Key Points
Phosphotyrosine-binding capacity of the Syk N-SH2 domain is essential for GPVI and CLEC-2, but not αIIbβ3, activation of mouse platelets.
Phosphorylation of Syk on Y519/20, but not of Y346, requires a functional Syk N-terminal SH2 domain.
Abstract
We have used a novel knockin mouse to investigate the effect of disruption of phosphotyrosine binding of the N-terminal SH2 domain of Syk on platelet activation by GPVI, CLEC-2, and integrin αIIbβ3. The SykR41Afl/fl mouse was crossed to a PF4-Cre+ mouse to induce expression of the Syk mutant in the megakaryocyte/platelet lineage. SykR41Afl/fl;PF4-Cre mice are born at approximately 50% of the expected frequency and have a similar phenotype to Sykfl/fl;PF4-Cre mice, including blood-lymphatic mixing and chyloascites. Anastomosis of the venous and lymphatic vasculatures can be seen in the mesenteric circulation accounting for rapid and continuous mixing of the 2 vasculatures. Platelet activation by CLEC-2 and GPVI is abolished in SykR41Afl/fl;PF4-Cre platelets. Syk phosphorylation on Tyr519/20 is blocked in CLEC-2–stimulated platelets, suggesting a model in which binding of Syk via its N-terminal SH2 domain regulates autophosphorylation. In contrast, outside-in signaling by integrin αIIbβ3 is not altered, but it is inhibited in the presence of inhibitors of Src and Syk tyrosine kinases. These results demonstrate that αIIbβ3 regulates Syk through an ITAM-independent pathway in mice and provide novel insight into the course of events underlying Syk activation and hemITAM phosphorylation by CLEC-2.
Introduction
Outside-in signaling by integrin αIIbβ3 in platelets was first described following observations that platelet aggregation induces tyrosine phosphorylation of several proteins,1 with similar results seen upon platelet adhesion to the αIIbβ3 ligand, fibrinogen.2,3 Shortly thereafter, Src family tyrosine kinases4-6 and the tyrosine kinase Syk7 were identified as the major kinases regulating outside-in signaling in platelets.
Independently, Src and Syk tyrosine kinases were shown to mediate platelet activation by FcγRIIA and the GPVI-FcRγ complex, both of which signal through an immunoreceptor tyrosine-based activation motif (ITAM), defined by the sequence YxxLx6-12YxxL.8,9 Ligand engagement leads to phosphorylation of the ITAM tyrosines by Src kinases and possibly also by Syk. The phosphorylated motif provides a binding site for the SH2 domains of Syk leading to Syk activation and phosphorylation of downstream targets, including PLCγ2.10 Studies have since shown that both SH2 domains in Syk are required for binding to a phospho-ITAM by FcγRIIA11 and GPVI.12
Studies on transfected cell lines provided evidence for a different mode of regulation of Syk by the C-terminal tail of the β subunit of αIIbβ3 that is independent of tyrosine phosphorylation. Thus, αIIbβ3 activates a C-terminal SH2 mutant of Syk in CHO cells that is unable to bind to phosphotyrosine.13 Furthermore, Syk associates with the β3 cytoplasmic tail via its N-terminal SH2 domain and interdomain A, and this association is inhibited by phosphorylation of the 2 conserved tyrosines in the β3-cytoplasmic tail.13-15 The 2 conserved tyrosines of the β3-cytoplasmic tail do not form an ITAM and, upon phosphorylation, mediate clot retraction through recruitment of myosin II,16,17 a process that is independent of Syk.18 The β3 C-terminal tail undergoes constitutive association with Src, which lies upstream of Syk.19
A functional role for Src and Syk in outside-in signaling by αIIbβ3 in mice platelets was shown using Src family kinase-deficient and Syk-deficient mice. Platelets from Syk-deficient radiation chimeras show reduced spreading and tyrosine phosphorylation on fibrinogen relative to litter-matched controls.19,20 Furthermore, a knockin mouse in which the last 3 amino acids in the β3 subunit were mutated to disrupt binding to Src exhibited defective outside-in signaling.21 Mouse platelets undergo partial spreading on fibrinogen, in contrast to human platelets that extend filopodia and form full lamellipodia. Mouse platelets can, however, be induced to generate full lamellipodia on fibrinogen in the presence of thrombin or adenosine 5′-diphosphate independently of Syk.19,20
Outside of the platelet field, an alternative view of integrin signaling began to emerge. Experiments in neutrophils and macrophages showed that β2 integrins are unable to signal in the absence of either Syk22 or the ITAM-containing molecules FcRγ and DAP12.23 Furthermore, a Syk C-terminal SH2 mutant with disrupted phosphotyrosine binding also blocked outside-in signaling.23 These results suggest that β2 integrins regulate Syk in neutrophils and macrophages through an ITAM-dependent pathway.
Evidence for a role of an ITAM in outside-in regulation of Syk in mouse platelets has since been reported. Bone marrow progenitors from Syk-deficient mice were retrovirally infected with either wild-type (WT) Syk or N-terminal SH2 or C-terminal SH2 domain loss-of-function mutants and transplanted into radiation chimeric mice. Only platelets expressing WT Syk were able to spread on fibrinogen.24 Biochemical measurements of protein phosphorylation were not performed because of the low proportion of transduced platelets. The nature of the ITAM-containing protein that is regulated by αIIbβ3 in mouse platelets was not identified.
Later, Boylan et al demonstrated a critical role for FcγRIIA in supporting spreading on fibrinogen in human platelets using a blocking antibody and platelets from a patient with a reduced level of FcγRIIA.25 Further, the same group also showed increased spreading of platelets from a transgenic mouse expressing human FcγRIIA on fibrinogen.26 These results demonstrate that αIIbβ3 regulates Syk in human platelets through the ITAM receptor, FcγRIIA, but this cannot be the case in mouse platelets, as FcγRIIA is absent from the mouse genome.
