Key Points
TGN1412-induced T-cell activation following high-density preculture of PBMCs is a consequence of FcγRIIb upregulation on monocytes.
In vivo, cytokine release syndrome may be due to the close association of FcγRIIb-bearing cells with T cells in lymphoid tissues.
Abstract
The anti-CD28 superagonist antibody TGN1412 caused life-threatening cytokine release syndrome (CRS) in healthy volunteers, which had not been predicted by preclinical testing. T cells in fresh peripheral blood mononuclear cells (PBMCs) do not respond to soluble TGN1412 but do respond following high-density (HD) preculture. We show for the first time that this response is dependent on crystallizable fragment gamma receptor IIb (FcγRIIb) expression on monocytes. This was unexpected because, unlike B cells, circulating monocytes express little or no FcγRIIb. However, FcγRIIb expression is logarithmically increased on monocytes during HD preculture, and this upregulation is necessary and sufficient to explain TGN1412 potency after HD preculture. B-cell FcγRIIb expression is unchanged by HD preculture, but B cells can support TGN1412-mediated T-cell proliferation when added at a frequency higher than that in PBMCs. Although low-density (LD) precultured PBMCs do not respond to TGN1412, T cells from LD preculture are fully responsive when cocultured with FcγRIIb-expressing monocytes from HD preculture, which shows that they are fully able to respond to TGN1412-mediated activation. Our novel findings demonstrate that cross-linking by FcγRIIb is critical for the superagonist activity of TGN1412 after HD preculture, and this may contribute to CRS in humans because of the close association of FcγRIIb-bearing cells with T cells in lymphoid tissues.
Introduction
Immunostimulatory monoclonal antibodies (mAbs) targeting T-cell costimulatory molecules are an emerging class of therapeutics designed to promote either endogenous or vaccine-mediated anticancer T-cell immunity. Although cited as a major step forward in the clinical use of mAbs, they are often associated with severe side effects, including autoimmunity and inflammatory reactions resulting from cytokine release syndrome (CRS).1
CD28 is a key T-cell costimulatory molecule and drives T-cell activation alongside T-cell receptor (TCR) engagement. There has been a keen focus on the development of therapeutic anti-CD28 mAbs for a range of diseases, including autoimmunity and cancer.1-3 However, anti-CD28 mAbs suffered a major setback when the first-in-man trial of TGN1412 caused life-threatening CRS.1 TGN1412, a so-called “superagonist,” is able to stimulate T-cell activation without TCR engagement.4 Preclinical in vitro testing using human peripheral blood mononuclear cells (PBMCs) and in vivo testing in cynomolgus macaques failed to predict this toxicity. Later evaluations revealed that macaques lack CD28 on effector memory T cells and so were unable to respond.5 In addition, further species differences were observed in rodent models in which CD28 superagonist mAbs had been found to preferentially activate regulatory T cells.3,6
After these failures, there has been a concerted effort to develop predictive in vitro assays that allow a better understanding of the in vivo action of superagonists and their preclinical prediction. The immobilization of TGN1412 onto plastic or the addition of anti-immunoglobulin G (IgG) antibody has been shown to induce cytokine release in PBMC assays.6,7 Both approaches provide extensive cross-linking of the mAb on the T-cell surface. In assays with soluble TGN1412, such cross-linking could be provided by co-engagement of the mAb crystallizable fragment (Fc) region with Fc γ receptors (FcγRs) expressed on various PBMC subsets. The inability of soluble TGN1412 to mediate cytokine release suggests that unmanipulated PBMCs lack sufficient capacity to allow TGN1412 to induce T-cell activation. Coculture of T cells with human umbilical vein endothelial cells (HUVECs) has also been shown to induce T-cell activation in response to TGN1412, although the level of cytokine release was low and, surprisingly, the interaction of the mAb Fc region with FcγRs was not required.8
In an interesting alternative approach to demonstrating TGN1412 activity in vitro, Romer et al9 showed that soluble TGN1412 is able to stimulate cytokine release after preculturing PBMCs at high density (HD) for 48 hours and proposed that this was the result of an increase in the tonic activation of the responder T cells, which lowers their threshold for stimulation. In our study, we used this HD preculture protocol to investigate the role of FcγRs in the activation of T cells by TGN1412. We show that HD preculture induces a remarkable increase in the expression of FcγRIIb on monocytes, but not B cells, and that this provides sufficient interaction with the Fc region of TGN1412 to induce T-cell activation. In contrast to previous studies9,10 our observations indicate that no enhancement of T-cell sensitivity is required, whereas co-engagement with FcγRIIb is crucial to the agonistic activity of soluble TGN1412. These findings provide an insight into the cellular and molecular requirements for superagonistic activity in the context of targeting CD28 with mAbs, and they have important implications for mAbs designed to enhance T-cell responses.
Materials and methods
Donors and PBMC preparation
Anonymized leukocyte cones were from the National Blood Service (Southampton, UK) and used within 4 hours for preparation of PBMCs by density gradient centrifugation (Lymphoprep). Use of human samples was approved by local ethical committees, in accordance with the Declaration of Helsinki.
