Key Points
A high survival rate was seen in primary or secondary MF patients transplanted from matched related donors using the FluMel regimen.
FluMel plus ATG in HSCT from unrelated donors for MF patients is associated with an increased risk of graft failure.
Abstract
From 2007 to 2011, 66 patients with primary myelofibrosis or myelofibrosis (MF) preceded by essential thrombocythemia or polycythemia vera were enrolled into a prospective phase 2 clinical trial of reduced-intensity allogeneic hematopoietic stem cell transplantation (AHSCT), Myeloproliferative Disorder Research Consortium 101 trial. The study included patients with sibling donors (n = 32) receiving fludarabine/melphalan (FluMel) as a preparative regimen and patients with unrelated donors (n = 34) receiving conditioning with FluMel plus anti-thymocyte globulin (ATG). Patient characteristics in the 2 cohorts were similar. Engraftment occurred in 97% of siblings and 76% of unrelated transplants, whereas secondary graft failure occurred in 3% and 12%, respectively. With a median follow-up of 25 months for patients alive, the overall survival (OS) was 75% in the sibling group (median not reached) and 32% in the unrelated group (median OS: 6 months, 95% confidence interval [CI]: 3, 25) (hazard ratio 3.9, 95% CI: 1.8,8.9) (P < .001). Nonrelapse mortality was 22% in sibling and 59% in unrelated AHSCT. Survival correlated with type of donor, but not with the degree of histocompatibility match, age, or JAK2V617F status. In patients with MF with sibling donors, AHSCT is an effective therapy, whereas AHSCT from unrelated donors with FluMel/ATG conditioning led to a high rate of graft failure and limited survival. This trial was registered at www.clinicaltrials.gov as #NCT00572897.
Introduction
Primary myelofibrosis (PMF) is a chronic myeloproliferative neoplasm that occurs more frequently in patients >60 years of age. The JAK2V617F mutation is present in approximately 50% of PMF patients and post–essential thrombocythemia (post-ET) myelofibrosis (MF) and virtually all patients with postpolycythemia vera (post-PV) MF.1,2 Among PMF and post-ET MF patients with wild-type JAK2, mutations of calreticulin have been identified in approximately 90% of the cases.3 Survival in patients with MF depends on the time to transformation to acute myeloid leukemia or complications due to progressive cytopenias and/or splenomegaly. In the past, PMF, post-ET, or PV MF patients enrolled in clinical trials were stratified by prognosis according to the Lille prognostic scoring system4 that divided patients into low-risk, intermediate, or high-risk prognostic categories based on the presence of anemia, leukopenia, or leukocytosis. More recently, additional independent variables affecting the survival of PMF patients have been identified, including constitutional symptoms, presence of circulating blasts, thrombocytopenia, transfusion requirements, and chromosomal abnormalities, which have served as the basis for the Dynamic International Prognostic Score System (DIPSS)5 and the DIPSS-Plus.6 At this time, a therapeutic agent that is capable of curing MF patients is not available. Clinical trials with small-molecule JAK1/2 inhibitors have proven beneficial in reducing the degree of splenomegaly and suppressing constitutional symptoms in a large fraction of patients with MF, but treatment with these agents does not extensively affect the degree of marrow fibrosis or eliminate molecular or cytogenetic abnormalities.7,8
The only therapeutic option that can reverse the marrow fibrosis in MF patients9,10 is allogeneic hematopoietic stem cell transplantation (AHSCT). Initially, AHSCT with myeloablative conditioning regimens was shown to be curative, especially in younger patients, thanks to a graft-versus-tumor effect from donor lymphocytes.11-14 Nevertheless, because of a very high transplant-related mortality in patients ≥45 years of age,15 this approach was not routinely offered to the majority of MF patients. Studies from the MPD-RC and others16-18 then demonstrated that reduced-intensity conditioning (RIC) regimens allow older patients to undergo AHSCT with limited treatment-related mortality and with a significant chance of long-term survival. However, only a single multicenter large prospective study19 of RIC AHSCT has been reported to date. In this study, patients were transplanted from related or unrelated donors after receiving a conditioning regimen including fludarabine, busulfan, and rabbit anti-thymocyte globulin (ATG). The adverse prognostic factors that were identified19,20 in patients who received this type of RIC HSCT included age ≥57 years, a mismatched unrelated donor, an intermediate or high Lille score, JAK2 wild-type, the presence of constitutional symptoms, and more recently the absence of calreticulin mutations.21
Based on the initial transplant experience using a standard RIC regimen with fludarabine and melphalan, the Myeloproliferative Disorders Research Consortium (MPD-RC) launched in 2007 a prospective study of RIC AHSCT using this regimen and investigated the efficacy of fludarabine/melphalan (FluMel)-based RIC regimens in 2 parallel cohorts for matched sibling or matched unrelated donor transplantation, where ATG was used only in the latter group.
Patients and methods
Patients
The MPD-RC 101 is a multicenter phase 2 prospective study of reduced-intensity AHSCT that was performed at 11 centers affiliated with the MPD-RC (6 from the United States, 4 from Europe, and 1 from Canada). Patients enrolled in the study had documented PMF or post-ET or post-PV MF (blasts <20%), age 18 to 65 years, no significant comorbidities, intermediate or high-risk Lille score or low-risk Lille score with a platelet count <100 × 109/L, a sibling or unrelated available stem cell donor, and a signed consent form according to the MPD-RC 101 protocol approved by each MPD-RC institution’s institutional review board or equivalent ethical committee (for European centers). The study included 2 parallel protocols: one for patients who received an AHSCT from a sibling donor and one who received grafts from unrelated donors. The study was conducted in accordance with the Declaration of Helsinki.