The C-type lectin-like receptor CLEC-2 was identified in 2006 and shown to activate human and mouse platelets via Src and Syk tyrosine kinases.27-29 CLEC-2 contains a single tyrosine upstream of a conserved leucine (YxxL) and downstream of 3 negatively charged amino acids.30 This motif has been named a hemITAM. Activation of Syk by CLEC-2 leads to phosphorylation of the hemITAM and subsequent binding of the kinase to adjacent CLEC-2 receptors via its SH2 domains.31-33 Confirmation that both SH2 domains in Syk are required for CLEC-2 signaling was shown using DT40 cells transfected with Syk-SH2 point mutants and a reporter assay.12,28
The CLEC-2 ligand, podoplanin, is widely expressed outside of the vasculature, including on lymphatic endothelium. Genetic deletion of podoplanin results in defective lymphatic development characterized by blood-lymphatic mixing and perinatal mortality due to pulmonary and cardiac defects.34-36 The phenotype is indistinguishable from that in mice lacking CLEC-237 or critical proteins in the CLEC-2 signaling cascade, namely Syk,38 SLP-76,39,40 or PLCγ2.41 Similar phenotypes are seen in mice with megakaryocyte/platelet-specific deletion of CLEC-2,37 Syk,42 or SLP-76,43 suggesting that it is mediated by loss of platelet activation by podoplanin.
In the present study, we have generated a novel floxed mouse containing a mutation (R41A) that, following Cre-mediated expression, disrupts binding of the Syk N-terminal SH2 domain to phosphotyrosine.10,44 We have used this mouse to investigate the role of an ITAM in Syk activation by αIIbβ3 in mouse platelets and to study the proximal events in the CLEC-2 signaling cascade. We find that platelet activation by GPVI and CLEC-2, but not by integrin αIIbβ3, is critically dependent on the phosphotyrosine-binding (therefore ITAM-binding) capacity of Syk. Furthermore, we demonstrate that a functional N-terminal SH2 domain in Syk is required for phosphorylation of Syk on Y519/520, but not on Y346, by the CLEC-2 ligand, rhodocytin.
Methods
Extensive methods can be found in the supplemental Methods (available on the Blood Web site).
Reagents
A full list of reagents can be found in supplemental the supplemental Methods.
Mice
A full description of mice can be found in the supplemental Methods.
Preparation of washed platelets
Washed platelets were obtained by centrifugation using prostacyclin and resuspended in modified Tyrode's buffer as previously described.29 Further details can be found in the supplemental Methods.
Biochemistry
Washed platelets were stimulated in suspension or on a monolayer prior to lysis. Further details can be found in the supplemental Methods.
Aggregation and dense granule secretion
Aggregation was monitored using Born aggregometry. Further details can be found in the supplemental Methods.
Flow cytometry
Protein surface expression and fibrinogen binding was performed as previously described.45
Static-adhesion assay
Coverslips were coated with matrix proteins. Platelets were allowed to spread for 45 minutes at 37°C as performed previously.29
Immune thrombocytopenia
Thrombocytopenia was induced as described previously.46 Blood samples were taken at days 3 to 6 following injection.
Clot retraction
Clot retraction was performed at 37°C for 60 minutes. Further details can be found in the supplemental Methods.
Tail bleeding
A 3-mm portion of the tail tip was excised with a razor blade. Further details can be found in the supplemental Methods.
Flow-adhesion assay
Blood was perfused through collagen-coated capillaries for 4 minutes. Further details can be found in the supplemental Methods.
Ferric chloride injury
Platelets were fluorescently labeled and the mesenteric circulation exteriorized. Injury was induced by topical application of 20% FeCl3 solution. Further details can be found in the supplemental Methods.
Results
SykR41Afl/fl;PF4-Cre mice have defective lymphatic development
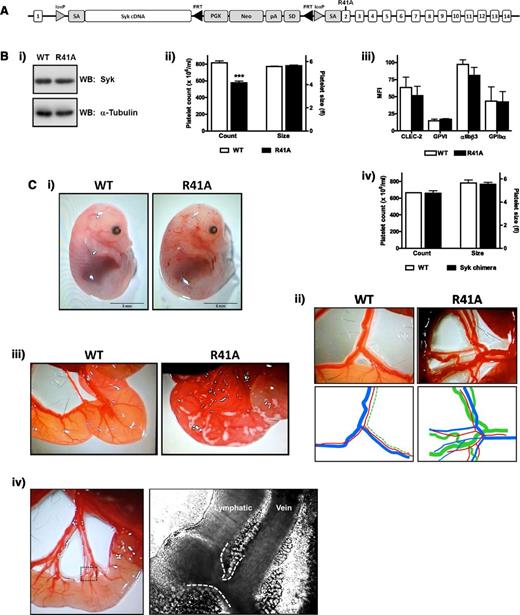
To investigate the role of the phosphotyrosine-binding capacity of the N-terminal SH2 domain of Syk in platelet activation, we developed a floxed knockin mutant mouse model (Figure 1A). The mutation R41A, which renders the N-terminal SH2 unable to bind to phosphotyrosine,10 was introduced into exon 2 of the Syk gene. WT complementary DNA for Syk was inserted after exon 1, flanked by loxP sites. Cre-recombinase expression induces excision of WT Syk complementary DNA and expression of SykR41A.
SykR41Afl/fl;PF4-Cre mouse development. (A) SykR41Afl/fl targeted locus. Following crossbreeding with PF4-Cre–expressing mice, the loxP flanked DNA is excised, allowing for expression of the knockin mutation. (B) Expression of Syk was measured by western blotting platelet whole-cell lysates (n = 3) (i). Platelet count and size (n > 10, ***P < .005) (ii). Surface expression of stated glycoproteins was measured on platelets by flow cytometry (n = 3) (iii). Platelet count and size for Syk radiation chimeras (n = 3) (iv). (C) Blood-lymphatic mixing is visible in the skin of embryonic day 14.5 embryos (i) and in the mesenteric vessels of adult mice (ii). For clarity, colored lines are traced below (red, arteriole; blue, venule; green, lymphatic). Chyle can be seen in the serosa of recently weaned R41A mice (iii). Connections between the lymphatic and vein were directly observed by intravital microscopy. The dotted box exemplifies the area near the serosa where the branching vessels are densely packed, and the dashed white lines are highlighting the vessel edges (iv). R41A, SykR41Afl/fl;PF4-Cre; WB, western blot.