Antibodies
OKT3 was obtained from the American Type Culture Committee (ATCC), UCHT1 was from eBioscience, and 28.1 was from Ancell. 10.1 (anti-FcγR1) was a gift from Nancy Hogg (London Research Institute, Cancer Research UK, London, UK); E05 (anti-FcγRIIa) and 6G11 (anti-FcγRIIb) with Fc regions mutated to eliminate FcγR binding were produced by BioInvent International AB (Malmo, Sweden) by using phage display technology11 (A.R., I. Teige, L. Mårtensson, K. L. Cox, M. Kovacek, A. Ljungars, J. Mattson, A. Sundberg, A. T. Vaughan, V. Shah, N. Smyth, B. Sheth, H.T.C.C., E. L. Williams, G. Manfredi, R.J.O., C.I.M., S. A. James, L. N. Dahal, K.H., J. S. Verbeek, G. Juliusson, M. Hansson, M. Jerkeman, P. W. M. Johnson, A. Davies, S. A. Beers, M.J.G., B.F., and M.S.C. manuscript in revision; A. L. Tutt, S. James, S. A. Laversin, T. R. W. Tipton, M. Ashton-Key, R.R.F., K.H., A. Vaughan, L. Dou, A. Earley, L. N. Dahal, C. Lu, M. Dunscombe, H.T.C.C., C. A. Penfold, J. H. Kim, E. Potter, C.I.M., A.R., R.J.O., K. L. Cox, I. Teige, B.F., M.J.G., S. A. Beers, and M.S.C., manuscript in preparation).
TGN1412 was produced by using published sequences (US patent number US7585960). Variable regions were subcloned into expression vectors (pEE6.4 heavy chain and pEE12.4 light chain; Lonza) containing constant regions of human IgG4. Heavy and light chain vectors were subcloned together before transfection into 293F cells for transient production or CHO-K1 cells for stable production. mAb was purified on Protein A-Sepharose, and aggregates were removed by gel filtration. Preparations were endotoxin low (<1 ng/mg protein) (Endosafe-PTS, Charles River Laboratories). Preparation of F(ab′)2 from IgG was described previously.12
Cell culture and T-cell proliferation assays
Cell culture was in serum-free medium (CTL-Test Medium, CTL Europe GmbH, Bonn, Germany) supplemented with glutamine (2 mM), pyruvate (1 mM), penicillin, and streptomycin (100 IU/mL) at 37°C in 5% CO2.
Fresh PBMCs were labeled with 2 μM carboxyfluorescein succinimidyl ester (CFSE). For HD preculture, cells were cultured in a 24-well plate at 1 × 107/mL as described by Romer et al9 for 48 hours prior to the stimulation assays. For low-density (LD) preculture, cells were cultured at 1 × 106/mL. For the PBMC stimulation, cells were transferred into round-bottomed 96-well plates at 1 × 105 per well. On day 4, cells were labeled with anti-CD8-APC (BioLegend) and anti-CD4-PE (in-house), and proliferation was assessed by CFSE dilution on a FACSCalibur or FACSCanto flow cytometer (BD Biosciences).
T-cell, B-cell, and monocyte isolation
Cell fractions were isolated from CFSE-labeled PBMCs by negative selection using EasySep for B cells and T cells (STEMCELL Technologies) and magnetic-activated cell sorting (MACS) for monocytes (Miltenyi Biotec).
Cytokine determination
Supernatants were taken 48 hours after stimulation, and cytokines were determined by using the V-plex Proinflammatory Panel 1 (human) Kit (Meso Scale Discovery, Rockville, MD).
Flow cytometry
FcγRIIb on monocytes and B cells was determined by using anti-CD19-APC-Cy7, anti-CD14-Pacific Blue (BD Biosciences), anti-FcγRIIb-APC, and human IgG1 isotype control (BioInvent International AB). FcγRIIb expression was determined by using a FACSCanto II or FACSCalibur flow cytometer and was analyzed by using FCS Express (De Novo Software) or Cellquest (BD Biosciences).
Western blot
Monocytes were isolated from HD and LD precultured PBMCs, resuspended in lysis buffer, and processed as previously described.13 Membranes were probed with rabbit anti-human FcγRIIb (Abcam) and goat anti-rabbit IgG horseradish peroxidase (HRP) F(ab′)2, and the signal was visualized by using enhanced chemiluminescence (GE Healthcare Life Sciences).
Transfection of CHO-K1 cells
CHO-K1 cells were transfected with FcγRIIb in plasmid pcDNA3, selected by using 1 mg/mL geneticin (Life Technologies), and screened by flow cytometry using the pan-FcγRII mAb AT10 F(ab′)2-fluorescein isothiocyanate (FITC) (in-house). Positive colonies were expanded and sorted by using a FACSAria II flow cytometer (BD Biosciences).
Statistics
Statistical analysis was performed by using a 2-tailed Spearman rank correlation on Graphpad Prism version 6 software.
Results
T-cell proliferation and cytokine responses induced by TGN1412 differ before and after HD preculture
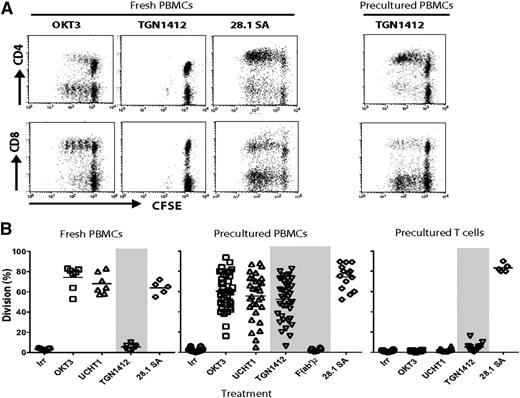
CD28 superagonistic mAbs induce polyclonal T-cell activation in vitro, independent of TCR engagement.14,15 TGN1412 was produced from the patented sequence. PBMCs from a large panel of donors were used to assess T-cell proliferation induced by soluble TGN1412 (hIgG4), a second anti-CD28 superagonist 28.1 (mIgG1), and 2 anti-CD3 mAbs, OKT3 (mIgG2a) and UCHT1 (mIgG1), before and after HD preculture. With fresh PBMCs, anti-CD3 mAbs induced predominantly CD8+ T-cell division whereas 28.1 induced predominantly CD4+ T-cell division (Figure 1A). TGN1412 induced a very low or no response in fresh PBMCs (Figure 1A), as has been reported previously.6 However, after PBMCs were precultured at HD for 48 hours as described by Romer et al,9 CD4+ T-cell division in response to TGN1412 was dramatically increased. The predominantly CD4 response to anti-CD28 mAbs may be a consequence of the differential expression of CD28 on CD4+ and CD8+ T cells, with only ∼50% of CD8+ cells expressing CD28 compared with most CD4+ cells (supplemental Figure 1, available on the Blood Web site).