Donors
HLA matching was determined by low-resolution molecular typing for sibling donors and by high-resolution molecular typing for both class I (A, B, and C) and class II (HLA-DRB1 and HLA-DQB1) antigens for unrelated donors. The donor graft consisted of either unmanipulated bone marrow or peripheral blood hematopoietic stem cells (PBSCs). The PBSC donors were mobilized with recombinant human granulocyte colony-stimulating factor 10 μg/kg subcutaneously per day for 5 days and underwent PBSC collection by leukapheresis.
Treatment plan
Patients were conditioned with fludarabine 30 mg/m2 per day intravenously (IV) for 5 days (day −6 to day −2) and melphalan 70 mg/m2 per day IV for 2 days (day −2 to day −1). Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus 0.03 mg/kg IV from day −2 and methotrexate 10 mg/m2 IV on day +1 and 8 mg/m2 on day +3 and day +6. Thymoglobulin (rabbit antithymocyte globulin; Genzyme, Cambridge, MA) at a 4.5-mg/kg total dose was used as additional GVHD prophylaxis only in patients receiving a graft from an unrelated donor.
Criteria for engraftment, response, and GVHD
Hematopoietic engraftment was defined as time to absolute neutrophil count ≥0.5 × 109/L and time to platelet count ≥50 × 109/L for 3 consecutive days. Posttransplantation donor-recipient chimerism was assessed by means of DNA microsatellite analysis of blood mononuclear cells. Clinical responses were categorized according to criteria developed by the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT)22 and included complete response (CR), partial response (PR), clinical improvement, stable disease, or progressive disease. Acute and chronic GVHD were graded according to standard criteria.23,24
Study design
The trial was designed to estimate progression-free survival and overall survival (OS) of patients with MF undergoing an AHSCT prepared with an RIC regimen in each of 2 cohorts separately (sibling and unrelated donors). With 32 patients in each stratum, an improvement in progression-free survival at 2 years without any evidence of disease from 50% of patients surviving to 75% is detectable with 2-sided α of .05 and power of 80%. Within each stratum, if 22 or more patients survive progression-free at 2 years, we would conclude that the transplant regimen had a progression-free survival rate of ≥75%; if there are ≤10 patients surviving progression-free at 2 years, we would conclude that the transplant regimen has a progression-free survival rate of ≤25%.
A group sequential stopping rule was used to test whether there was evidence that the transplant-related mortality rate (ie, mortality within 6 months of transplant) exceeded 50%. This stopping rule was applied separately to each stratum. The trial would have been terminated for a stratum if the stopping rule demonstrated that transplant-related mortality exceeded 50%.
Statistical analysis
Baseline patient characteristics, disease history, and treatment-related variables were summarized separately for each cohort defined by donor type using descriptive summary statistics and graphical approaches. Summary test statistics were used to provide additional descriptive information regarding the differences between the sibling and unrelated groups of patients. OS was calculated from the date of transplant to the date of death or last follow-up date. Event-free survival (EFS) was calculated from date of transplant to the date of the first occurrence of failure to engraftment (primary graft failure), loss of donor graft (secondary graft failure), death, or last follow-up date. Survival curves were estimated using Kaplan-Meier methods. The association of OS and current diagnosis (PMF or MF secondary to ET/PV), Lille score (0/1 or 2), gender, age-adjusted DIPSS (low/intermediate-1 [int-1] risk or intermediate-2 [int-2]/high risk), donor HLA compatibility (matched or mismatched), and baseline JAKV617F status (positive or negative) was examined using the log-rank test. The association of OS and age at transplant, time from diagnosis to transplant, baseline white blood cell count, platelet count, percentage of blasts, and the dose of CD34+ cells within the graft was examined using the Cox proportional hazards model.
Results
Patient characteristics
The characteristics of patients in the sibling (n = 32) and unrelated (n = 34) groups are shown in Table 1. Although the study was not designed to compare these 2 groups, the clinical characteristics of patients were similar. Of 66 patients, 63 had intermediate or high-risk MF according to the Lille score system, whereas 3 patients in the sibling group had low-risk disease with thrombocytopenia. The median age at the time of transplant was 55 years in the sibling group and 56 years in the unrelated group. Bone marrow biopsy specimens from 51 of 66 patients were reviewed by a central group of pathologists to confirm the histologic diagnosis and to grade the fibrosis according to the European consensus scale (0-3).25 A Jak2V617F mutational analysis was available for 63 of 66 patients and was positive in approximately 50% of the patients. Of the 66 patients, 52 had splenomegaly at the time of transplant, whereas 10 had been previously splenectomized (5 patients in each group). Karyotypic analyses just prior to transplant were available in 49 patients. A total of 44% patients in the sibling group and 41% in the unrelated group had a normal karyotype. The median time from diagnosis to transplant in the 2 groups was 16 months (range: 1-247) in recipients with a sibling donor and 20 months (range: 2-341) in recipients with an unrelated donor.