SykR41Afl/fl;PF4-Cre mouse development. (A) SykR41Afl/fl targeted locus. Following crossbreeding with PF4-Cre–expressing mice, the loxP flanked DNA is excised, allowing for expression of the knockin mutation. (B) Expression of Syk was measured by western blotting platelet whole-cell lysates (n = 3) (i). Platelet count and size (n > 10, ***P < .005) (ii). Surface expression of stated glycoproteins was measured on platelets by flow cytometry (n = 3) (iii). Platelet count and size for Syk radiation chimeras (n = 3) (iv). (C) Blood-lymphatic mixing is visible in the skin of embryonic day 14.5 embryos (i) and in the mesenteric vessels of adult mice (ii). For clarity, colored lines are traced below (red, arteriole; blue, venule; green, lymphatic). Chyle can be seen in the serosa of recently weaned R41A mice (iii). Connections between the lymphatic and vein were directly observed by intravital microscopy. The dotted box exemplifies the area near the serosa where the branching vessels are densely packed, and the dashed white lines are highlighting the vessel edges (iv). R41A, SykR41Afl/fl;PF4-Cre; WB, western blot.
SykR41Afl/fl mice were crossed with PF4-Cre+ mice to induce megakaryocyte/platelet-specific expression of the mutant. SykR41Afl/fl;PF4-Cre mice survived beyond postnatal day 0 at approximately 50% of the expected Mendelian frequency (Table 1). Syk expression was similar in platelets from both WT and SykR41Afl/fl;PF4-Cre mice (Figure 1B). Platelet size and surface expression of CLEC-2, GPVI, αIIbβ3, and GPIbα was similar to littermate controls (Figure 1B). SykR41Afl/fl;PF4-Cre mice had a ∼25% decrease in platelet count (Figure 1B), which is most likely the result of mixing of the blood and lymphatic vasculatures (see below). There was a smaller decrease in erythrocyte count, whereas leukocyte counts were unchanged (supplemental Figure 1). In contrast, the platelet count is normal in Syk-deficient radiation chimeras at 6 weeks postirradiation, demonstrating that Syk is not required for platelet formation (Figure 1B). In confirmation of this, the recovery in platelet count following immune thrombocytopenia was not altered in SykR41Afl/fl;PF4-Cre mice (supplemental Figure 2).
Mendelian breeding ratios of R41A mice
| . | Genotype . | ||||||
|---|---|---|---|---|---|---|---|
| Breeding strategy . | +/+ . | +/+; PF4-Cre . | fl/+ . | fl/+; PF4-Cre . | fl/fl . | fl/fl; PF4-Cre . | Total . |
| fl/+;PF4-Cre × fl/+;PF4-Cre | |||||||
| Expected frequency | 6.25% | 18.75% | 12.50% | 37.50% | 6.25% | 18.75% | 100.00% |
| Actual count | 15 | 51 | 28 | 118 | 19 | 24 | 255 |
| Actual frequency | 5.88% | 20.00% | 10.98% | 46.27% | 7.45% | 9.41% | 100.00% |
| fl/fl × fl/+;PF4-Cre | |||||||
| Expected frequency | 0% | 0% | 25% | 25% | 25% | 25% | 100% |
| Actual count | 0 | 0 | 16 | 14 | 14 | 6 | 50 |
| Actual frequency | 0% | 0% | 32% | 28% | 28% | 12% | 100% |
| . | Genotype . | ||||||
|---|---|---|---|---|---|---|---|
| Breeding strategy . | +/+ . | +/+; PF4-Cre . | fl/+ . | fl/+; PF4-Cre . | fl/fl . | fl/fl; PF4-Cre . | Total . |
| fl/+;PF4-Cre × fl/+;PF4-Cre | |||||||
| Expected frequency | 6.25% | 18.75% | 12.50% | 37.50% | 6.25% | 18.75% | 100.00% |
| Actual count | 15 | 51 | 28 | 118 | 19 | 24 | 255 |
| Actual frequency | 5.88% | 20.00% | 10.98% | 46.27% | 7.45% | 9.41% | 100.00% |
| fl/fl × fl/+;PF4-Cre | |||||||
| Expected frequency | 0% | 0% | 25% | 25% | 25% | 25% | 100% |
| Actual count | 0 | 0 | 16 | 14 | 14 | 6 | 50 |
| Actual frequency | 0% | 0% | 32% | 28% | 28% | 12% | 100% |
Blood-filled lymphatics were observed in midgestation (embryonic day 14.5) and adult mice (Figure 1C). Furthermore, in recently weaned mice, chyle is visible in the intestine, further demonstrating defective lymphatic function (Figure 1C). Anastomosis of the lymphatic and blood vessels was observed at multiple places in the mesentery of SykR41Afl/fl;PF4-Cre mice and gave rise to rapid mixing of the 2 vasculatures (Figure 1C and supplemental Video 1). These connections were located close to the intestinal serosa, where densely packed vessels branch from the main conducting vessels. This observation extends previous immunohistochemical observations of connections of the 2 vasculatures using specific markers of blood and lymphatic vessels.40,41,47
Platelets expressing Syk-R41A do not respond to GPVI and CLEC-2 agonists
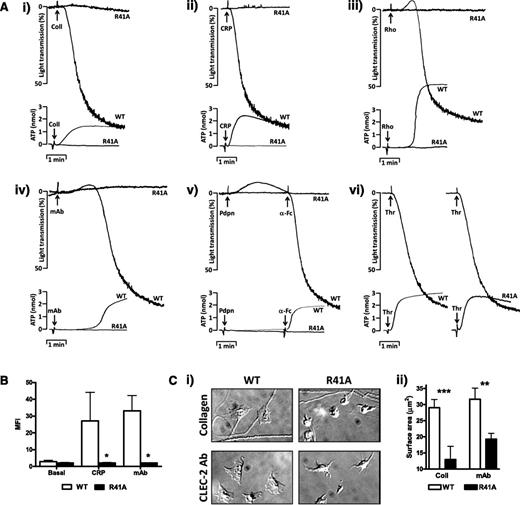
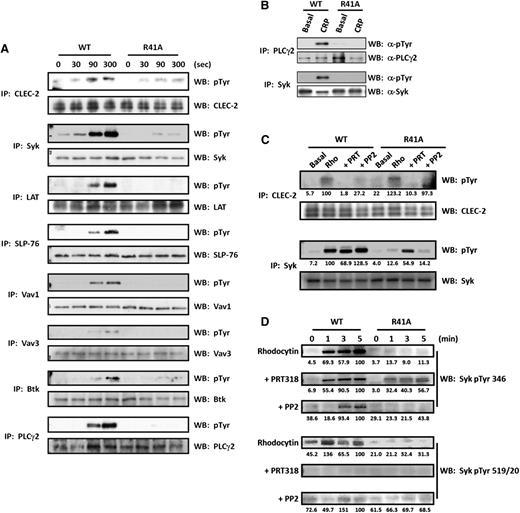
SykR41Afl/fl;PF4-Cre platelets failed to aggregate or secrete dense granules in response to collagen, collagen-related peptide (CRP), rhodocytin, podoplanin, or an α-CLEC-2 monoclonal antibody (mAb) (Figure 2A). Fibrinogen binding induced by CRP or by a CLEC-2 mAb (Figure 2B) and spreading on collagen-coated or CLEC-2 mAb-coated surfaces (Figure 2C) were also abrogated. In contrast, aggregation and secretion induced by thrombin was similar to that of controls (Figure 2A). Phosphorylation of proteins in the linker for activation of T cells (LAT) signalosome (namely Btk, LAT, SLP-76, Vav1/3, and PLCγ2) induced by rhodocytin was blocked in CLEC-2–stimulated SykR41Afl/fl;PF4-Cre platelets (Figure 3A). In contrast, partial phosphorylation of CLEC-2 and Syk was retained. Phosphorylation of Syk and PLCγ2 was abolished downstream of GPVI, in agreement with previous studies48,49 (Figure 3B).