T-cell proliferation in response to anti-CD3 and anti-CD28 mAbs with fresh and precultured PBMCs. Freshly prepared PBMCs were CFSE-labeled and used in proliferation assays either immediately or after HD preculture for 48 hours. (A-B) PBMCs were incubated with OKT3 and UCHT1 (anti-CD3; 0.1 μg/mL), TGN1412 (5 μg/mL), TGN1412 F(ab′)2 (5 μg/mL; precultured PBMCs only), and CD28.1 superagonist (SA) (1 μg/mL) for 4 days. Cells were then labeled with anti-CD8-APC and anti-CD4-PE mAbs and analyzed by flow cytometry to determine proliferation by CFSE dilution. Results are shown as the percentage of cells having undergone one or more divisions. (A) Representative dot plots and (B) the responses from a panel of donors; n = 5 to 7 for fresh PBMCs and n = 30 for precultured PBMCs. (B, right panel) Responses of T cells isolated after HD preculture of PBMCs to the same mAbs.
T-cell proliferation in response to anti-CD3 and anti-CD28 mAbs with fresh and precultured PBMCs. Freshly prepared PBMCs were CFSE-labeled and used in proliferation assays either immediately or after HD preculture for 48 hours. (A-B) PBMCs were incubated with OKT3 and UCHT1 (anti-CD3; 0.1 μg/mL), TGN1412 (5 μg/mL), TGN1412 F(ab′)2 (5 μg/mL; precultured PBMCs only), and CD28.1 superagonist (SA) (1 μg/mL) for 4 days. Cells were then labeled with anti-CD8-APC and anti-CD4-PE mAbs and analyzed by flow cytometry to determine proliferation by CFSE dilution. Results are shown as the percentage of cells having undergone one or more divisions. (A) Representative dot plots and (B) the responses from a panel of donors; n = 5 to 7 for fresh PBMCs and n = 30 for precultured PBMCs. (B, right panel) Responses of T cells isolated after HD preculture of PBMCs to the same mAbs.
A comparison of the proliferative responses in fresh and precultured PBMCs from the panel of donors is shown in Figure 1B. To reflect the predominant T-cell response in this and subsequent figures, the percentage division refers to CD8 division for anti-CD3 mAbs and CD4 division for anti-CD28 mAbs. There was considerable variation between donors in the level of proliferation induced by TGN1412 and the anti-CD3 mAbs, with a small proportion of donors showing poor responses.
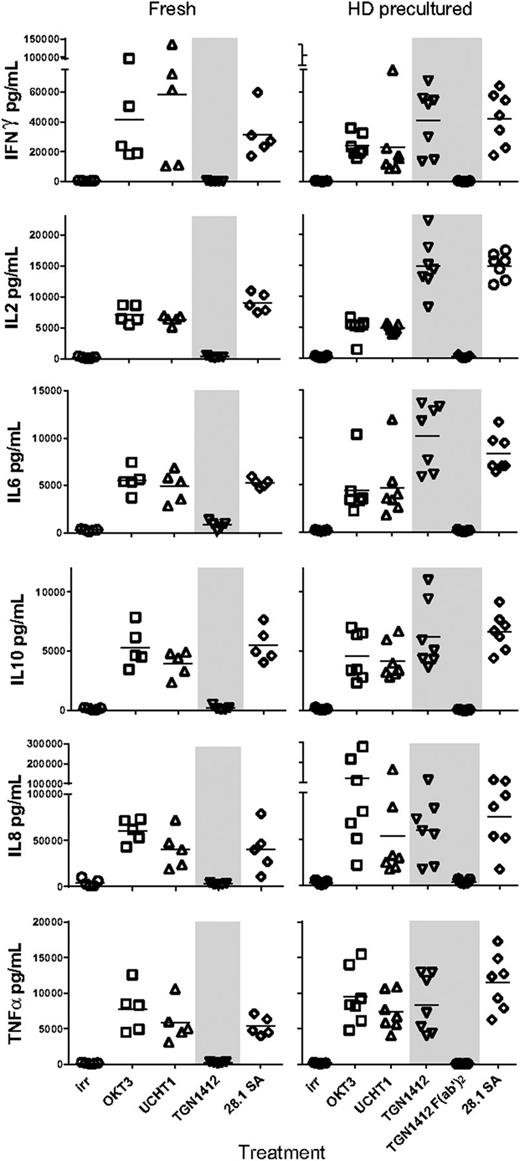
Supernatants were taken after 48 hours for measuring interferon gamma (IFN-γ), interleukin-2 (IL-2), IL-10, IL-6, IL-8, and tumor necrosis factor α (TNF-α) (Figure 2), confirming the release of inflammatory cytokines in response to TGN1412 after HD preculture, with the level and range of TNF-α and IFN-γ release comparable to those reported previously.9 Thus, our proliferation assays showed the same response profile as that shown by the measurement of cytokine release with the additional discrimination of T-cell subset responsiveness. As a consequence, we used T-cell proliferation as a robust surrogate readout for cytokine release to assess the functional requirements of TGN1412.
Cytokine release in response to anti-CD3 and anti-CD28 mAbs with fresh and precultured PBMCs. Supernatants from assays described in the legend for Figure 1 were taken at 48 hours for the determination of cytokine concentrations using the V-plex Proinflammatory Panel 1 (human) Kit (Meso Scale Discovery, Rockville, MD); n = 5 for fresh and n = 7 for HD precultured PBMCs.
Cytokine release in response to anti-CD3 and anti-CD28 mAbs with fresh and precultured PBMCs. Supernatants from assays described in the legend for Figure 1 were taken at 48 hours for the determination of cytokine concentrations using the V-plex Proinflammatory Panel 1 (human) Kit (Meso Scale Discovery, Rockville, MD); n = 5 for fresh and n = 7 for HD precultured PBMCs.
It has previously been shown that the function of anti-CD3 mAbs is dependent on their interaction with FcγRs.16 To investigate the requirement of FcγR interactions for TGN1412 activity, F(ab′)2 was produced free from contaminating IgG. Biacore analysis confirmed that its binding to CD28 matched that of the parent IgG (supplemental Figure 2). The failure of TGN1412 F(ab′)2 to induce T-cell proliferation (Figure 1B, middle panel) indicated that Fc-FcγR interaction was probably necessary for activity. This was reinforced by the inability of TGN1412 IgG to induce responses in T cells isolated after HD preculture (Figure 1B, right panel). In contrast, the anti-CD28 superagonist 28.1 induced proliferation in isolated T cells, indicating that this mAb does not require interaction with FcγRs on accessory cells, as previously reported.17
CD28 expression on CCR7– effector memory T cells was not altered during HD preculture (supplemental Figure 3), and therefore we next investigated the role of specific FcγRs in facilitating TGN1412 activity.
Inhibition of T-cell proliferation by anti-FcγR mAbs
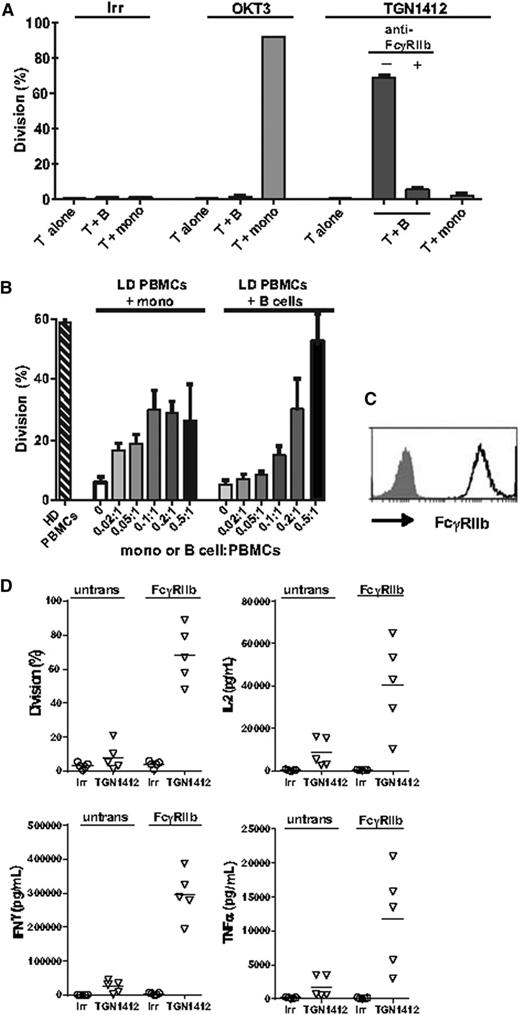
To investigate the requirement for FcγR co-engagement by TGN1412, we used a panel of anti-FcγR mAbs to block specific FcγR-Fc interactions: anti-FcγRI, anti-FcγRIII, and two novel anti-FcγRII mAbs binding to FcγRIIa and FcγRIIb. The anti-FcγRIIb mAbs were engineered with the N297Q mutation to abrogate binding of their Fc region to FcγRs. The observed inhibition of the responses to OKT3 and UCHT1 was in agreement with their known FcγR requirements16,18 : OKT3 (mIgG2a) requires FcγRI, whereas UCHT1 (mIgG1) requires FcγRIIa (Figure 3A). In contrast, we found that TGN1412 activity was inhibited by the anti-FcγRIIb mAb (Figure 3A), and this was confirmed in 8 donors (Figure 3B).
TGN1412 requires interaction with FcγRIIb for activity and this can be provided by B cells and monocytes. (A) T-cell proliferation in response to OKT3, UCHT1 and TGN1412 in the presence of anti-FcγRI, IIa, IIb, and IIIa mAb. PBMCs were incubated with stimulating mAb as described in Figure 1 in the absence or presence of anti-FcγRI, IIa, IIb, and IIIa blocking mAb (50 μg/mL). Error bars show mean and range of duplicate wells. (B) Effect of anti-FcγRIIa and IIb on response to TGN1412. Results are expressed as % of division with TGN1412 alone and error bars represent mean and SD from 7 donors. (C) Responsiveness of isolated T cells to TGN1412 is restored by B cells and monocytes. T cells, monocytes and B cells were isolated from PBMCs after 48 h HD preculture. T cells were incubated with OKT3, UCHT1 and TGN1412 either alone, or in coculture with B cells or monocytes at a monocyte or B cell:T cell ratio of 0.3:1; TGN1412 responses were determined in the absence or presence of anti-FcγRIIb blocking mAb (50 μg/mL). Results show mean and range of 2 donors. (D) B cells and monocytes were added to isolated T cells at mono or B:T cell ratios from 0.02:1 to 0.5:1. Results show mean and range from 2 donors.
TGN1412 requires interaction with FcγRIIb for activity and this can be provided by B cells and monocytes. (A) T-cell proliferation in response to OKT3, UCHT1 and TGN1412 in the presence of anti-FcγRI, IIa, IIb, and IIIa mAb. PBMCs were incubated with stimulating mAb as described in Figure 1 in the absence or presence of anti-FcγRI, IIa, IIb, and IIIa blocking mAb (50 μg/mL). Error bars show mean and range of duplicate wells. (B) Effect of anti-FcγRIIa and IIb on response to TGN1412. Results are expressed as % of division with TGN1412 alone and error bars represent mean and SD from 7 donors. (C) Responsiveness of isolated T cells to TGN1412 is restored by B cells and monocytes. T cells, monocytes and B cells were isolated from PBMCs after 48 h HD preculture. T cells were incubated with OKT3, UCHT1 and TGN1412 either alone, or in coculture with B cells or monocytes at a monocyte or B cell:T cell ratio of 0.3:1; TGN1412 responses were determined in the absence or presence of anti-FcγRIIb blocking mAb (50 μg/mL). Results show mean and range of 2 donors. (D) B cells and monocytes were added to isolated T cells at mono or B:T cell ratios from 0.02:1 to 0.5:1. Results show mean and range from 2 donors.
Monocytes and B cells from HD cultures confer responsiveness to TGN1412
To investigate which immune cells were capable of restoring TGN1412 responsiveness to isolated T cells, T cells from HD precultured PBMCs were cocultured with monocytes or B cells from the same HD precultures, and their response to anti-CD3 mAbs and TGN1412 was determined. Under these conditions, monocytes restored the responsiveness of T cells to OKT3, UCHT1, and TGN1412; at a monocyte:T cell ratio of 0.3:1, the proliferation was comparable to that in total PBMCs (Figure 3C). In contrast, B cells isolated from HD preculture were unable to restore the activity of OKT3 and UCHT1 but did restore activity to TGN1412 (Figure 3C). The restoration of TGN1412 responsiveness with both monocytes and B cells was almost totally blocked by anti-FcγRIIb mAb (Figure 3C). The ability of monocytes to confer responsiveness to OKT3 and UCHT1 is consistent with their expression of FcγRI and FcγRIIa and the requirement of cross-linking by these FcγRs. In contrast, B cells express only FcγRIIb, so they are unable to restore responsiveness to OKT3 and UCHT1. In donors who show a medium-high response to TGN1412, a titration of monocytes or B cells from HD PBMCs into isolated T cells showed that whereas with B cells the threshold for a TGN1412 response was not reached until a B-cell:T-cell ratio of more than 0.1:1, monocytes induced a response at a ratio of 0.02:1 (Figure 3D).
Expression of FcγRIIb is increased on HD precultured monocytes
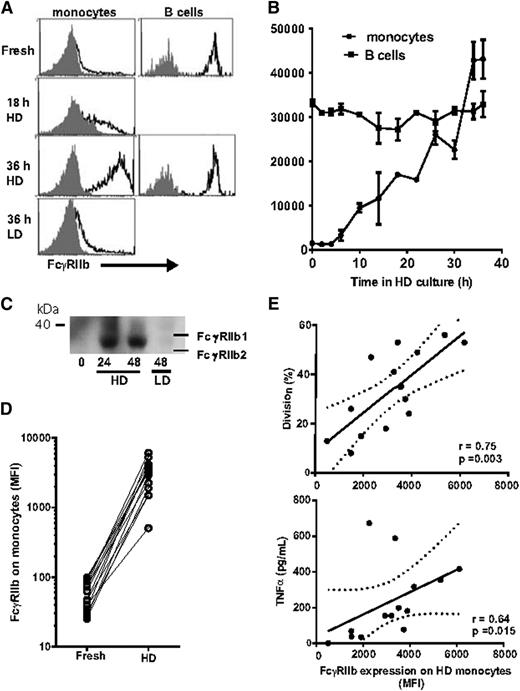
The finding that the TGN1412 response could be restored by monocytes as well as B cells and that this response was blocked by anti-FcγRIIb was unexpected because FcγRIIb is expressed on only a fraction of monocytes and at very low density.19 We then compared the expression of FcγRIIb on B cells and monocytes from fresh and HD precultured PBMCs. FcγRIIb expression was similar on fresh and HD B cells. In contrast, we found that FcγRIIb expression on monocytes was up to 50-fold greater after HD preculture compared with monocytes in fresh PBMCs (Figure 4A); when PBMCs were precultured at LD, FcγRIIb on monocytes was only modestly increased (Figure 4A). This increase in FcγRIIb expression was detectable by 10 hours of HD preculture and reached a maximum by 36 hours (Figure 4B). Western blotting confirmed that the upregulation of FcγRIIb was the result of the increased expression of the FcγRIIb2 isoform (Figure 4C).
FcγRIIb expression on monocytes is upregulated during HD preculture. (A) Histograms showing expression of FcγRIIb on monocytes from fresh PBMCs, and taken after 18 and 36 hours of HD preculture, and 36 hours of LD preculture, and on B cells from fresh PBMCs and after HD preculture. (B) Time course of the increase in FcγRIIb expression on monocytes during HD preculture. In contrast, B cells show no change in FcγRIIb expression during HD preculture. Bars show mean and range of 2 donors. Analysis in A and B was using a FACSCantoII. (C) Western blot showing an increase in the expression of the b2 isoform of FcγRIIb during HD preculture. (D) Comparison of FcγRIIb expression on fresh and HD precultured monocytes. (E) Plots showing relationship between FcγRIIb expression on monocytes after HD preculture and proliferation and TNFα release in response to TGN1412; n = 14, analyzed using 2-tailed Spearman rank correlation (Graphpad Prism 6). FcγRIIb expression and proliferation plots from all donors are shown in supplemental Figure 4. Analysis in D and E was using a FACSCalibur flow cytometer.
FcγRIIb expression on monocytes is upregulated during HD preculture. (A) Histograms showing expression of FcγRIIb on monocytes from fresh PBMCs, and taken after 18 and 36 hours of HD preculture, and 36 hours of LD preculture, and on B cells from fresh PBMCs and after HD preculture. (B) Time course of the increase in FcγRIIb expression on monocytes during HD preculture. In contrast, B cells show no change in FcγRIIb expression during HD preculture. Bars show mean and range of 2 donors. Analysis in A and B was using a FACSCantoII. (C) Western blot showing an increase in the expression of the b2 isoform of FcγRIIb during HD preculture. (D) Comparison of FcγRIIb expression on fresh and HD precultured monocytes. (E) Plots showing relationship between FcγRIIb expression on monocytes after HD preculture and proliferation and TNFα release in response to TGN1412; n = 14, analyzed using 2-tailed Spearman rank correlation (Graphpad Prism 6). FcγRIIb expression and proliferation plots from all donors are shown in supplemental Figure 4. Analysis in D and E was using a FACSCalibur flow cytometer.
Analysis of the monocyte expression of the other FcγRs showed that FcγRIIa and FcγRI were unchanged by preculture, whereas FcγRIIIa was high on a small population of monocytes from fresh PBMCs but was at an intermediate density on most monocytes after preculture (data not shown).
The upregulation of FcγRIIb was seen consistently over the panel of donors (Figure 4D and supplemental Figure 4). It should be noted that even in donors showing the lowest FcγRIIb expression after HD preculture, there was still at least a 10-fold increase relative to fresh monocytes. There was a statistically significant positive correlation between FcγRIIb expression on monocytes and T-cell proliferation and TNF-α release (Figure 4E). Together with the blocking results (Figure 3), these data explain how monocytes from HD precultures are able to engage with and cross-link TGN1412 through FcγRIIb.
T cells do not require HD preculture to respond to TGN1412 in the presence of HD precultured monocytes
It has previously been suggested that changes in the activation state of T cells themselves might be responsible for the responsiveness to TGN1412 after HD preculture.9 We next examined whether the changes we observed in the monocytes alone were sufficient. T cells were isolated from PBMCs after HD preculture, and their response to TGN1412 in the presence of monocytes from autologous HD and LD precultured PBMCs was compared (Figure 5A). With monocytes from HD precultures, TGN1412 induces T-cell proliferation comparable to that in total PBMCs whereas it induces only a very low level of stimulation with monocytes from LD preculture. In contrast, T cells responded to OKT3 with both LD and HD precultured monocytes, and in many donors, responses were higher with the LD monocytes. We also determined the response of T cells isolated from PBMCs after LD preculture in the presence of the HD and LD monocytes (Figure 5B). Importantly, with monocytes from HD preculture, T cells from LD preculture gave responses to TGN1412 that were comparable to those of the HD T cells, but they gave no response with monocytes from LD preculture. In both cases, the response was blocked by anti-FcγRIIb mAb. These results strongly point to the ability of T cells to respond to TGN1412 after HD preculture being primarily the result of an increase in the availability of FcγRIIb on monocytes rather than an increase in the responsiveness of the T cells themselves. This was supported by the finding that T cells isolated from fresh PBMCs were responsive to TGN1412 in the presence of autologous HD but not LD precultured monocytes (supplemental Figure 5).
T cells respond to TGN1412 in the presence of monocytes from HD preculture. CFSE-labeled PBMCs were cultured either at HD or LD for 48 hours. T cells and monocytes were then isolated from each culture. (A) T cells isolated from HD preculture were cocultured with monocytes isolated from LD (light bars) and HD (dark bars) cultures in the presence of OKT3 or TGN1412 at a monocyte:T cell ratio of 0.3:1. T-cell division was determined on day 4. Responses to TGN1412 were determined in the absence and presence of anti-FcγRIIb mAb. (B) As in (A) but with T cells isolated from LD preculture. The results show mean and SD from 3 donors.
T cells respond to TGN1412 in the presence of monocytes from HD preculture. CFSE-labeled PBMCs were cultured either at HD or LD for 48 hours. T cells and monocytes were then isolated from each culture. (A) T cells isolated from HD preculture were cocultured with monocytes isolated from LD (light bars) and HD (dark bars) cultures in the presence of OKT3 or TGN1412 at a monocyte:T cell ratio of 0.3:1. T-cell division was determined on day 4. Responses to TGN1412 were determined in the absence and presence of anti-FcγRIIb mAb. (B) As in (A) but with T cells isolated from LD preculture. The results show mean and SD from 3 donors.
Fresh T cells respond to TGN1412 in the presence of sufficient FcγRIIb
Figure 3 shows that when cocultured with B cells at a high B-cell:T-cell ratio, T cells isolated from HD preculture were responsive to TGN1412. Because the level of FcγRIIb on B cells is unchanged by HD preculture (Figure 4), we next determined whether fresh T cells were also responsive to TGN1412 when cocultured with B cells at a similarly high ratio. The results in Figure 6A clearly show that in the presence of B cells, fresh T cells were responsive to TGN1412. In contrast, fresh T cells did not respond to TGN1412 when cocultured with fresh monocytes, consistent with the monocytes’ lack of FcγRIIb. However, these monocytes were able to induce a T-cell response to OKT3, consistent with its requirement for FcγRI.
T cells from fresh and LD cultured PBMCs respond to TGN1412 in the presence of sufficient FcγRIIb. (A) T and B cells and monocytes were isolated from fresh PBMCs. T cells were then cultured with OKT3 or TGN1412 either alone or in coculture with B cells or monocytes at a B-cell or monocyte:T-cell ratio of 0.3:1. T-cell division was determined on day 4. (B) LD precultured PBMCs were cocultured with B cells or monocytes isolated from HD preculture at B-cell or monocyte:T-cell ratios of 0.02:1 to 1:1 in the presence of TGN1412. Division was determined on day 4. Results show mean and range from 2 donors. (C) FcγRIIb expression on transfected CHO-K1 cells. (D) T cells isolated from fresh PBMCs were cocultured with untransfected or FcγRIIb transfected CHO-K1 cells and TGN1412. Supernatant was taken for cytokine determination at 48 hours, and proliferation was determined on day 4. Results from 5 donors are shown.
T cells from fresh and LD cultured PBMCs respond to TGN1412 in the presence of sufficient FcγRIIb. (A) T and B cells and monocytes were isolated from fresh PBMCs. T cells were then cultured with OKT3 or TGN1412 either alone or in coculture with B cells or monocytes at a B-cell or monocyte:T-cell ratio of 0.3:1. T-cell division was determined on day 4. (B) LD precultured PBMCs were cocultured with B cells or monocytes isolated from HD preculture at B-cell or monocyte:T-cell ratios of 0.02:1 to 1:1 in the presence of TGN1412. Division was determined on day 4. Results show mean and range from 2 donors. (C) FcγRIIb expression on transfected CHO-K1 cells. (D) T cells isolated from fresh PBMCs were cocultured with untransfected or FcγRIIb transfected CHO-K1 cells and TGN1412. Supernatant was taken for cytokine determination at 48 hours, and proliferation was determined on day 4. Results from 5 donors are shown.
Like monocytes in fresh PBMCs, those in LD precultured PBMCs express little FcγRIIb (Figure 4A). Titrating monocytes or B cells from HD preculture into LD precultured PBMCs restored the T-cell mitogenic capacity of TGN1412 (Figure 6B), showing that the unresponsiveness to TGN1412 was the result of a lack of FcγRIIb availability in the LD precultures. Furthermore, in comparison with T cells cocultured with untransfected CHO-K1 cells, marked T-cell proliferation, IL-2, IFN-γ, and TNF-α release were observed in response to TGN1412 when T cells were cocultured with CHO-K1 cells transfected with FcγRIIb (Figure 6C-D).
Discussion
In this study, we show that HD preculture of PBMCs induces an exponential increase in the expression of FcγRIIb by monocytes, and that this is a prerequisite for mediating the superagonistic activity of TGN1412 in vitro. Our findings add to the growing body of evidence showing the importance of Fc-FcγRIIb interactions for the activity of agonistic mAbs targeting tumor necrosis factor receptor (TNFR) superfamily members such as CD40, TNF-related apoptosis-inducing ligand (TRAIL), and death receptor 5 (DR5), where Fc-FcγRIIb interactions are crucial for activity.20-23 In these experiments, we did not observe any change in the sensitivity of the T cells themselves to TGN1412 after HD preculture in contrast to previous studies, which suggested that HD preculture resulted in enhanced tonic TCR signaling causing a reduction in the activation threshold of the T cells in vitro.9,24,25 Ongoing studies show that other immunostimulatory mAbs, including anti-OX40 and anti-4-1BB, show similar HD preculture dependency for increased activatory activity (data not shown), suggesting that this assay will have considerable utility for predicting the potency and perhaps toxicity for many FcγRIIb-dependent agonistic mAbs.
Lühder et al4 proposed that anti-CD28 superagonists oligomerize CD28 on the T-cell surface and that the position of the recognized epitope influences the proximity of intracellular effector molecules leading to TCR-independent T-cell activation. We postulate that this increase in FcγRIIb after HD preculture enables efficient clustering of CD28 on the T-cell surface, which presumably mimics the activity of CD80/CD86 at the APC:T-cell synapse leading to T-cell activation.
In our experiments, TGN1412 F(ab′)2 failed to induce T-cell proliferation or cytokine release in accordance with findings by Ball et al.26 However, a recent study reported that TGN1412 F(ab′)2 still induces residual intracellular TNF-α expression in T cells at 0.5 μg/mL.10 The reason for this discrepancy is unclear. Taken together, these findings suggest that TGN1412 should be defined as an FcγR-dependent superagonist. In contrast, the activity of a second anti-CD28 mAb, 28.1, appears to be independent of FcγR interaction and so should be considered a true superagonist.
Our data support a mechanism by which HD monocytes mediate TGN1412 activity as a result of their dramatically increased FcγRIIb expression and the extent of proliferation and cytokine release correlates with the level of FcγRIIb. This upregulation was observed on monocytes from HD PBMC cultures and on HD precultured isolated monocytes (data not shown). FcγRIIb expression on HD monocytes was higher than on HD B cells (Figure 4B) and predominantly of the b2 isoform (Figure 4C). In vitro, both IL-4 and IL-10 have been shown to drive the upregulation of FcγRIIb on monocytes, although they increased expression of both the b1 and b2 isoforms.21,27,28 However, we did not see any increase in the levels of IL-4 or IL-10 when comparing supernatants from LD and HD precultures (data not shown), and it remains to be seen what is responsible for the increase in monocyte FcγRIIb expression.
In the presence of monocytes from HD precultures, T cells from LD precultures gave responses to TGN1412 that were comparable to those of HD T cells. This strongly suggests that the HD preculture enhances responses through a direct effect on the monocyte population independent of any change in the responsiveness of the T cells themselves.9 This is further supported by experiments using fresh PBMCs in which T cells were responsive to TGN1412 in the presence of autologous HD but not LD monocytes (supplemental Figure 5). This is in agreement with a recent study in which cross-linking anti-IgG modified responsiveness to TGN1412 irrespective of whether the T cells were isolated from HD or LD PBMCs.10 Together these observations suggest that enhanced tonic TCR signaling during HD preculture24,25 is not critical for responsiveness to TGN1412 in vitro. However, our observations are in accordance with previous findings in which monocyte maturation in HD PBMC cultures mediates full T-cell responsiveness to TGN1412.9
FcγRIIb is the only immunoreceptor tyrosine-based inhibitory motif (ITIM)–bearing FcγR. Although generally inhibitory, it may also act as a positive regulator independent of any signaling activity by providing cross-linking, and it confers activity for several agonistic mAbs (reviewed in White et al21 ). Here, we show a similar FcγRIIb requirement for T-cell proliferation and cytokine release in response to TGN1412. Although human IgG4 mAbs show little or no binding to FcγRIIb as monomers, Bruhns et al29 have shown that they are able to bind as immune complexes; their binding to FcγRIIb when presented as an array on the T cell may be similarly favorable. Whether this requirement is the result of some inherent property of FcγRIIb or whether any FcγR would serve this function if expressed at the right level, the right time, and with high enough IgG binding affinity, was discussed recently.21 Work with CD40 has shown that simply cross-linking the receptors is sufficient for activity and that no downstream signaling is required.20,23,30 Whether the same holds true for TGN1412 and CD28 remains to be confirmed.
In HD precultured PBMCs, TGN1412 activity was dependent on monocytes, but activity could also be mediated by B cells when added at a high enough frequency. However, monocytes were more active on a cell:cell basis. Although this may be the result of the higher expression of FcγRIIb on monocytes, it may be associated with qualitative differences between the two cell types. One intrinsic difference is their expression of alternate FcγRIIb isoforms, with B cells expressing predominantly b1 and monocytes expressing predominantly b2 (Figure 4C). The b1 isoform prevents association with clathrin-coated pits and internalization when co-ligated with the B-cell receptor.31,32 In contrast, the b2 isoform localizes to clathrin-coated pits and adopts a more clustered appearance (G. Manfredi, I. Teige, A.R., I. S. Findlow, P. Duriez, L. Douglas, C.I.M., L. Mårtensson., A. Ljungars, B.F., M.J.G, and M.S.C., unpublished data). It is possible that the more clustered b2 isoform on monocytes is a more efficient cross-linker giving more potent CD28 signaling.
Peripheral blood is composed of 1% to 7% B cells and 7% to 24% T cells; however, in lymphoid tissues, the B-cell:T-cell ratio may be as high as 2:1. Preclinical mAb testing typically relies on PBMCs in which the FcγR availability and distribution is very different from that in lymphoid tissues (A. L. Tutt, S. James, S. A. Laversin, T. R. W. Tipton, M. Ashton-Key, R.R.F., K.H., A. Vaughan, L. Dou, A. Earley, L. N. Dahal, C. Lu, M. Dunscombe, H.T.C.C., C. A. Penfold, J. H. Kim, E. Potter, C.I.M., A.R., R.J.O., K. L. Cox, I. Teige, B.F., M.J.G., S. A. Beers, and M.S.C., manuscript in preparation). Increasing the B-cell:T-cell ratio of PBMCs to 0.5:1 restored TGN1412 activity regardless of their preculture status, indicating that in conventional PBMC cultures, the availability of FcγRIIb is insufficient purely because of low B-cell frequency rather than because of FcγRIIb expression per se. This is supported by the observation that culturing T cells isolated from fresh PBMCs with FcγRIIb-transfected CHO-K1 cells also restored TGN1412 activity.
In the TGN1412 clinical trial, the most pronounced feature was the early onset of respiratory distress and pulmonary infiltrates.1 It is plausible that the tissue architecture of the bronchial-associated lymphoid tissue, in which large numbers of FcγRIIb-expressing B cells reside in close proximity to T cells, provided the ideal environment for the rapid cross-linking of TGN1412 leading to T-cell activation and associated events. Other lymphoid organs such as the spleen and lymph nodes show similar architecture in which B-cell–rich areas such as germinal centers and primary follicles are surrounded by T-cell dense periarteriolar lymphoid sheaths, and therefore these may be additional sites in which TGN1412 rapidly induces T-cell activation.
Our study offers insights on how TGN1412 may be cross-linked in vivo leading to adverse events and has implications for in vitro testing of a wider range of therapeutic mAbs targeting T-cell costimulatory molecules. Furthermore, we propose that TGN1412 can be specifically redefined as an FcγR-dependent superagonist, and together, our observations indicate that FcγRIIb-targeted Fc engineering of mAbs such as TGN1412, which target T-cell costimulatory molecules, may be used to improve their safe clinical use.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Christine Penfold, Jinny Kim, and Elizabeth Potter for their excellent technical support.
This work was supported by Cancer Research UK (grant C1477/A10834), Leukaemia & Lymphoma Research (grants 08014 and 12050), and the United Kingdom National Centre for the Replacement, Refinement and Reduction of Animals in Research CRACK IT Programme (award NC/C011204/1). R.J.O. is the recipient of a Medical Research Council-funded CASE studentship with Huntingdon Life Sciences.
Authorship
Contribution: K.H. designed and performed experiments, analyzed and interpreted results, and wrote the article; C.E.H., A.R., C.I.M., and R.J.O. performed experiments; H.T.C.C. and B.F. produced and provided vital reagents; F.C., K.S.H., and J.C.S. advised on assays and provided useful discussion; M.S.C. and M.J.G. designed the research, evaluated results, and edited the article; A.P.W. designed the research and wrote the article; and R.R.F. designed and performed experiments, analyzed and interpreted results, prepared figures, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruth R. French, MP88, Antibody and Vaccine Group, Cancer Sciences Unit, Southampton General Hospital, Southampton, SO16 6YD, United Kingdom; e-mail: rrf@soton.ac.uk.
References
Author notes
R.R.F. and A.P.W. contributed equally to this study and are the senior authors.