Characteristics of 66 patients with MF in the MPD-RC 101 study
| . | Sibling donor (n = 32) . | Unrelated donor (n = 34) . | |
|---|---|---|---|
| Diagnosis | |||
| PMF | 14 (44%) | 25 (74%) | |
| PV-MF | 3 (9%) | 5 (15%) | |
| ET-MF | 15 (47%) | 4 (12%) | |
| Lille score | |||
| 0 | 3 (9%) | 0 (0%) | |
| 1 | 20 (63%) | 23 (68%) | |
| 2 | 9 (28%) | 11 (32%) | |
| Age at transplant (y), median (range) | 55 (40-65) | 56 (30-65) | |
| Gender | |||
| Female | 13 (41%) | 15 (44%) | |
| Male | 19 (59%) | 19 (56%) | |
| Time from diagnosis (mo), median (range) | 16.1 (1-247) | 20 (2-341) | |
| Patient:donor gender | |||
| Female:Female | 6 (19%) | 7 (21%) | |
| Male:Male | 7 (22%) | 8 (24%) | |
| Male:Female | 10 (31%) | 6 (18%) | |
| Female:Male | 9 (28%) | 13 (38%) | |
| Age-adjusted DIPSS | |||
| Low | 5 (16%) | 5 (15%) | |
| Int-1 | 13 (41%) | 14 (41%) | |
| Int-2 | 8 (29%) | 10 (29%) | |
| High | 3 (9%) | 2 (6%) | |
| Unknown | 3 (9%) | 3 (9%) | |
| WBC ×109/L, median (range) | 5.1 (2-43) | 6.8 (1.3-70) | |
| Circulating blasts %, median (range) | 1.0 (0-10) | 0.12 (0-16) | |
| Platelets ×109/L, median (range) | 104 (28-927) | 120 (19-1662) | |
| JAK-2 V617F | |||
| Positive | 12 (38%) | 18 (53%) | |
| Negative | 17 (53%) | 16 (47%) | |
| Unknown | 3 (9%) | 0 (0%) | |
| Bone marrow fibrosis | |||
| Grade 1 | 0 | 2 (6%) | |
| Grade 2 | 2 (6%) | 6 (18%) | |
| Grade 3 | 18 (56%) | 23 (68%) | |
| Unknown | 12 (38%) | 3 (9%) | |
| Splenomegaly | |||
| Yes | 24 (75%) | 28 (82%) | |
| No | 3 (9%) | 1 (3%) | |
| Splenectomy | 5 (16%) | 5 (15%) | |
| Karyotype | |||
| Normal | 14 (44%) | 14 (41%) | |
| One abnormality | 7 (22%) | 9 (26%) | |
| Complex abnormality | 5 (16%) | 0 (0%) | |
| Unknown | 6 (19%) | 11 (32%) | |
| Stem cell source | |||
| Peripheral blood | 26 (81%) | 31 (91%) | |
| BM | 6 (19%) | 3 (9%) | |
| CD34 cells (×106/kg), PBSC median (range) | 5.9 (3.2-14) | 6.5 (2.9-11) | |
| TNC (×108/kg), BM median (range) | 3.7 (2.6-7.8) | 2.3 (1.9, 2.7) | |
| Full HLA matched | 30 (94%) | 25 (74%) | |
| HLA 1 Ag mismatched, no allele mismatched | 2 (6%) | 4 (12%) | |
| HLA Ag matched, 1 or 2 alleles mismatched | 0 (0%) | 5 (15%) | |
| . | Sibling donor (n = 32) . | Unrelated donor (n = 34) . | |
|---|---|---|---|
| Diagnosis | |||
| PMF | 14 (44%) | 25 (74%) | |
| PV-MF | 3 (9%) | 5 (15%) | |
| ET-MF | 15 (47%) | 4 (12%) | |
| Lille score | |||
| 0 | 3 (9%) | 0 (0%) | |
| 1 | 20 (63%) | 23 (68%) | |
| 2 | 9 (28%) | 11 (32%) | |
| Age at transplant (y), median (range) | 55 (40-65) | 56 (30-65) | |
| Gender | |||
| Female | 13 (41%) | 15 (44%) | |
| Male | 19 (59%) | 19 (56%) | |
| Time from diagnosis (mo), median (range) | 16.1 (1-247) | 20 (2-341) | |
| Patient:donor gender | |||
| Female:Female | 6 (19%) | 7 (21%) | |
| Male:Male | 7 (22%) | 8 (24%) | |
| Male:Female | 10 (31%) | 6 (18%) | |
| Female:Male | 9 (28%) | 13 (38%) | |
| Age-adjusted DIPSS | |||
| Low | 5 (16%) | 5 (15%) | |
| Int-1 | 13 (41%) | 14 (41%) | |
| Int-2 | 8 (29%) | 10 (29%) | |
| High | 3 (9%) | 2 (6%) | |
| Unknown | 3 (9%) | 3 (9%) | |
| WBC ×109/L, median (range) | 5.1 (2-43) | 6.8 (1.3-70) | |
| Circulating blasts %, median (range) | 1.0 (0-10) | 0.12 (0-16) | |
| Platelets ×109/L, median (range) | 104 (28-927) | 120 (19-1662) | |
| JAK-2 V617F | |||
| Positive | 12 (38%) | 18 (53%) | |
| Negative | 17 (53%) | 16 (47%) | |
| Unknown | 3 (9%) | 0 (0%) | |
| Bone marrow fibrosis | |||
| Grade 1 | 0 | 2 (6%) | |
| Grade 2 | 2 (6%) | 6 (18%) | |
| Grade 3 | 18 (56%) | 23 (68%) | |
| Unknown | 12 (38%) | 3 (9%) | |
| Splenomegaly | |||
| Yes | 24 (75%) | 28 (82%) | |
| No | 3 (9%) | 1 (3%) | |
| Splenectomy | 5 (16%) | 5 (15%) | |
| Karyotype | |||
| Normal | 14 (44%) | 14 (41%) | |
| One abnormality | 7 (22%) | 9 (26%) | |
| Complex abnormality | 5 (16%) | 0 (0%) | |
| Unknown | 6 (19%) | 11 (32%) | |
| Stem cell source | |||
| Peripheral blood | 26 (81%) | 31 (91%) | |
| BM | 6 (19%) | 3 (9%) | |
| CD34 cells (×106/kg), PBSC median (range) | 5.9 (3.2-14) | 6.5 (2.9-11) | |
| TNC (×108/kg), BM median (range) | 3.7 (2.6-7.8) | 2.3 (1.9, 2.7) | |
| Full HLA matched | 30 (94%) | 25 (74%) | |
| HLA 1 Ag mismatched, no allele mismatched | 2 (6%) | 4 (12%) | |
| HLA Ag matched, 1 or 2 alleles mismatched | 0 (0%) | 5 (15%) | |
Patients who received a stem cell transplant from a sibling (n = 32) or an unrelated (n = 34) donor were enrolled. Patients at low risk were enrolled only if they were thrombocytopenic.
Ag, antigen; BM, bone marrow; TNC, total nucleated cells; WBC, white blood cell.
Donors
A total of 57 patients (26/32 in the sibling group and 31/34 in the unrelated group) received PBSCs, and 9 patients (6/32 in sibling and 3/34 in the unrelated group) received marrow grafts. The median dose of CD34+ cells infused in recipients of PBSCs was comparable in the sibling and unrelated groups (Table 1). In recipients of bone marrow grafts, the median dose of total nucleated cells infused in 6 sibling transplants (3.78 × 108/kg; range: 2.65, 7.87) was slightly higher than in 3 unrelated transplants (2.37 × 108/kg; range: 1.9, 2.7). Donors were HLA matched (10/10 antigens) in 30 out of 32 sibling transplants and 25 out of 34 unrelated transplants (P = .02). The remaining donors were mismatched for either 1 HLA antigen or 1 HLA antigen + 1 allele or only 1 or 2 alleles.
Engraftment
Donor cell engraftment was analyzed in each of the 2 groups separately (Table 2). In the sibling group, neutrophil and platelet engraftment was achieved in 31 out of 32 patients (97%) and 28 out of 32 patients (88%), respectively. The median time to engraftment for neutrophils was 22 days (range: 0-62) and for platelets was 28 days (range: 0-62). In the unrelated group, neutrophil and platelet engraftment occurred in 26 of 34 patients (76%) and 20 of 34 patients (59%). Two patients in this group died prior to day 30 without hematopoietic cell engraftment. The median time to engraftment for neutrophils was 18 days (range: 11-43) and for platelets was 28 days (range: 9-365).
Outcomes in patients with MF in MPD-RC 101
| . | Sibling donor (n = 32) . | Unrelated donor (n = 34) . |
|---|---|---|
| Primary graft failure, n (%) | 1 (3%) | 8 (24%) |
| Patients with ANC ≥0.5 × 109/L, n | 31 | 26 |
| Days to ANC engraftment, median (range) | 22 (0-62) | 18 (11-43) |
| Patients with PLT ≥20 × 109/L, n | 28 | 20 |
| Days to PLT engraftment, median (range) | 28 (0-62) | 29 (9-365) |
| Secondary graft failure, n (%) | 1 (3%) | 4 (12%) |
| aGVHD grade II-IV, % | 12 (38%) | 14 (41%) |
| aGVHD grade III-IV, n % | 4 (13%) | 7 (21%) |
| cGVHD, n % | 10/28 (36%) | 5/15 (38%) |
| Patients alive, n (%) | 24 (75%) | 11 (32%) |
| Deaths <6 mo, n (%) | 3 (9%) | 17 (50%) |
| Clinical response, n (%) | 29 | 17 |
| Overall response rate, n (%) | 26/28 (93%) | 11 (69%) |
| Clinical CR, n (%) | 7 (24%) | 6 (35%) |
| PR, n (%) | 8 (28%) | 1 (6%) |
| Clinical improvement, n (%) | 11 (38%) | 5 (29%) |
| Stable disease, n (%) | 2 (7%) | 4 (24%) |
| Progressive disease, n (%) | 0 | 1 (6%) |
| Unknown response, n (%) | 1 (3%) | — |
| . | Sibling donor (n = 32) . | Unrelated donor (n = 34) . |
|---|---|---|
| Primary graft failure, n (%) | 1 (3%) | 8 (24%) |
| Patients with ANC ≥0.5 × 109/L, n | 31 | 26 |
| Days to ANC engraftment, median (range) | 22 (0-62) | 18 (11-43) |
| Patients with PLT ≥20 × 109/L, n | 28 | 20 |
| Days to PLT engraftment, median (range) | 28 (0-62) | 29 (9-365) |
| Secondary graft failure, n (%) | 1 (3%) | 4 (12%) |
| aGVHD grade II-IV, % | 12 (38%) | 14 (41%) |
| aGVHD grade III-IV, n % | 4 (13%) | 7 (21%) |
| cGVHD, n % | 10/28 (36%) | 5/15 (38%) |
| Patients alive, n (%) | 24 (75%) | 11 (32%) |
| Deaths <6 mo, n (%) | 3 (9%) | 17 (50%) |
| Clinical response, n (%) | 29 | 17 |
| Overall response rate, n (%) | 26/28 (93%) | 11 (69%) |
| Clinical CR, n (%) | 7 (24%) | 6 (35%) |
| PR, n (%) | 8 (28%) | 1 (6%) |
| Clinical improvement, n (%) | 11 (38%) | 5 (29%) |
| Stable disease, n (%) | 2 (7%) | 4 (24%) |
| Progressive disease, n (%) | 0 | 1 (6%) |
| Unknown response, n (%) | 1 (3%) | — |
Engraftment, GVHD, and survival data are shown. Primary graft failure is defined as lack of engraftment of donor neutrophils and secondary graft failure as loss of the graft. Clinical response was assessed according to the IWG criteria in patients with at least 180 days of follow-up.
ANC, absolute neutrophil count; PLT, platelet.
An analysis of donor cell chimerism of blood mononuclear cells was available in 56 patients. By day 30 posttransplant, 23 out of 28 patients in the sibling group and 21 out of 28 in the unrelated cohort had ≥98% donor cell chimerism in the peripheral blood. Primary graft failure was observed in 1 out of 32 (3%) sibling transplants and in 8 out of 34 (24%) unrelated transplants. Secondary graft failure was observed in 1 sibling and 4 unrelated transplants, after a median of 32 and 48 days, respectively. Therefore, the overall graft failure rate was 6% in the sibling group and 36% in the unrelated transplant group. Within the 12 patients with graft failure in the unrelated group, 8 had received an HLA-matched graft and 4 an HLA-mismatched graft.
GVHD
Overall, 61% of the patients participating in the study did not experience greater than grade I acute GVHD (aGVHD). In the sibling group, 12 out of 32 patients (38%) experienced aGVHD grade II to IV, with 4 cases (12%) at grade III or IV. In the unrelated group, 14 out of 34 patients (41%) had aGVHD grade II to IV, with 7 cases (21%) at grade III or IV (Table 2). The degree of chronic GVHD (cGVHD) could be assessed in 43 patients (28 in the sibling group and 15 in the unrelated group). Of patients in the sibling cohort, 36% experienced cGVHD (extensive in 25%), whereas in the unrelated cohort, 38% of the patients had cGVHD and in 20% it was extensive.
Mortality
The overall median follow-up for patients alive at last follow-up was 25 months (range: 10-73). In the sibling group, 24 out of 32 patients (75%) were alive. Progression of disease caused the death of 1 patient in this group, whereas causes of nonrelapse mortality (NRM) in the remaining 7 patients (22%) included secondary malignancy (n = 1), aGVHD (n = 3), hemorrhage (n = 1), respiratory failure (n = 1), and heart failure (n = 1). In the unrelated group, 11 out of 34 patients (32%) are alive. Among the causes of death, 3 were related to progression of disease (in 2 cases after a second transplant) (9%), whereas 20 (59%) were due to transplant-related complications, including aGVHD (n = 5), hemorrhage (n = 3), renal failure (n = 2), pneumonia/respiratory failure (n = 2), venous occlusive disease (n = 1), viral infection (n = 1), and other events secondary to graft failure (n = 6).
Clinical response
Clinical responses were assessed according to the IWG-MRT 2006 criteria in 46 patients (29 sibling and 17 unrelated transplants) who survived at least 180 days (Table 2). In the sibling group, the overall response rate was 93%, with 7 patients (25%) achieving a clinical CR, 8 patients a PR (29%), and 11 patients (39%) a clinical improvement. Two patients had stable disease and 1 patient had an unknown response. In the unrelated group, the overall response rate was 69% with 6 out of 17 (35%) CRs, 1 PR (6%), and 5 clinical improvements (29%). One patient experienced progression of the disease 180 days posttransplant. These results show that the clinical result of AHSCT from sibling or unrelated donors may be comparable for patients who achieve a sustained stem cell engraftment.
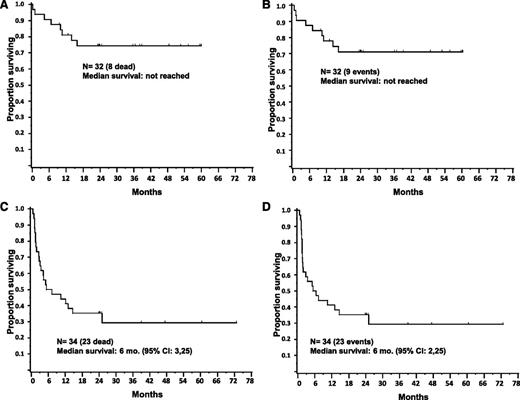
Survival
The median OS for the sibling group has not been reached, whereas for the unrelated group it was 6 months (95% confidence interval [CI]: 3,25). A significantly higher risk of death was observed for patients receiving a transplant from an unrelated as compared with a sibling donor (hazard ratio 3.9; 95% CI: 1.8, 8.9) (P < .001). (Figure 1 top). Median EFS has not been reached in the sibling group and was 6 months (95% CI: 2, 25) in the unrelated group (Figure 1 bottom). Analysis by Cox proportional hazards model in sibling and unrelated transplants did not show any association between OS and age of patient at transplant, time from diagnosis to transplant, baseline white blood cell count, baseline platelet count, baseline percentage of circulating blasts, and CD34+ cell dose in the graft. Survival curves based on diagnosis (primary or secondary MF), degree of donor HLA match, presence of JAK2V617F, and age ≥57 years showed no statistical difference within the sibling or unrelated groups (Figure 2). Patients with or without the JAK2V617F mutation had a similar survival in the sibling cohort. In the unrelated cohort, however, the presence of JAK2V617F mutation was associated with a trend for worse survival, but this relationship did not reach statistical significance (P = .29).
Survival after AHSCT with a FluMel conditioning regimen in MF patients. Cumulative OS (A) and EFS (B) in 32 MF patients who received a transplant from a sibling donor. Median survival has not been reached, because 75% of patients were alive at last follow-up and 71% were without disease progression. Cumulative OS (C) and EFS (D) in 34 MF patients who received a transplant from an unrelated donor. Median OS and EFS in unrelated transplants are shown.
Survival after AHSCT with a FluMel conditioning regimen in MF patients. Cumulative OS (A) and EFS (B) in 32 MF patients who received a transplant from a sibling donor. Median survival has not been reached, because 75% of patients were alive at last follow-up and 71% were without disease progression. Cumulative OS (C) and EFS (D) in 34 MF patients who received a transplant from an unrelated donor. Median OS and EFS in unrelated transplants are shown.
Diagnosis, HLA matching, JAK2V617F, and age do not correlate with survival after AHSCT with a FluMel conditioning regimen in MF patients. OS in recipients with grafts from sibling (Sib) and unrelated (Unrel) donors based on diagnosis (PMF, ET-MF, or PV-EF) (A), HLA-matched or HLA-mismatched donor (B) (*2/32 patients in the HLA-matched Sib group received a 1-antigen-mismatched transplant from their sibling, and none of them died), presence of JAK2V617F mutation (Jak2 pos) (C), and age < or ≥57 years (D).
Diagnosis, HLA matching, JAK2V617F, and age do not correlate with survival after AHSCT with a FluMel conditioning regimen in MF patients. OS in recipients with grafts from sibling (Sib) and unrelated (Unrel) donors based on diagnosis (PMF, ET-MF, or PV-EF) (A), HLA-matched or HLA-mismatched donor (B) (*2/32 patients in the HLA-matched Sib group received a 1-antigen-mismatched transplant from their sibling, and none of them died), presence of JAK2V617F mutation (Jak2 pos) (C), and age < or ≥57 years (D).
Therefore, none of these variables predicted survival in these patients. When patients in each group were stratified based on the age-adjusted DIPSS26,27 (Figure 3), patients with low/int-1 and int-2/high risk in the sibling cohort had comparable survival, whereas patients in the unrelated cohort at int-2/high risk had lower survival rates than those at low/int-1 risk (2-year survival: 42% vs 17%, P = .1). Survival was examined in each of the 2 transplant groups in relation to diagnosis, age, gender, Lille score and DIPSS score at the time of transplant, donor HLA compatibility, and presence of Jak2V617F mutation. The results, shown in Table 3, demonstrate that none of these factors predicted survival individually in either group of patients.
OS in sibling (Sib) or unrelated (Unrel) transplants by age-adjusted DIPSS categories. Patients in each group were classified as low/int-1 or int-2/high risk.
OS in sibling (Sib) or unrelated (Unrel) transplants by age-adjusted DIPSS categories. Patients in each group were classified as low/int-1 or int-2/high risk.
Univariate survival analysis in sibling and unrelated donor groups
| Sibling donors (n = 32) . | Unrelated donors (n = 34) . | |||||
|---|---|---|---|---|---|---|
| n . | 2-y survival . | Log-rank P . | n . | 2-y survival . | Log-rank P . | |
| Current diagnosis | ||||||
| PMF | 14 (44%) | 71% (48%, 95%) | .67 | 25 (74%) | 36% (17%, 55%) | .94 |
| ET/PV | 18 (56%) | 76% (56%, 97%) | — | 9 (26%) | 33% (3%, 64%) | — |
| Lille score | ||||||
| 0/1 | 23 (72%) | 73% (54%, 91%) | .81 | 23 (68%) | 35% (15%, 54%) | .88 |
| 2 | 9 (28%) | 78% (51%, 100%) | — | 11 (32%) | 36% (8%, 65%) | — |
| Gender | ||||||
| Female | 13 (41%) | 69% (44%, 94%) | .55 | 15 (44%) | 40% (15%, 65%) | .81 |
| Male | 19 (59%) | 78% (58%, 97%) | — | 19 (56%) | 32% (11%, 52%) | — |
| Age-adjusted DIPSS | ||||||
| Low-risk/int-1 | 18 (56%) | 71% (49%, 92%) | .67 | 19 (56%) | 42% (20%, 64%) | .14 |
| Int-2/high-risk | 11 (34%) | 82% (59%, 100%) | — | 12 (35%) | 17% (0%, 38%) | — |
| Donor HLA | ||||||
| Match | 30 (94%) | 72% (56%, 89%) | .43 | 25 (74%) | 40% (21%, 59%) | .33 |
| Mismatch | 2 (6%) | 100% | — | 9 (26%) | 22% (0%, 49%) | — |
| Baseline JAK-2 V617F | ||||||
| Positive | 12 (38%) | 76% (56%, 97%) | .68 | 18 (53%) | 28% (7%, 48%) | .29 |
| Negative | 17 (53%) | 64% (36%, 92%) | — | 16 (47%) | 44% (19%, 68%) | — |
| Sibling donors (n = 32) . | Unrelated donors (n = 34) . | |||||
|---|---|---|---|---|---|---|
| n . | 2-y survival . | Log-rank P . | n . | 2-y survival . | Log-rank P . | |
| Current diagnosis | ||||||
| PMF | 14 (44%) | 71% (48%, 95%) | .67 | 25 (74%) | 36% (17%, 55%) | .94 |
| ET/PV | 18 (56%) | 76% (56%, 97%) | — | 9 (26%) | 33% (3%, 64%) | — |
| Lille score | ||||||
| 0/1 | 23 (72%) | 73% (54%, 91%) | .81 | 23 (68%) | 35% (15%, 54%) | .88 |
| 2 | 9 (28%) | 78% (51%, 100%) | — | 11 (32%) | 36% (8%, 65%) | — |
| Gender | ||||||
| Female | 13 (41%) | 69% (44%, 94%) | .55 | 15 (44%) | 40% (15%, 65%) | .81 |
| Male | 19 (59%) | 78% (58%, 97%) | — | 19 (56%) | 32% (11%, 52%) | — |
| Age-adjusted DIPSS | ||||||
| Low-risk/int-1 | 18 (56%) | 71% (49%, 92%) | .67 | 19 (56%) | 42% (20%, 64%) | .14 |
| Int-2/high-risk | 11 (34%) | 82% (59%, 100%) | — | 12 (35%) | 17% (0%, 38%) | — |
| Donor HLA | ||||||
| Match | 30 (94%) | 72% (56%, 89%) | .43 | 25 (74%) | 40% (21%, 59%) | .33 |
| Mismatch | 2 (6%) | 100% | — | 9 (26%) | 22% (0%, 49%) | — |
| Baseline JAK-2 V617F | ||||||
| Positive | 12 (38%) | 76% (56%, 97%) | .68 | 18 (53%) | 28% (7%, 48%) | .29 |
| Negative | 17 (53%) | 64% (36%, 92%) | — | 16 (47%) | 44% (19%, 68%) | — |
Discussion
We report here the results of a large prospective phase 2 multicenter study of RIC AHSCT in patients with PMF-, post-ET–, or post-PV–related MF that included 66 patients transplanted in 11 centers within the MPD-RC.
That HSCT is the only curative intervention for patients with MF has been known for over 15 years. However, the parameters that should guide transplant physicians in defining treatment plans or assessment of risk associated with transplant remain to be validated. In fact, most of the data related to factors that determine the outcome of transplant in MF are derived from retrospective studies28-35 of cohorts of patients who have been transplanted at single centers or larger numbers of patients in national or international registries. These studies often include data related to transplants performed over decades, thus adding additional variables related to significant changes in supportive therapy in blood and marrow transplantation over the years. The only large prospective transplant study in primary or secondary MF was the European Society for Blood and Marrow Transplant (EBMT) trial19 that used an RIC regimen with fludarabine, busulfan, and rabbit ATG in 33 transplants from sibling donors and 70 from unrelated donors. The results of this study differed from others, especially because of the high survival rate (approximately 70%) and low NRM in transplants from matched unrelated donors. However, because in that study patients >55 years of age had a 48% survival as opposed to 82% for younger patients, it is conceivable that a large portion of patients who received a matched unrelated transplant may have been younger than those participating in the present study. Survival correlated also with HLA-mismatched donors and intermediate- and high-risk Lille score at the time of transplant. Relapse after transplant was influenced by Lille score and splenectomy before transplant. Based on this trial, the authors then reported a prognostic scoring system20 that included age ≥57, absence of JAK2V617F (JAK2 wild-type), and presence of constitutional symptoms at the time of transplant as independent adverse indicators. In our study, we prospectively transplanted 66 patients in 2 groups based on the type of donor: 32 patients with a sibling donor (94% were HLA matched) and 34 with an unrelated donor (74% HLA matched). As compared with the EBMT study, the present study included a lower number of patients with a low-risk Lille score (4.5 vs 16.5%). Moreover, low-risk patients in our study had more advanced disease according to a modified Lille score37 based on each of them being thrombocytopenic. Our conditioning regimen included melphalan instead of busulfan, and ATG was administered only to recipients of the group receiving unrelated grafts. The present results in the sibling group showed 75% OS and are consistent with the EBMT study as well as with our prior retrospective study16 of transplants from matched siblings. However, in transplants from unrelated donors, the OS in the present study was 32%, significantly inferior to the sibling group (hazard ratio 3.9; 95% CI: 1.8, 8.9) (P < .001). As opposed to the EBMT trial, in our study we did not detect a difference between HLA-matched and HLA-mismatched unrelated transplants. Because the relapse-related mortality was only 6% in the study (3% in the sibling group and 9% in the unrelated group), the high rate of NRM in the unrelated group was frequently secondary to graft failure. Previous studies have reported controversial conclusions concerning the risk associated with utilizing an unrelated donor. Similar to the EBMT prospective trial, a retrospective study from Seattle37 did not find a significant difference of NRM with matched sibling or unrelated donors. However, a recent retrospective analysis of the EBMT registry in 250 patients with post-ET MF or post-PV MF who received an AHSCT from 1994 to 2010 showed that NRM in unrelated transplants was 34% and that using an unrelated donor was an independent adverse prognostic factor.38 Given that in our study we did not observe any difference in the outcome of PMF and post-ET or post-PV MF, our findings seem consistent with the EBMT retrospective report. Prior retrospective data from the Italian (GITMO)35 and French (SFGM-TC)34 registries also showed significantly higher rates of NRM in transplants from nonsibling donors. An initial analysis39 from the Center for International Blood and Marrow Transplant Registry including MF patients receiving mostly a myeloablative regimen found a 55% and 70% overall mortality in sibling and unrelated transplants, respectively. A more recent retrospective study40 from the Center for International Blood and Marrow Transplant Registry of 233 patients with MF who received an RIC transplant showed a better outcome for those who received a transplant from a matched sibling as opposed to a matched unrelated donor. In another study of RIC HSCT, Bacigalupo et al41 reported that the 3 independent adverse prognostic factors for outcome of AHSCT transplant for MF were any type of donor other than a matched sibling, a large spleen, and an excessive number of red blood cell transfusions prior to transplant. This scoring system suggests that other factors related to the disease can affect the transplant outcome and will be validated in future prospective trials. However, consistent with this study, we found that a transplant from an unrelated donor carried a higher risk of death.
None of the studies of transplantation in MF have evaluated the possible role of HLA antibodies, especially in the unrelated setting, as a possible factor correlating with graft failure. Unfortunately these data were not measured in our study, and we cannot rule out that this may have influenced the excessive rate of rejection in our cohort of unrelated transplants. Based on risk factors identified in prior studies, we analyzed whether the limited survival in unrelated transplants was related to HLA mismatch, age >57, or absence of JAK2V617F. None of these factors correlated with survival, and only the type of donor (sibling vs unrelated) predicted the outcome. As opposed to the EBMT results, JAKV617F mutation actually showed a trend toward a worse outcome in unrelated transplants. This, however, could be related to a smaller number of patients in the unrelated cohort in our study. Finally, we found that in transplants from unrelated but not sibling donors, a DIPSS int-2/high–risk status for the recipient correlated with an inferior survival. A correlation between advanced DIPSS scores and NRM was reported in the retrospective analysis by Scott et al in 170 patients transplanted in Seattle from sibling or unrelated donors between 1990 and 2009.26 However, this study included a variety of conditioning regimens ranging from nonmyeloablative to reduced intensity or fully myeloablative.
It is possible that differences between the only 2 prospective cooperative studies (ours and the EBMT one) may be due primarily to the different conditioning regimens. However, other possible differences could include a lower number of low-risk patients in our study or the different type of ATG used. Because the FluMel regimen was associated with low rates of graft failure, relapse, and NRM in sibling transplants, we assume that the different results obtained in the unrelated group may be due, in part, to a strong in vivo immunomodulatory effect or in vivo T-cell depletion of the graft due to the combination of FluMel/ATG as a conditioning regimen that could have favored a host-antidonor immune response. The immune effect of the conditioning may play an important role in the success that will be encountered with new protocols that are being designed to include ruxolitinib or other JAK 1/2 inhibitors (MPD-RC114).42-45 In fact, these agents are being investigated in AHSCT not only because they can rapidly reduce constitutional symptoms and spleen size thanks to a marked suppression of proinflammatory cytokines8 but also for a possible immunosuppressive activity that may limit GVHD.46 Because of these considerations and the high rate of graft failure in unrelated transplants prepared with FluMel/ATG observed in this study, the MPD-RC has recently launched a new prospective study combining ruxolitinib with a fludarabine/busulfan ATG RIC regimen.
Based on the results of 2 large prospective studies of RIC AHSCT in MF, regimens including melphalan or busulfan are both very effective in transplants from sibling donors. However, a busulfan-based regimen seems preferable in case of transplant from an unrelated donor, because comparable results in sibling or unrelated transplants were observed in the EBMT study using a reduced-intensity regimen and previously in a large retrospective study using a myeloablative regimen. These studies also suggest that an initial search for matched donors should be performed for all the MF patients at ≥int-1 risk. In patients at int-2 or high risk, AHSCT should be offered immediately. In patients at int-1, especially if they have only a matched unrelated donor, AHSCT should be offered as soon as the disease shows any sign of overall progression, such as worsening of anemia or symptoms or increase in spleen size, even before they meet the criteria for the int-2 risk category.
Our prospective study exploited for the first time the use of a standard RIC regimen such as FluMel in AHSCT for MF. More large prospective studies testing new strategies to reduce NRM in high-risk AHSCT, such as from nonsibling or HLA-mismatched donors or in patients with more advanced disease, are warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Institutes of Health, National Cancer Institute grant 1 P01 CA108671-01A2 (principal investigator: R.H.).
Authorship
Contribution: D.R., J.D.G., L.R.S., and R.H. contributed to the study design; D.R., J.D.G., L.S.P., and R.H. contributed to the writing of the manuscript; M.S., C.M., G.P., and R.M. collected and assembled the data; V.N., A.O., and R.S.W. collected and analyzed patient samples; J.D.G. and L.S.P. performed statistical analysis; D.R., L.I., T.B.S., M.B., A.B., A.R., R.B.K., V.G., B.A., J.M., M.W., A.M.V., J.T.P., G.B., and R.H. enrolled patients; and all authors assisted in the critical review of the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Damiano Rondelli, Division of Hematology/Oncology, University of Illinois Hospital & Health Sciences System, 840 S Wood St, 820-E-CSB, Chicago, IL; e-mail: drond@uic.edu.