SykR41Afl/fl;PF4-Cre Syk platelets have functional defects in response to GPVI and CLEC-2 agonists. (A) Platelets from SykR41Afl/fl;PF4-Cre (R41A) and WT mice (2 × 108/mL) were stimulated with collagen (30 μg/mL), CRP (3 μg/mL), rhodocytin (300 nM), CLEC-2 mAb (10 μg/mL), Fc-Pdpn (10 μg/mL plus 10 μg/mL α-Fc to crosslink), or thrombin (0.1 U/mL). Adenosine triphosphate secretion was monitored with Chrono-lume (n = 3). (B) R41A platelets (2 × 107/mL) were stimulated with either CRP (3 μg/ml) or CLEC-2 mAb (10 μg/mL) followed by staining with Alexa 488–labeled fibrinogen. Fibrinogen binding was measured by flow cytometry (n = 3, *P < .05). (C) R41A platelets (2 × 107/mL) were allowed to spread on coverslips coated with either collagen (100 μg/mL) or CLEC-2 mAb (10 μg/mL) for 45 minutes (n = 3, **P < .01, ***P < .005).
SykR41Afl/fl;PF4-Cre Syk platelets have functional defects in response to GPVI and CLEC-2 agonists. (A) Platelets from SykR41Afl/fl;PF4-Cre (R41A) and WT mice (2 × 108/mL) were stimulated with collagen (30 μg/mL), CRP (3 μg/mL), rhodocytin (300 nM), CLEC-2 mAb (10 μg/mL), Fc-Pdpn (10 μg/mL plus 10 μg/mL α-Fc to crosslink), or thrombin (0.1 U/mL). Adenosine triphosphate secretion was monitored with Chrono-lume (n = 3). (B) R41A platelets (2 × 107/mL) were stimulated with either CRP (3 μg/ml) or CLEC-2 mAb (10 μg/mL) followed by staining with Alexa 488–labeled fibrinogen. Fibrinogen binding was measured by flow cytometry (n = 3, *P < .05). (C) R41A platelets (2 × 107/mL) were allowed to spread on coverslips coated with either collagen (100 μg/mL) or CLEC-2 mAb (10 μg/mL) for 45 minutes (n = 3, **P < .01, ***P < .005).
SykR41Afl/fl;PF4-Cre signaling is inhibited in response to GPVI and CLEC-2 agonists. WT and SykR41Afl/fl;PF4-Cre platelets (R41A) (5 × 108/mL) were stimulated with either rhodocytin (300 nM for 300 seconds) (A,C) or CRP (3 μg/mL for 90 seconds) (B) followed by lysis. Where stated, platelets were preincubated with either PP2 (20 μM) or PRT318 (5 μM). Proteins were subsequently immunoprecipitated (IP). For the time course (D), platelets were stimulated with rhodocytin (300 nM), and samples were removed after 1, 3, and 5 minutes to prepare whole-cell lysates. Precipitated proteins and whole-cell lysates were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot (WB) for phosphotyrosine (n = 3). Blots were exposed with either autoradiographic film (A-B) or using a Licor Odyssey FC (C-D). Quantitation was performed with Licor Image Studio and normalized to percentage of the WT/maximal response. Where absent, there was no difference compared with background.
SykR41Afl/fl;PF4-Cre signaling is inhibited in response to GPVI and CLEC-2 agonists. WT and SykR41Afl/fl;PF4-Cre platelets (R41A) (5 × 108/mL) were stimulated with either rhodocytin (300 nM for 300 seconds) (A,C) or CRP (3 μg/mL for 90 seconds) (B) followed by lysis. Where stated, platelets were preincubated with either PP2 (20 μM) or PRT318 (5 μM). Proteins were subsequently immunoprecipitated (IP). For the time course (D), platelets were stimulated with rhodocytin (300 nM), and samples were removed after 1, 3, and 5 minutes to prepare whole-cell lysates. Precipitated proteins and whole-cell lysates were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot (WB) for phosphotyrosine (n = 3). Blots were exposed with either autoradiographic film (A-B) or using a Licor Odyssey FC (C-D). Quantitation was performed with Licor Image Studio and normalized to percentage of the WT/maximal response. Where absent, there was no difference compared with background.
The partial phosphorylation of Syk by CLEC-2 in SykR41Afl/fl;PF4-Cre platelets contrasts with the complete loss of Syk phosphorylation downstream of GPVI. We have previously observed phosphorylation of Syk in the presence of inhibitors of the kinase,32,33 suggesting that Syk is initially phosphorylated by 1 or more Src kinases. Partial Syk phosphorylation, however, is also seen in mouse platelets in the presence of the Src kinase inhibitor PP231 (a result that has been confirmed in Figure 3C), suggesting that Syk is also regulated by autophosphorylation prior to hemITAM phosphorylation.
Using SykR41Afl/fl;PF4-Cre mice, we were able to further investigate the sequence of events underlying Syk and CLEC-2 phosphorylation in comparison with the effect of Src and Syk inhibitors. Retention of CLEC-2 phosphorylation by rhodocytin in SykR41Afl/fl;PF4-Cre platelets indicates that phosphorylation is independent of the N-terminal SH2 domain of Syk (Figure 3C). This phosphorylation is blocked in the presence of the Syk inhibitor, PRT318, and reduced in the presence of the Src inhibitor, PP2 (Figure 3C), consistent with previous reports that Syk mediates phosphorylation of CLEC-2, with Src mediating optimal activation of Syk. Confirmation that PRT318 does not have off-target effects on Src kinases is shown by western blotting of whole-cell lysates with an antibody against Y424 in the Src activation loop (supplemental Figure 3). In contrast, phosphorylation of CLEC-2 was abrogated in SykR41Afl/fl;PF4-Cre platelets in response to an activating CLEC-2 antibody (supplemental Figure 4). This is likely to reflect the weaker signaling of the antibody, which induces dimerization rather than clustering of multiple CLEC-2 receptors as seen with rhodocytin (see “Discussion”).
To investigate Syk phosphorylation by rhodocytin in more detail, we monitored the time course of phosphorylation of Syk using phosphospecific antibodies (Figure 3D). Rhodocytin stimulated phosphorylation of Y346 and Y519/20 in the linker region and activation loop of Syk, respectively. Phosphorylation of Y346 was dramatically reduced, but not blocked, in SykR41Afl/fl;PF4-Cre platelets, whereas phosphorylation of Y519/20 was blocked. Phosphorylation of Y346 could be more clearly seen in SykR41Afl/fl;PF4-Cre platelets in the presence of the Syk inhibitor, PRT-318, whereas again there was no phosphorylation of Y519/20. The increase in phosphorylation of Syk on Y346 in the presence of PRT318 may reflect, for example, loss of phosphorylation and activation of an inhibitory phosphatase, such as SHP1 or SHP-2. These results provide unequivocal evidence that Src kinases phosphorylate Y346 in the linker region in SykR41Afl/fl;PF4-Cre platelets. On the other hand, phosphorylation of Y346 is seen in WT platelets in the presence of the Src kinase inhibitor PP2, albeit delayed relative to controls (Figure 3D). This indicates that both kinases phosphorylate Y346, with Src kinases mediating phosphorylation at early times. Syk also undergoes autophosphorylation on Y519/20, and this is lost in Syk-R41A–expressing platelets, indicating that this is dependent on a functional N-terminal SH2 domain, possibly following binding to phosphorylated CLEC-2. These results therefore provide a unique insight into the sequence of events under phosphorylation of Syk and CLEC-2 (see “Discussion”).
SykR41Afl/fl;PF4-Cre mice have a mild impairment in thrombosis
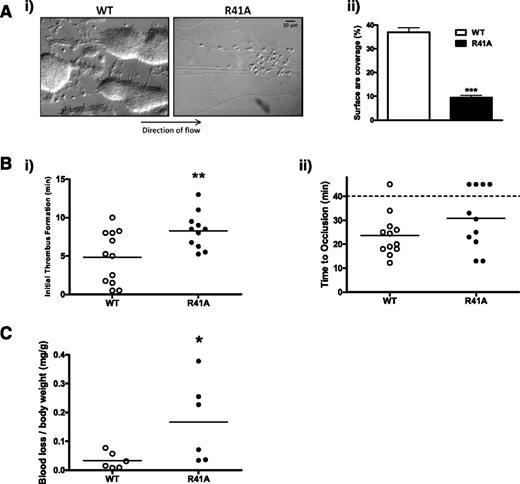
Aggregation was absent and only weak adhesion was observed when blood from SykR41Afl/fl;PF4-Cre mice was flowed over collagen-coated glass capillaries at an arteriolar shear rate of 1000 s−1. In contrast, WT blood formed large, stable platelet aggregates (Figure 4A). These results are similar to those for Syk- and GPVI-deficient platelets.50,51
SykR41Afl/fl;PF4-Cre mice have a mild thrombosis defect. (A) Anticoagulated whole blood from wild-type (WT) and SykR41Afl/fl;PF4-Cre (R41A) mice was labeled with DiOC6 prior to flow over capillary tubes coated with collagen (100 μg/ml) at 1000 s−1 for 4 minutes. Fluorescence thresholding was used to calculate surface area coverage (n = 3, ***P < .005). (B) FeCl3 (20%) was used to induce an injury in mesenteric arterioles. Platelets were labeled with DyLight488-labeled α-GPIbβ antibody. Thrombus formation was monitored for 40 minutes. Time to the initiation of a thrombus was measured (i), as was time to vessel occlusion (ii). If a vessel did not occlude within 40 minutes, the experiment was terminated (n ≥ 11, **P < .01). (C) Mice were anesthetized with isoflurane followed by removal of 3 mm of tail. Blood loss was monitored for up to 20 minutes or until 15% of total blood volume was lost (n = 6, *P < .05).
SykR41Afl/fl;PF4-Cre mice have a mild thrombosis defect. (A) Anticoagulated whole blood from wild-type (WT) and SykR41Afl/fl;PF4-Cre (R41A) mice was labeled with DiOC6 prior to flow over capillary tubes coated with collagen (100 μg/ml) at 1000 s−1 for 4 minutes. Fluorescence thresholding was used to calculate surface area coverage (n = 3, ***P < .005). (B) FeCl3 (20%) was used to induce an injury in mesenteric arterioles. Platelets were labeled with DyLight488-labeled α-GPIbβ antibody. Thrombus formation was monitored for 40 minutes. Time to the initiation of a thrombus was measured (i), as was time to vessel occlusion (ii). If a vessel did not occlude within 40 minutes, the experiment was terminated (n ≥ 11, **P < .01). (C) Mice were anesthetized with isoflurane followed by removal of 3 mm of tail. Blood loss was monitored for up to 20 minutes or until 15% of total blood volume was lost (n = 6, *P < .05).
A ferric chloride injury to the mesenteric arteriolar wall was used to investigate the functional consequence of the Syk-R41A mutation (Figure 4B). SykR41Afl/fl;PF4-Cre mice had a significant delay in the initiation of thrombus formation in response to injury, with the median increased to 8.50 ± 0.71 minutes relative to 5.13 ± 0.98 minutes in the control. Further, 4 out of 11 of the injuries failed to form an occlusive thrombus within 40 minutes compared with 1 out of 12 in the controls. Excision of a portion of the tail revealed a small but significant increase in blood loss in SykR41Afl/fl;PF4-Cre mice (Figure 4C).
These results demonstrate that phosphotyrosine binding by the Syk N-terminal SH2 domain is critical for platelet aggregation on collagen at arteriolar shear in vitro and are consistent with previous reports of a milder phenotype in vivo due the presence of other pathways of platelet activation.
SykR41Afl/fl;PF4-Cre platelets respond normally to αIIbβ3 stimulation
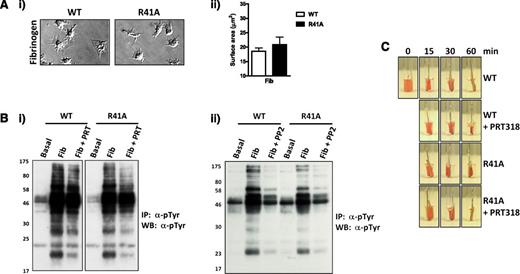
The effect of the Syk-R41A mutation on αIIbβ3 outside-in signaling was investigated by allowing platelets to spread on fibrinogen-coated coverslips. Mutant platelets spread to a similar extent as WT platelets, both in the absence (Figure 5A) and presence of thrombin (supplemental Figure 5). Clot retraction was not altered in blood from SykR41Afl/fl;PF4-Cre mice or in blood treated with a Syk inhibitor (Figure 5C), consistent with previous reports that Syk is not involved in this response.18 Whole-cell tyrosine phosphorylation was not altered in SykR41Afl/fl;PF4-Cre platelets that had been allowed to spread on fibrinogen, whereas treatment with the Syk and Src inhibitors, PRT318 and PP2, partially and completely inhibited phosphorylation, respectively (Figure 5B). The inhibitors had a similar effect on platelet spreading (not shown). Syk phosphorylation was unaffected by PRT318 but blocked with PP2, whereas phosphorylation of the downstream effector molecule, PLCγ2, was blocked in platelets treated with either inhibitor (supplemental Figure 6). Significantly, we saw no evidence of FcRγ phosphorylation (Figure 5B), even on long exposures (not shown). These results show that although Syk mediates outside-in signaling by integrin aIIbβ3, this is independent of the phosphotyrosine-binding capacity of the N-SH2 domain.
SykR41Afl/fl;PF4-Cre platelets respond normally to integrin αIIbβ3 outside-in signaling. (A) WT and SykR41Afl/fl;PF4-Cre (R41A) platelets (2 × 107/mL) were allowed to spread on coverslips coated with fibrinogen (100 μg/mL) for 45 minutes (n = 3). (B) WT and R41A platelets (5 × 108/mL) were allowed to spread in 6-cm wells coated with fibrinogen (100 μg/mL) for 45 minutes in the presence of PRT318 (5 μM) (i), PP2 (20 μM) (ii), or vehicle. Adherent platelets were then lysed, followed by phosphotyrosine (4G10) immunoprecipitation. Precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot for phosphotyrosine (n = 3). (C) A clot was initiated in WT and R41A PRP with 10 U/mL thrombin and allowed to retract for 60 minutes (n = 3).
SykR41Afl/fl;PF4-Cre platelets respond normally to integrin αIIbβ3 outside-in signaling. (A) WT and SykR41Afl/fl;PF4-Cre (R41A) platelets (2 × 107/mL) were allowed to spread on coverslips coated with fibrinogen (100 μg/mL) for 45 minutes (n = 3). (B) WT and R41A platelets (5 × 108/mL) were allowed to spread in 6-cm wells coated with fibrinogen (100 μg/mL) for 45 minutes in the presence of PRT318 (5 μM) (i), PP2 (20 μM) (ii), or vehicle. Adherent platelets were then lysed, followed by phosphotyrosine (4G10) immunoprecipitation. Precipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot for phosphotyrosine (n = 3). (C) A clot was initiated in WT and R41A PRP with 10 U/mL thrombin and allowed to retract for 60 minutes (n = 3).
FcγRIIA potentiates spreading on fibrinogen through a Src and Syk-dependent pathway
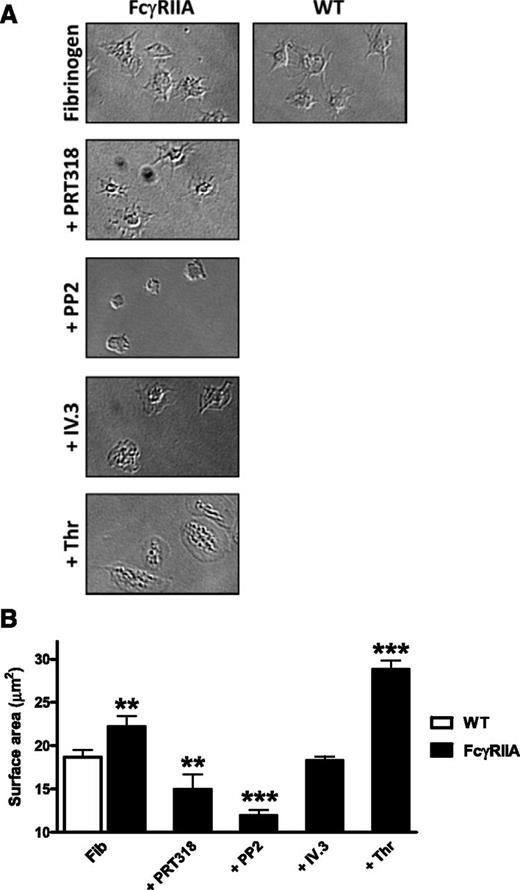
In human platelets, αIIbβ3 has been shown to mediate outside-in signaling through association with the ITAM-containing immunoglobulin G receptor, FcγRIIA, with a similar result also seen in FcγRIIA-transgenic mouse platelets.25,26 We have confirmed these observations under our experimental conditions using FcγRIIA-transgenic mouse platelets. FcγRIIA-transgenic mouse platelets exhibit a significant increase (∼25%) in surface area on fibrinogen (Figure 6), although this is less than that seen following activation with thrombin. The increase in spreading is blocked by fragments against FcγRIIA (IV.3), confirming previous results.26 The increase in spreading is also blocked by inhibition of Src and Syk kinases using PP2 or PRT318, respectively, consistent with involvement of the FcγRIIA ITAM as previously shown.11 Spreading on fibrinogen was not altered in mouse platelets deficient in FcRγ (Fcer1g−/− mice) compared with WT controls (supplemental Figure 7), demonstrating that the ITAM-containing protein does not couple to the integrin.
FcγRIIA-mediated spreading is Syk dependent. FcγRIIA transgenic platelets (2 × 107/mL) were allowed to spread on coverslips coated with fibrinogen (100 μg/mL) for 45 minutes in the presence of PP2 (20 μM), PRT318 (5 μM), thrombin (0.1 U/mL), IV.3 fragment antigen-binding fragments (10 μg/mL), or vehicle (n = 3, **P < .01, ***P < .005).
FcγRIIA-mediated spreading is Syk dependent. FcγRIIA transgenic platelets (2 × 107/mL) were allowed to spread on coverslips coated with fibrinogen (100 μg/mL) for 45 minutes in the presence of PP2 (20 μM), PRT318 (5 μM), thrombin (0.1 U/mL), IV.3 fragment antigen-binding fragments (10 μg/mL), or vehicle (n = 3, **P < .01, ***P < .005).
Discussion
This study has used a novel knockin mouse to address the requirement for an ITAM in the regulation of Syk by integrin αIIbβ3 and the functional consequence of loss of ITAM signaling in platelets during development and in models of thrombosis and hemostasis. The data demonstrate that phosphotyrosine binding by the Syk N-terminal SH2 domain is critical for platelet activation by GPVI and CLEC-2 signaling, but not by αIIbβ3. Loss of (hem)ITAM signaling results in blood-lymphatic mixing as shown by dynamic mixing of the lymphatic and venous circulations, and mild inhibition of hemostasis and thrombosis. The developmental and hemostatic/thrombotic defects observed in Syk-deficient mice are therefore mediated by loss of ITAM signaling rather than by a combined loss of (hem)ITAM and integrin signaling.
The observations that αIIbβ3-mediated spreading and tyrosine phosphorylation are unchanged in SykR41Afl/fl;PF4-Cre mouse platelets, but that both are reduced in the presence of a Syk inhibitor, demonstrates that the integrin regulates Syk through an ITAM-independent pathway. This is further supported by the absence of tyrosine phosphorylation of the only ITAM protein in mouse platelets, FcRγ, on fibrinogen. These results support the model of Syk regulation by αIIbβ3 that was originally proposed by Shattil and colleagues.15 The observation that platelet spreading on fibrinogen is increased in FcγRIIA-transgenic mouse platelets through a Syk-dependent pathway is consistent with the model of Newman and colleagues in human platelets in which αIIbβ3 signals through the low-affinity immune receptor. FcγRIIA and FcRγ are the only 2 ITAM molecules expressed in human platelets, with no evidence for other known ITAM molecules such as DAP-10, DAP-12, and CD247 (TCR-CD3 ζ-chain).52,53
The demonstration that αIIbβ3 regulates Syk in mouse platelets independently of ITAMs contrasts with the observations of Abtahian et al24 using virally infected rescue experiments in Syk-deficient progenitor cells. In this study, integrin-mediated spreading was rescued by WT, but not by Syk mutants with loss-of-function mutations in the N- and C-terminal SH2 domains. This is a different approach to that used in the present study in that only a low proportion of cells were infected, preventing biochemical measurement of protein expression levels and tyrosine phosphorylation. In contrast, in the present study, Syk-R41A was expressed at a similar level to WT protein in the entire platelet population. Thus, one explanation for the differing results could be a reduced level of expression of the virally infected proteins in the study by Abtahian et al,24 although this could not be directly tested due to the low level of infection. Abtahian et al24 also demonstrated that disruption of the Syk N- or C-terminal SH2 domains prevented integrin signaling in neutrophils and macrophages, and it will be of interest to investigate whether a similar result is seen in mice in which the SykR41Afl/fl mutant has been expressed in these 2 cell types.
A further difference with the results of Abtahian et al24 is the report that expression of either Syk-SH2 mutant resulted in a partial rescue of the blood-lymphatic mixing phenotype. Not only does this contradict the results of the present study, as the phenotype of SykR41Afl/fl;PF4-Cre mice is indistinguishable from that of Clec1bfl/fl;PF4-Cre mice,37 but also it is difficult to reconcile this with the complete ablation of CLEC-2 signaling in the presence of Syk-R41A, as shown in the present study. Abtahian et al24 did not investigate the effect of either the N- or C-terminal SH2 domain mutants on CLEC-2 signaling, as the receptor was not known to be expressed in platelets at this time.
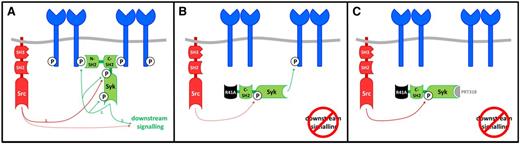
The present study provides the first functional demonstration in platelets of the importance of binding of the N-terminal SH2 domain of Syk to a phosphorylated hemITAM in CLEC-2. Loss of Syk binding in SykR41Afl/fl;PF4-Cre platelets is associated with blockade of phosphorylation of numerous proteins downstream of Syk, including PLCγ2. This is consistent with a model in which binding of Syk through its tandem SH2 domains to 2 phosphorylated CLEC-2 molecules is essential for phosphorylation of LAT and other downstream proteins. On the other hand, the increase in phosphorylation of Syk on Y346 and of the CLEC-2 hemITAM, but not Y519/20, demonstrates that these lie upstream of binding of the N-terminal SH2 domain of Syk to phosphorylated CLEC-2. These results are summarized in the model shown in Figure 7. In this model, engagement of CLEC-2 leads to Src kinase phosphorylation of Y346 in the linker region of Syk, which is later reinforced by Syk-dependent autophosphorylation of the same site. Additionally, Syk mediates phosphorylation of CLEC-2, which recruits Syk to the hemITAM receptor via its N-SH2 domain, leading to autophosphorylation of Y519/20 in the activation loop. This initiates a downstream signaling cascade that culminates in phosphorylation and activation of PLCγ2.
Model of Syk-mediated CLEC-2 signaling. (A) In WT platelets, Syk is phosphorylated after ligand binding initially on Y346 by the action of 1 or more Src kinases, followed by autophosphorylation of Y346. Phosphorylation of the CLEC-2 hemITAM leads to Syk recruitment and autophosphorylation at 519/20. This leads to activation of a downstream signaling pathway by Syk and Src (dotted line) that culminates in activation of PLCγ2. (B) In SykR41Afl/fl;PF4-Cre platelets, Syk is weakly phosphorylated on Y346 (dotted line) by Src, and Syk phosphorylates the CLEC-2 hemITAM in response to strong agonists (rhodocytin), but not weak agonists (antibody). Syk-R41A is not stably recruited to the phosphorylated hemITAM on CLEC-2 and is therefore unable to undergo autophosphorylation on Y519/20 and activate downstream signaling events. (C) In the presence of the Syk inhibitor PRT318, Syk-R41A is partially phosphorylated on Y346 by Src but is unable to phosphorylate the CLEC-2 hemITAM or undergo autophosphorylation. The increase in Src kinase phosphorylation of Y346 may reflect the loss of translocation of Syk to CLEC-2 through binding of its C-terminal SH2 domain.
Model of Syk-mediated CLEC-2 signaling. (A) In WT platelets, Syk is phosphorylated after ligand binding initially on Y346 by the action of 1 or more Src kinases, followed by autophosphorylation of Y346. Phosphorylation of the CLEC-2 hemITAM leads to Syk recruitment and autophosphorylation at 519/20. This leads to activation of a downstream signaling pathway by Syk and Src (dotted line) that culminates in activation of PLCγ2. (B) In SykR41Afl/fl;PF4-Cre platelets, Syk is weakly phosphorylated on Y346 (dotted line) by Src, and Syk phosphorylates the CLEC-2 hemITAM in response to strong agonists (rhodocytin), but not weak agonists (antibody). Syk-R41A is not stably recruited to the phosphorylated hemITAM on CLEC-2 and is therefore unable to undergo autophosphorylation on Y519/20 and activate downstream signaling events. (C) In the presence of the Syk inhibitor PRT318, Syk-R41A is partially phosphorylated on Y346 by Src but is unable to phosphorylate the CLEC-2 hemITAM or undergo autophosphorylation. The increase in Src kinase phosphorylation of Y346 may reflect the loss of translocation of Syk to CLEC-2 through binding of its C-terminal SH2 domain.
The developmental defects in the SykR41Afl/fl;PF4-Cre mice are indistinguishable from those reported in megakaryocyte/platelet-specific CLEC-2–, Syk-, and SLP-76–deficient mice and include mixing between the blood and lymphatic vasculatures during development and in adult mice.37,40,43 Until now, direct linkage of the lymphatic and venous system has only been seen by immunochemistry.40,41,47,54 In the present study, we observed areas of dynamic blood flow between the 2 vasculatures in the densely packed intestinal serosa of SykR41Afl/fl;PF4-Cre mice. This study therefore represents the first real-time visualization of functional anastomoses between the venous and lymphatic vasculatures, and as such, it provides an explanation to the rapid mixing shown previously between the 2 circulations.37 It also explains why flow rates approach those in the venous system as reported by Kahn and colleagues.55 This observation should be considered alongside a recent study from the Kahn group proposing that the venous and lymphatic systems are connected by retrograde flow through the thoracic duct.56 Although the 2 sets of results are not mutually exclusive, it is unclear how retrograde flow can explain the rapid mixing and high flow rates in the blood-filled lymphatics.
The SykR41Afl/fl;PF4-Cre mice were observed to have platelet counts reduced by ∼25%, with a modest reduction in erythrocyte counts. A similar reduction in platelet count is seen in CLEC-2-deficient mice.57 The reduction in platelet count is thought to be caused by blood-lymphatic mixing, as platelet production is normal in both SykR41Afl/fl;PF4-Cre and CLEC-2–deficient mice (supplemental Figure 2). Furthermore, platelet counts in CLEC-2– or Syk-deficient radiation chimeras are similar to controls in the first few weeks after transplantation.45 It is likely that red and white blood cell production compensates for the linked vasculatures, resulting in largely unaffected cell counts, whereas platelets, with their short lifetime (∼5 days), and the steady production of thrombopoietin, are unable to compensate.
SykR41Afl/fl;PF4-Cre mice exhibit only mild defects in tail bleeding and in thrombus formation despite loss of CLEC-2 and GPVI/FcRγ signaling. Similar observations are also seen in the presence of the Syk inhibitor PRT318,50 and a slightly greater increase in bleeding is seen in mice with genetic depletion of GPVI and CLEC-2,57 possibly reflecting loss of adhesion as well as signaling by the 2 (hem)ITAM receptors. A greater defect in thrombus formation is, however, seen in mice depleted of GPVI and CLEC-2 using specific antibodies, which may reflect additional effects of the antibody treatment, although this is not directly mediated by the transient thrombocytopenia, as platelet levels had returned to normal levels before experimentation.57 Overall, these results are consistent with a relatively mild role for ITAM receptors in supporting hemostasis and thrombosis.
In conclusion, the present study confirms a critical role of the N-terminal SH2 domain of Syk in (hem)ITAM, but not integrin, signaling in mouse platelets. In addition, the results demonstrate that phosphorylation of Syk on Y346 and of the CLEC-2 hemITAM are upstream of autophosphorylation of Y519/20, which likely requires binding of Syk to 2 phosphorylated CLEC-2 receptors through its tandem SH2 domains.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Dr A. Pollitt for production of the Fc-Pdpn, Drs S. Thomas and N. Poulter for assistance with flow adhesion studies, and Dr B. Grygielska for genotyping of mice.
This work was supported by the Wellcome Trust (073107 and 088410) and the British Heart Foundation (PG/07/116 and PG/05/134). S.P.W. holds a BHF Chair (CH/03/003).
Authorship
Contribution: C.E.H. designed and performed research, collected data, analyzed and interpreted data, made the figures, and wrote the manuscript; B.A.F. and K.L.L. designed and performed research, collected data, and analyzed and interpreted data; F.K. generated knockin mice; and S.P.W. designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: F.K. is the chief executive officer of Ozgene Pty Ltd. The remaining authors declare no competing financial interests.
Correspondence: Craig E. Hughes, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: c.e.hughes@bham.ac.uk; and Steve P. Watson, Centre for Cardiovascular Sciences, Institute for Biomedical Research, College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, United Kingdom; e-mail: s.p.watson@bham.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal