPH1 inhibition alone (Figure 7E).
Discussion
PPF is associated with morphologic changes that require profound cytoskeleton reorganization. The mammalian Diaphanous-related formins directly promote actin filament growth. They could also stabilize microtubules or coordinate the dynamics of the microtubules and actin cytoskeleton.13,14,32 These properties make DIAPH (mDia) molecules ideal candidates for the cytoskeletal assembly machines that build the structures supporting PPF. Further, DIAPH1(mDia1) is one of the major effectors of the Rho signaling pathway, which is a key regulator of PPF as outlined by in vitro and in vivo models.16,17 Moreover, 2 genes encoding Rho GTPase guanine nucleotide exchange factors, DOCK8 (dedicator of cytokinesis 8) and ARHGEF3 (Rho guanine nucleotide exchange factor 3), were identified among 68 genomic loci reliably associated with platelet count and volume.33 Mutations in MYH9, another important effector of Rho pathway, can lead to macrothrombocytopenia.18 Until now, there has been no direct evidence that DIAPH1 mutations are associated with disorders in platelet biogenesis. However, DIAPH1 as a main Rho effector is implicated in PPF, either directly or in cooperation with other Rho effectors, such as Myosin II or Rock.
During MK differentiation, DIAPH1 expression increases and DIAPH2 and DIAPH3 expression diminish. The different expression profiles suggest that these family members play specific roles in the differentiation process. MKs first become polyploid by endomitosis before PPF, with a corresponding increase in cell size and a concomitant elevation of actin content.16,34 DIAPH1 expression also increases to allow normal actin assembly, a process required for normal cell function at the end of MK differentiation. DIAPH1 is a potent activator of serum response factor, which senses changes in actin monomer concentrations to induce the expression of cell structure genes.35 Our data suggest a specific role of DIAPH1 in PPF and platelet shedding. In contrast, DIAPH2 (mDia3) and DIAPH3 (mDia2) are downregulated, possibly to facilitate MK polyploidization and PPF.
mDia1 and mDia2 are required for cytokinesis depending on the cell type,36-38 and mDia3 is involved in stabilization of the kinetochores onto spindle microtubules.39,40 Moreover, mDia2 contributes to the generation of an actin structure that maintains the cortical actin cytoskeleton,41 and inhibition of mDia2 leads to nonapoptotic membrane blebbing, a process that resembles PPF in MKs. Other studies have shown that formin activation impairs the ability of oncogenic Ras to induce membrane extrusions that bud off from cells to form microvesicles (Julie Davis Turner and Arthur S. Alberts, manuscript submitted August 2014). A persistent mitogen-activated protein kinase activation also inhibits PPF in humans.42 Therefore, hyperactivation of actin assembly may impair the ability of MKs to alter their membrane plasticity and to generate platelets. A selective decrease in the expression of some DIAPH could facilite PPF. Another notable point is that when extracts of MKs from different culture days were analyzed by western blotting in 8% SDS-PAGE gels, the 3 DIAPHs each appeared as 2 distinct bands. These may correspond to different splice variants, as described for mDia3,43 or to posttranslational changes. The mDia1 and mDia2 can be both ubiquinated (Aaron D. Deward and Arthur S. Alberts, unpublished observation) and phosphorylated.44
DIAPH1 expression increases during MK differentiation, but DIAPH1 knockdown increases PPF, which seems contradictory. It is likely that the increase during differentiation is related to the MKs becoming polyploid, which produces a large increase in cell size and is accompanied by an elevation in actin content.1,34 An increase in DIAPH1 expression is required to regulate actin polymerization and maintain a normal cell shape. At the end of MK differentiation, MKs promote membrane protrusion to form proplatelets. The contractile forces generated by Myosin II and F-actin restrain cytoplasmic extensions and maintain cell rigidity that must be inactivated to trigger PPF and platelet shedding. That is why the knockdown or inhibition of DIAPH1 or MYH9 increases the PPF.
As we were unable to detect modification in MLC2 phosphorylation level after DIAPH1 knockdown, we infer that mDia1 may contribute to actomyosin assembly only by promoting actin polymerization, but not by activating myosin II. It is possible that Rho activation regulates PPF by 2 independent pathways, the Rho/Rock/Myosin IIA and the Rho/DIAPH1 pathways. This is in line with our observation that the simultaneous inhibition of Rock and DIAPH1 induced a marked increase in PPF. Our results revealed that the 2 important effectors of Rho pathway, DIAPH1 and ROCK, cooperate to create the contractile forces that restrain cytoplasmic extensions and to inhibit PPF during MK differentiation. Our data also revealed that the Rho pathway must be shut down to allow appropriate PPF.
We examined whether DIAPH1 can regulate microtubule reorganization during PPF. Surprisingly, our results suggest that DIAPH1 plays a negative role in microtubule polymerization and stability. Indeed, it has been clearly shown that proplatelet extension is a microtubule-driven process with a continuous polymerization of microtubules at the free (plus) end. This contributes to PPF and to the sliding of overlapping microtubules, which likely drives proplatelet elongation.17-20 Activated DIAPH1 could restrict PPF by 2 mechanisms, first by inducing stress fiber formation (contractile forces) and second by destabilizing microtubules.
The mechanism by which DIAPH1 (mDia1) contributes to microtubule stabilization is still a matter of debate. Early studies have suggested that actin and microtubules compete for mDia because the mDia FH2 domain functions in both actin assembly and microtubule stabilization.13,45 There is also evidence that mDia regulates microtubule stability independently of actin nucleation.14,32 DIAPH1 can also complex with other microtubule-binding proteins, including EB1 and the product of the familial colon cancer gene APC1, or it can regulate GSK3beta through a novel class of protein kinase Cs to promote microtubule stabilization.46-49 Simultaneous loss of both mDia1 and adenomatous polyposis coli function profoundly impacts the myelodysplatic syndrome phenotype in mice (Julie Davis Turner, Arthur S. Alberts, and Susan Kitchen-Goosen, unpublished observation), suggesting a collaboration in tumor suppression, as well as in actin assembly.49
Moreover, inhibition of either Rock or myosin II could also increase microtubule elongation and stability. More importantly, their dual inhibition increased microtubule stability even more, as indicated by the Glu-tubulin/Tyr-tubulin ratio. The combined inhibition of Rock/myosin II and DIAPH1 also has a more significant effect on PPF inhibition than either single inhibition. However, there is no clear evidence for direct interactions between Rock and microtubules. It has been reported that inhibition of Rock or myosin II can increase acetyl-MT levels, a posttranslational modification associated with microtubule stabilization, via the phosphorylation of tubulin polymerization that promotes protein 1 and histone deacetylase 6 activity.50-52 In MKs, Rock/myosin II inhibition increased the Glu-/Tyr-tubulin ratio and increased tubulin polymerization. However, we did not find an obvious change in the acetyl-tubulin level when Y27632 or blebbistatin is added. The molecular mechanism by which Rock regulates microtubules needs to be further studied.
Another interesting question is raised by our work because DIAPH usually increases microtubule stability in interphase cells, but we observed the opposite in MKs. A similar result was also reported in osteoclasts, in which Rho inhibition is correlated with an increased microtubule stabilization that is mediated by mDia2 through tubulin acetylation.53 However, we did not observe any significant changes in microtubule acetylation level when we knocked down DIAPH1 or expressed the active form of DIAPH1 in human MK, suggesting that regulation of tubulin acetylation may be more linked to mDia2 (DIAPH3) or to formins other than mDia1 (DIAPH1). All these observations suggest the complexity of the DIAPH pathway, whose effects on microtubule may depend on the cellular context and thus on cell type.
Finally, DIAPH expression profiles are different between human and mouse MKs with a much higher DIAPH2 and DIAPH3 expression level in mouse than in human MK (unpublished results). The functional redundancy among different DIAPH molecules or other formin family members may explain why there is no evident abnormality in PPF (unpublished results), platelet number, and function in DIAPH1 knockout mice.5,54
Together, our work shows that the 2 main effectors of the Rho-GTPase pathway (DIAPH1 and Rock/myosin II) are involved in Rho-mediated stress fiber assembly and in the regulation of microtubule stability and dynamics during PPF.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Françoise Wendling and Jacques Bertoglio for reviewing the manuscript, Kirin Breweries and A.B. Biovitrum for their kind gifts of recombinant human TPO and SCF, This work was supported by the Institut National de la Santé et de la Recherche Médicale, by the Agence Nationale de la Recherche (Jeune chercheur Y.C.), by grants from la Ligue Nationale Contre le Cancer (Equipe Labellisée), by the China Scholarship Council and Sociéte Française d'hématologie (J.P.), and by the support of the Van Andel Endowment and the Grand Rapids Community foundation (S.M.K.-G. and A.S.A.).
Author
Contribution: Y.C. directed the studies; Y.C. and W.V. designed the experiments; P.J.J., Y.C., L.L., and D.M. performed research; P.R., Y.L., S.M.K.-G., I.B., and H.M. contributed to the performance of certain experiments; S.N. provided mDia1ΔN3; and Y.C., A.S.A., and W.V. wrote and revised the manuscript with contribution of all the authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yunhua Chang, 114 rue Edouard Vaillant, 94805 Villejuif Cedex, France; e-mail: yunhua.chang-marchand@inserm.fr.
References
Author notes
J.P. and L.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal