Key Points
AURKA is essential for adult hematopoiesis.
AURKA is dispensable for megakaryocyte polyploidization and differentiation.
Abstract
Aurora kinase A (AURKA) is a therapeutic target in acute megakaryocytic leukemia. However, its requirement in normal hematopoiesis and megakaryocyte development has not been extensively characterized. Based on its role as a cell cycle regulator, we predicted that an Aurka deficiency would lead to severe abnormalities in all hematopoietic lineages. Here we reveal that loss of Aurka in hematopoietic cells causes profound cell autonomous defects in the peripheral blood and bone marrow. Surprisingly, in contrast to the survival defects of nearly all hematopoietic lineages, deletion of Aurka was associated with increased differentiation and polyploidization of megakaryocytes both in vivo and in vitro. Furthermore, in contrast to other cell types examined, megakaryocytes continued DNA synthesis after loss of Aurka. Thus, like other cell cycle regulators such as Aurkb and survivin, Aurka is required for hematopoiesis, but is dispensable for megakaryocyte endomitosis. Our work supports a growing body of evidence that the megakaryocyte endomitotic cell cycle differs significantly from the proliferative cell cycle.
Introduction
Aurora kinase A (AURKA) is a member of an evolutionary conserved family of serine/threonine protein kinases that plays essential roles in mitosis and meiosis.1 Mammals possess 3 Aurora kinases: Aurora A, B, and C. AURKA functions at multiple stages of mitosis and is required for centrosome maturation, mitotic spindle formation, and accurate chromosome segregation. Frequently upregulated in human cancers, AURKA is an attractive target for anticancer therapies. Indeed, there are several AURKA inhibitors in clinical trials. We recently reported that AURKA is a novel therapeutic target in acute megakaryocytic leukemia and that AURKA inhibitors selectively enforce differentiation of normal and malignant megakaryocytes.2 Although several studies have examined the role of AURKB during maturation of this lineage,3-6 less is known about the contributions of AURKA to megakaryopoiesis.
Using germ-line and conditional deletion mouse models, several groups have studied the requirement for Aurka in development and cancer. The first reports of a germ-line deletion of Aurka found that the null mice die early during embryonic development, before the blastocyst stage, due to defects in spindle assembly and other mitotic defects.7 A second study confirmed these results and found that Aurka-null mouse embryos show severe defects as early as embryonic day 3.5. Defects including growth retardation, misaligned and lagging chromosomes, and a disorganized mitotic spindle were observed.8 Several conditional and tissue-specific deletion studies have also demonstrated that Aurka is required for viability. One study reported that early loss of Aurka leads to embryonic lethality and impaired mitotic entry with defects in bipolar spindle formation.9 A follow-up study found that inactivation of Aurka in the epiblast or visceral endoderm leads to apoptosis and inhibition of embryo growth and is lethal by E9.5.10 As for tissue-specific deletions, loss of Aurka selectively in the skin resulted in perinatal lethality caused by damaged, fragile, and thin skin. Increased apoptosis and polyploidy were also observed in the skin.11 Finally, a 4-hydroxytamoxifen-inducible Cre deletion of Aurka demonstrated that its loss increased apoptosis and led to formation of polyploid cells following tamoxifen treatment. In this model, loss of Aurka also prevented the progression of skin and mammary gland tumors.12 Of note, the study detected defects in hematopoiesis, including decreased hematocrit, platelet, white cell, and red cell counts, as well as a reduction in splenic red pulp that was accompanied by mitotic arrest, aneuploidy, and apoptosis. However, thorough analysis of specific lineages was not undertaken. Moreover, survival curves revealed that ∼35% of the mice died by 20 weeks after treatment. In this model, death was not attributed to hematologic defects.
Here we provide a detailed analysis of an inducible, hematopoietic specific Aurka deletion in adult mice. We found that loss of Aurka resulted in profound cell autonomous defects in the bone marrow and peripheral blood, accompanied by a rapid and fully penetrant survival defect. Unexpectedly, we discovered that instead of depleting all blood lineages, loss of Aurka led to a specific enrichment of differentiated megakaryocytes. These results are similar to observations that survivin13 and Aurkb3 are dispensable for survival and polyploidization of postmitotic megakaryocytes and indicate that unique cell cycle regulatory mechanisms accompany endomitosis.
Material and methods
Cell culture
Bone marrow cells were flushed from mouse femurs and tibias with RPMI, and the cells were separated by passage through a 20-gauge needle. Lin− cells were isolated with the EasySep mouse hematopoietic progenitor cell enrichment kit (Stem Cell Technologies) following the manufacturer’s instructions and grown overnight in StemSpan media (StemCell Technologies) supplemented with 100 mg/mL penicillin/streptomycin, interleukin-3 (IL-3; 10 ng/mL), erythropoietin (1 ng/mL), human low-density lipoprotein (1:200), and stem cell factor (SCF; 100 ng/mL) (R&D Systems). Megakaryocytes were expanded in a serum-free system as described previously.14 For western blotting, proteins were extracted from primary megakaryocyte cultures over 4 days. The blot was probed with the anti-AURKA antibody (GeneTex; clone 35C1). All cultured cells were maintained in a humidified atmosphere at 37°C with 5% CO2. For retroviral transduction, cell lines and primary cells were transduced on consecutive days with the indicated packaged retrovirus in the presence of polybrene (4 μg/mL) by spinoculation for 90 minutes at 2600 rpm. Retroviral packaging was achieved by transient transfection of Plat-E cells with appropriate retroviral vectors using Extremegene (Roche).
Flow cytometry
Cells were resuspended in phosphate-buffered saline containing 1 mM EDTA and 1% bovine serum albumin (Sigma-Aldrich) to reduce aggregates and nonspecific staining, respectively. Surface marker expression was analyzed using an LSRII cytometer (Becton Dickinson Biosciences) and FlowJo software (Tree Star). Sorting was performed with an Aria II flow cytometer (Becton Dickinson). For surface staining, cells were incubated with c-kit (eBioscience), Sca1 (eBioscience), CD41 (eBioscience), and CD42 antibodies (EmFret) for 30 minutes. Lineage-positive cells were excluded using a cocktail of biotinylated antibodies and staining with streptavidin (eF450; eBioscience). The lineage cocktail included CD3, CD19, NK1.1, Ter119, CD11b, Gr1, and B220. Staining for DNA content (Hoechst), CD41 (allophycocyanin [APC] or phycoerythrin [PE]-Cy7) and CD42 (PE) was performed prior to staining with annexin V-APC (BD Pharmingen). Cells were resuspended in annexin binding buffer with annexin-V APC 1:100. Data were acquired on LSR II and analyzed with FlowJo. For myeloid progenitor analysis, lineage-positive cells were excluded using a cocktail of biotinylated antibodies as above and streptavidin secondary antibody (eF450; eBioscience). Lineage-negative cells were subsequently stained for c-kit, Sca-1, CD9, CD41, FcRγ, and CD34. Populations were identified following the gating strategy of Nakorn et al.15
Colony forming assays
A total of 25 000 cells were plated in MethoCult 3434 (StemCell Technologies), containing 10 ng/mL IL-3, 10 ng/mL SCF, and 10 U/mL erythropoietin. Burst-forming unit erythroid and myeloid colonies were enumerated after 7 to 10 days. For colony-forming unit megakaryocyte (CFU-MK) assays, 100 000 bone marrow mononuclear cells were seeded in Megacult-C medium supplemented with 10 ng/mL IL-3, 10 ng/mL of IL-6, 10 ng/mL of IL-11, and 50 ng/mL of thrombopoietin (THPO) and cultured for 10 to 12 days. Slides of Megacult cultures were fixed with acetone and stained according to the manufacturer’s instructions (StemCell Technologies). Stained colonies were then enumerated microscopically.
Bone marrow transplantation
C57BL/6 CD45.1+ congenic recipients (6 to 8 weeks) were purchased from The Jackson Laboratory and placed on bactrim-containing water 1 week before irradiation (1100 rads). One million CD45.2+ total bone marrow cells from Aurka+/+/Mx1-Cre or Aurkafl/fl/Mx1-Cre mice, along with 250 000 CD45.1+ support cells, were injected retroorbitally into lethally irradiated CD45.1+ recipients that were anesthetized with isoflurane (Abbott Laboratories). CD45.2+ and CD45.1+ bone marrow cells were allowed to engraft for 6 weeks after transplantation. After 6 weeks, peripheral blood from recipient mice was obtained by retroorbital or tail vein bleeding, subjected to red blood cell lysis, and stained with APC-conjugated CD45.1 (eBioscience) and fluorescein isothiocyanate-conjugated CD45.2 (BD Biosciences) antibodies to verify donor cell engraftment by flow cytometric analysis. Recipients were then injected with 3 doses of polyinosine-polycytidylic acid (pI-pC) every other day. Bone marrow cells from pI-pC-treated animals were isolated 4 weeks after pI-pC administration and analyzed for the presence of CD45.2+ and CD45.1+ donor cells. For Lin−cKit+Sca1+ (LSK) analysis, bone marrow cells from transplanted mice were stained for mature hematopoietic lineages, expressing surface antigens CD5, CD11b, B220, Gr-1, and Ter119 with EasySep (StemCell Technologies) to exclude mature cells. Lineage-negative cells were stained with APC anti-c-kit, PE-Cy7 anti-Sca-1, fluorescein isothiocyanate anti-CD45.2, and PE-Cy5.5 anti-CD45.1 antibodies and analyzed by flow cytometry on an LSRII.
Quantitative reverse transcriptase-polymerase chain reaction
RNA was prepared using the RNeasy mini kit (Qiagen) and reverse transcribed using iScript reverse transcriptase supermix (Biorad) according to manufacturer procedures. Quantitative polymerase chain reaction (PCR) was performed with SYBR Green.
Animal experiments
Animal studies were approved by the Northwestern University Institutional Care and Use Committee. Aurka floxed mice were obtained from Terry van Dyke (National Cancer Institute) and have been described previously.9 C57Bl/6 and Mx1-Cre transgenic mice were purchased from The Jackson Laboratory. Aurka floxed mice were genotyped using the following PCR conditions: denaturing, 95°C for 30 seconds; annealing, 55°C for 30 seconds; and elongation, 68°C for 1 minute and 30 seconds for 35 cycles. Taq polymerase (Invitrogen) was used with 2 mM MgCl2, 1 mM dNTP, and 500 nM primers following the manufacturer’s instructions. For blood parameter data, blood (50 µL) was collected in EDTA-coated tubes and analyzed with a Hemavet 850.
Histological analysis
Six-week-old mice received 3 intraperitoneal injections (150 µg) of pI-pC (GE Healthcare) every other day to induce Aurka deletion. Sternum bone marrow, liver, and spleen were isolated at various timepoints, fixed overnight in formalin, and processed for histologic analysis. For supplemental Figure 3, bone marrows were collected 6 days after pI-pC treatment and processed as above. Sections were stained with hematoxylin and eosin (H&E), and peripheral blood smears were stained with May-Grünwald Giemsa using standard protocols. Details on the microscopic methods are provided in the figure legends.
Statistical analyses
We performed statistical analysis using the Student's t test, and we considered results with P < .05 as statistically significant. Survival in animal experiments is represented with Kaplan-Meier curves, and significance is estimated with the log-rank test (Prism GraphPad).
Results
Aurka is required for adult hematopoiesis
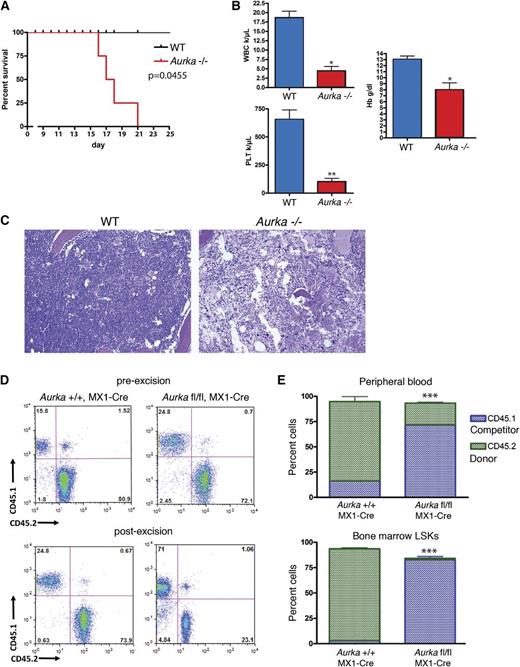
Because complete loss of Aurka results in preimplantation embryonic lethality, we studied the in vivo requirement of Aurka in hematopoiesis using a conditional knockout model. Aurka mutant mice in which exon 2 is flanked by loxP sites were bred to mice expressing Cre recombinase under the control of the Mx1 locus (Mx1-Cre), which can be transiently induced in hematopoietic cells in response to pI-pC.9,16 We treated Aurkafl/fl (WT) heterozygous Aurkafl/+/Mx1-Cre (Aurka+/−) and homozygous Aurkafl/fl/Mx1-Cre (Aurka−/−) mice with pI-pC to trigger an inducible deletion of the floxed Aurka alleles in hematopoietic cells. We observed excision of the second exon of the Aurka gene and robust reductions in protein in bone marrow cells from Aurka+/− and Aurka−/− mice (Figure 1A). Strikingly, we found that homozygous loss of Aurka mice was rapidly lethal: all of the Aurka−/− animals became moribund and were euthanized within 22 days of pI-pC treatment (Figure 1B). In comparison, the heterozygous littermates receiving pI-pC survived as well as controls.
AURKA is required for hematopoiesis. (A) Western blot of Aurka in bone marrow lysates prepared from Aurkafl/fl (WT), Aurkafl/+/Mx1-Cre (Aurka+/−), and Aurkafl/f/Mx1-Cre (Aurka−/−) mice 14 days after 3 doses of pI-pC treatment. Heat shock protein 70 (Hsc70) is included as a loading control. (B) Survival curve for WT, Aurka+/−, and Aurka−/− mice after pI-pC treatment. Day 1 refers to the day of the first pI-pC injection. The significance of the difference in survival curves was calculated by log-rank test. P = .0002. n = 4 to 5 mice per group, respectively. (C-E) Analysis of the peripheral blood white blood cell count, hemoglobin, and platelet counts from mice 14 days after the first pI-pC injection. Data are shown as the means ± standard deviation (SD). (F) H&E-stained sections of bone marrow, spleen, and liver from control and homozygous-deleted mice 14 days after the first pI-pC injection. Peripheral blood smears from control and homozygous-deleted mice were stained with May-Grünwald Giemsa. Slides were viewed with a Leica DM4000B microscope fitted with a 10× Leica HCX PL Fluorotar objective. Images were acquired with Leica DFC320 camera and Leica LAS v4.4 software at room temperature. (G) Bone marrow cellularity 14 days after pI-pC treatment of control, heterozygous, and homozygous-deleted mice. Data are shown as the means ± SD. n = 6. (H) Loss of Aurka in vivo results in increased apoptosis as measured by annexin V staining 14 days after pI-pC treatment. (Left) Representative flow cytometry plot. (Right) Bar graph with mean ± SD. n = 3. *P < .05; **P < .01; ***P < .001.
AURKA is required for hematopoiesis. (A) Western blot of Aurka in bone marrow lysates prepared from Aurkafl/fl (WT), Aurkafl/+/Mx1-Cre (Aurka+/−), and Aurkafl/f/Mx1-Cre (Aurka−/−) mice 14 days after 3 doses of pI-pC treatment. Heat shock protein 70 (Hsc70) is included as a loading control. (B) Survival curve for WT, Aurka+/−, and Aurka−/− mice after pI-pC treatment. Day 1 refers to the day of the first pI-pC injection. The significance of the difference in survival curves was calculated by log-rank test. P = .0002. n = 4 to 5 mice per group, respectively. (C-E) Analysis of the peripheral blood white blood cell count, hemoglobin, and platelet counts from mice 14 days after the first pI-pC injection. Data are shown as the means ± standard deviation (SD). (F) H&E-stained sections of bone marrow, spleen, and liver from control and homozygous-deleted mice 14 days after the first pI-pC injection. Peripheral blood smears from control and homozygous-deleted mice were stained with May-Grünwald Giemsa. Slides were viewed with a Leica DM4000B microscope fitted with a 10× Leica HCX PL Fluorotar objective. Images were acquired with Leica DFC320 camera and Leica LAS v4.4 software at room temperature. (G) Bone marrow cellularity 14 days after pI-pC treatment of control, heterozygous, and homozygous-deleted mice. Data are shown as the means ± SD. n = 6. (H) Loss of Aurka in vivo results in increased apoptosis as measured by annexin V staining 14 days after pI-pC treatment. (Left) Representative flow cytometry plot. (Right) Bar graph with mean ± SD. n = 3. *P < .05; **P < .01; ***P < .001.
We next examined hematopoiesis in the peripheral blood, bone marrow, and spleen. Peripheral blood counts from Aurka−/− animals showed marked reductions in white blood cell, hemoglobin, and platelet counts 14 days after pI-pC treatment (Figure 1C-E). Stained peripheral blood smears and histological analysis of the bone marrow confirmed that Aurka deletion led to pancytopenia and hypocellularity of the bone marrow at this time point (Figure 1F). In contrast, there were no obvious differences in the liver and spleen histology between the Aurka deleted and wild-type (WT) mice. Together, these findings suggest that the lethality may be due to anemia and/or bleeding. Consistent with the histology, analysis of the bone marrow by flow cytometry revealed a sixfold reduction of total bone marrow cells in the Aurka−/− animals 14 days after pI-pC treatment (Figure 1G). In contrast, Aurka+/− mice did not display a significant change in bone marrow cellularity. In addition, annexin V staining was significantly increased in Aurka-deficient bone marrow cells, indicating that loss of Aurka leads to an increase in apoptotic cell death (Figure 1H).
To determine whether the observed defects in hematopoiesis were linked to deficits in hematopoietic stem and progenitor cells, we performed methylcellulose colony-forming assays. The erythroid, myeloid, and megakaryocytic colony-forming ability of Aurka−/− bone marrow cells 14 days after pI-pC treatment was greatly diminished compared with WT cells (Figure 2A). We also performed flow cytometry to assess the level of myeloid progenitors in the bone marrow. As we observed no colony forming unit activity at day 14, we assayed the presence of the cells at day 6 and observed a striking decrease in the frequency of granulocyte macrophage progenitors and common myeloid progenitors, with a less pronounced, but significant decline in megakaryocyte erythroid progenitors (Figure 2B; supplemental Figure 1, available on the Blood Web site). The frequency of the megakaryocyte progenitor was also reduced in the Aurka deleted mice.
Knockout of Aurka leads to a striking reduction in myeloid progenitors. (A) Colony forming assays of bone marrow cells 14 days after pI-pC treatment from control (WT) or Aurkafl/fl/Mx1-Cre (Aurka−/−) mice; 25 000 and 100 000 cells were plated for the burst forming unit-erythroid (BFU-E), CFU-M, and CFU-MK assays. Colonies were enumerated 7 to 10 days after plating. Data are shown as mean ± SD. (B) Flow cytometry analysis of myeloid and megakaryocyte progenitors in the bone marrow. Means + SD of the different populations in WT vs Aurka−/− mice analyzed 6 days after the first pI-pC treatment are shown; n = 2. Progenitor counts were normalized per 10 000 bone marrow cells. *P < .05; **P < .01. Representative FACS plots are shown in supplemental Figure 1.
Knockout of Aurka leads to a striking reduction in myeloid progenitors. (A) Colony forming assays of bone marrow cells 14 days after pI-pC treatment from control (WT) or Aurkafl/fl/Mx1-Cre (Aurka−/−) mice; 25 000 and 100 000 cells were plated for the burst forming unit-erythroid (BFU-E), CFU-M, and CFU-MK assays. Colonies were enumerated 7 to 10 days after plating. Data are shown as mean ± SD. (B) Flow cytometry analysis of myeloid and megakaryocyte progenitors in the bone marrow. Means + SD of the different populations in WT vs Aurka−/− mice analyzed 6 days after the first pI-pC treatment are shown; n = 2. Progenitor counts were normalized per 10 000 bone marrow cells. *P < .05; **P < .01. Representative FACS plots are shown in supplemental Figure 1.
Aurka has a cell autonomous requirement in HSCs
To confirm that Aurka has a cell autonomous requirement in hematopoietic stem and progenitor cells, we collected bone marrow from uninduced Aurkafl/fl/Mx1-Cre and Aurka+/+/Mx1-Cre donor mice and transplanted 1 million total bone marrow cells into irradiated WT recipients. After 5 weeks, we confirmed engraftment by analysis of the peripheral blood and then treated the animals with pI-pC. The mice transplanted with bone marrow from Aurkafl/fl/Mx1-Cre mice became moribund and were euthanized within 21 days after pI-pC treatment (Figure 3A), mirroring the phenotype of the primary Aurka−/− deleted mice. Similar to the Mx1-Cre conditional knockout mice, the white blood cell, hemoglobin, and platelet counts were all significantly lower in the mice transplanted with Aurkafl/fl/Mx1-Cre bone marrow 17 days after pI-pC treatment compared with the mice transplanted with bone marrow from Aurka+/+/Mx1-Cre mice (Figure 3B). In addition, the bone marrow of mice that received Aurkafl/fl/Mx1-Cre cells was extremely hypocellular on histological examination following pI-pC treatment (Figure 3C).
The hematopoietic defect in Aurka−/− mice is cell autonomous. (A) In noncompetitive transplant experiments, mice engrafted with Aurkafl/fl/Mx1-Cre (Aurka−/−) bone marrow died within 21 days of pI-pC treatment. (B) Complete blood counts from mice transplanted with Aurkafl/fl/Mx1-Cre (Aurka−/−) and Aurka+/+/MX1-Cre (WT) bone marrow 17 days after pI-pC treatment. Data are shown as mean ± SD. (C) H&E-stained sections of sternum bone marrow from WT and Aurka−/− mice. Images were acquired as described in Figure 1F. (D) CD45.1 and CD45.2 stained bone marrow cells from representative Aurka+/+/MX1-Cre and Aurkafl/fl/Mx1-Cre mice before and 4 weeks after pI-pC treatment were analyzed by flow cytometry. Percentages of CD45.1+ cells and CD45.2+ cells are shown. (E) Bar graph depicts percentage of donor CD45.2+ (green) or WT CD45.1+ competitor (blue) cells after pI-pC administration, as determined by CD45.1 and CD45.2 staining of peripheral blood and bone marrow LSK cells. The genotype of the donor cells is indicated on the x-axis. Data are shown as the means ± SD, n = 6. *P < .05; **P < .01; ***P < .001.
The hematopoietic defect in Aurka−/− mice is cell autonomous. (A) In noncompetitive transplant experiments, mice engrafted with Aurkafl/fl/Mx1-Cre (Aurka−/−) bone marrow died within 21 days of pI-pC treatment. (B) Complete blood counts from mice transplanted with Aurkafl/fl/Mx1-Cre (Aurka−/−) and Aurka+/+/MX1-Cre (WT) bone marrow 17 days after pI-pC treatment. Data are shown as mean ± SD. (C) H&E-stained sections of sternum bone marrow from WT and Aurka−/− mice. Images were acquired as described in Figure 1F. (D) CD45.1 and CD45.2 stained bone marrow cells from representative Aurka+/+/MX1-Cre and Aurkafl/fl/Mx1-Cre mice before and 4 weeks after pI-pC treatment were analyzed by flow cytometry. Percentages of CD45.1+ cells and CD45.2+ cells are shown. (E) Bar graph depicts percentage of donor CD45.2+ (green) or WT CD45.1+ competitor (blue) cells after pI-pC administration, as determined by CD45.1 and CD45.2 staining of peripheral blood and bone marrow LSK cells. The genotype of the donor cells is indicated on the x-axis. Data are shown as the means ± SD, n = 6. *P < .05; **P < .01; ***P < .001.
Next, we performed competitive transplants to assay the repopulating activity of the Aurka deficient cells. One million Aurkafl/fl/Mx1-Cre or Aurka+/+/Mx1-Cre CD45.2+ total bone marrow cells were isolated, mixed with 250 000 CD45.1+ competitor bone marrow cells, and transplanted into lethally irradiated CD45.1+ congenic recipient mice. Engraftment was confirmed at 6 weeks after transplant by flow cytometry of the peripheral blood after which Aurka deletion was induced with pI-pC. Four weeks after pI-pC treatment, reconstitution was determined by flow cytometric analysis of the peripheral blood and bone marrow CD45.1+ and CD45.2+ populations. The CD45.2+Aurkafl/fl/Mx1-Cre population engrafted well, but was greatly decreased in its percentage following pI-pC treatment, indicating that Aurka deleted hematopoietic stem cells are severely compromised in competitive repopulating ability (Figure 3D-E). Additionally, LSK stem and progenitor cells were significantly decreased in frequency in the mice transplanted with bone marrow from the Aurkafl/fl/Mx1-Cre mice following pI-pC treatment (Figure 3E). Together these experiments demonstrate that the Aurka hematopoietic specific knockout phenotype is intrinsic to hematopoietic cells.
Aurka loss is associated with an increase in differentiated megakaryocytes
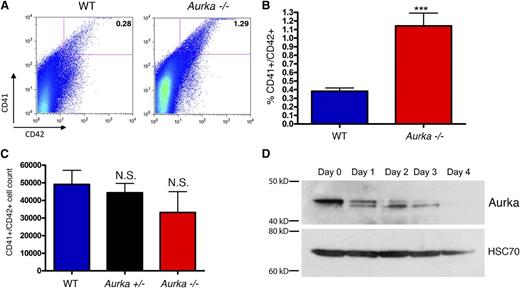
Given the pro-differentiating effect of the AURKA inhibitor MLN8237 on megakaryocytes,2 we next analyzed the effect of deletion on this lineage in more detail 14 days after pI-pC treatment. We discovered that loss of Aurka led to a significant increase in the proportion of bone marrow cells that express the megakaryocyte markers CD41+ (GPIIb) and CD42+ (GP1bα) compared with control mice (Figure 4A-B). Notably, the absolute number of CD41+/CD42+ cells in the bone marrow was not significantly reduced following Aurka deletion, despite an overall sixfold decrease in total bone marrow cells at this time point (compare Figures 1G and 4C). We saw a similar preservation of megakaryocytes in the bone marrow of mice 6 and 9 days after pI-pC treatment (supplemental Figure 2). Importantly, consistent with a specific effect of Aurka loss on megakaryocyte development, the number and proportion of Gr1+/Mac1+ cells in the bone marrow markedly decreased at 6 and 9 days after excision (supplemental Figure 2). These results were confirmed by histologic analysis of bone marrow sections taken 6 days after pI-pC treatment, which highlight the persistence of megakaryocytes and the loss of virtually all other lineages (supplemental Figure 3). Together these findings suggest that either megakaryocytes are less sensitive to loss of Aurka or that there is an increase in the generation of CD41+ polyploid cells when Aurka is deleted. Of note, Aurka protein decreases with megakaryocyte differentiation (Figure 4D), consistent with a lack of an absolute requirement during megakaryocyte maturation.
Megakaryocytes persist in the absence of Aurka. (A-B) Fourteen days after pI-pC treatment, bone marrow cells were isolated from control and knockout mice, erythrocytes were lysed, and cells were stained with CD41 and CD42. Representative (A) flow plots and (B) bar graph with means ± SD are shown; n = 8. (C) Absolute numbers of CD41+/CD42+ cells in the bone marrow 14 days after pI-pC treatment revealed no significant difference between the WT, Aurka+/−, and Aurka−/− samples. Means ± SD are shown; n = 5. (D) Western blot of Aurka levels during THPO-induced megakaryocytic differentiation of murine bone marrow cells. Hsc70 is shown as a loading control. ***P < .001.
Megakaryocytes persist in the absence of Aurka. (A-B) Fourteen days after pI-pC treatment, bone marrow cells were isolated from control and knockout mice, erythrocytes were lysed, and cells were stained with CD41 and CD42. Representative (A) flow plots and (B) bar graph with means ± SD are shown; n = 8. (C) Absolute numbers of CD41+/CD42+ cells in the bone marrow 14 days after pI-pC treatment revealed no significant difference between the WT, Aurka+/−, and Aurka−/− samples. Means ± SD are shown; n = 5. (D) Western blot of Aurka levels during THPO-induced megakaryocytic differentiation of murine bone marrow cells. Hsc70 is shown as a loading control. ***P < .001.
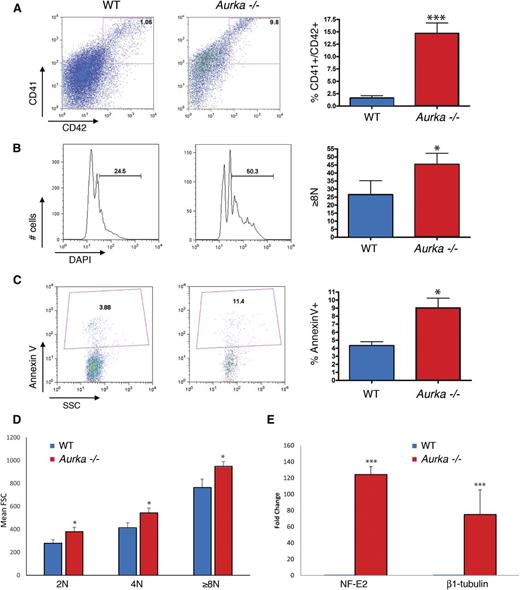
As a way to determine whether loss of Aurka merely led to survival of fully differentiated megakaryocytes, we treated mice with pI-pC and analyzed in vivo 5-bromo-2′-deoxyuridine (BrdU) incorporation in the bone marrow 3 days after pI-pC treatment. The proportion of CD41+ cells that stained for BrdU in the Aurka-deficient mice was on average nearly threefold higher than that of the control population (Figure 5A-B). In contrast, there was a significant reduction in BrdU staining in the Gr1+/Mac1+ lineage (Figure 5C-D). This result indicates that the megakaryocyte lineage is undergoing DNA replication at a higher rate with Aurka deletion. This is most likely reflective of the increased polyploidization of the megakaryocytes with Aurka loss, as we observed a significant decline in 2N CD41+ cells coupled with a significant increase in the proportion of CD41+ cells with DNA content ≥ 8N following excision (Figure 5E). Together these results show that hematopoietic progenitors and the mature myeloid lineages require Aurka at all stages of development, whereas committed megakaryocytes do not. Furthermore, we conclude that surviving megakaryocytes are not static but are undergoing DNA replication in the absence of Aurka.
Megakaryocytes continue cycling in the absence of Aurka. (A) Representative flow plots of BrdU incorporation in CD41+ cells revealed that deletion of Aurka significantly increased the proportion of cycling megakaryocytes. (B) Percentage of BrdU-positive CD41+ cells in the bone marrow of WT or Aurka−/− mice 3 days after pI-pC treatment. Means ± SD are shown; n = 3. (C) In contrast, cycling Gr1+/Mac1+ cells decrease following pI-pC treatment. Representative flow plots are shown. (D) Percentage of BrdU-positive Gr1+/Mac1+ cells in the bone marrow 3 days after pI-pC treatment. Means ± SD are shown; n = 3. (E) DNA content of CD41+ cells following pI-pC treatment. (Left) Bar graph depicting means ± SD; n = 3. (Right) Representative flow cytometry plots of DNA content. Numbers refer to the 2N, 4N, and ≥8N populations. ***P < .001; **P < .01; *P < .05.
Megakaryocytes continue cycling in the absence of Aurka. (A) Representative flow plots of BrdU incorporation in CD41+ cells revealed that deletion of Aurka significantly increased the proportion of cycling megakaryocytes. (B) Percentage of BrdU-positive CD41+ cells in the bone marrow of WT or Aurka−/− mice 3 days after pI-pC treatment. Means ± SD are shown; n = 3. (C) In contrast, cycling Gr1+/Mac1+ cells decrease following pI-pC treatment. Representative flow plots are shown. (D) Percentage of BrdU-positive Gr1+/Mac1+ cells in the bone marrow 3 days after pI-pC treatment. Means ± SD are shown; n = 3. (E) DNA content of CD41+ cells following pI-pC treatment. (Left) Bar graph depicting means ± SD; n = 3. (Right) Representative flow cytometry plots of DNA content. Numbers refer to the 2N, 4N, and ≥8N populations. ***P < .001; **P < .01; *P < .05.
We next studied the effect of Aurka deletion on megakaryopoiesis in more detail by ex vivo culture assays. We isolated Aurkafl/fl and Aurka+/+ bone marrow cells, enriched for stem and progenitor cells, and then transduced the cells with green fluorescent protein (GFP)-Cre or GFP-expressing retroviruses to induce excision. Transduced cells were then expanded and differentiated in liquid culture with THPO, IL-3, IL-6, and SCF for 3 days to produce megakaryocytes. After 3 days, cells were stained for CD41 and CD42 expression and DNA content and analyzed by flow cytometry. Compared with the Aurkafl/fl (WT) population, Aurkafl/fl cells with Cre (Aurka−/−) exhibited significant increases in the percentage of megakaryocytes expressing CD41 and CD42 and an increased proportion of ≥ 8N megakaryocytes (Figure 6A-B). We also observed a significant increase in annexin V staining in the Aurka−/− culture compared with the WT culture (Figure 6C). To assess the state of differentiation of these cells, we analyzed cell size, as a marker of maturation, and expression of megakaryocyte genes. We found that the Aurka deleted megakaryocytes were significantly larger than the control cells across all ploidy classes (Figure 6D). Furthermore, we observed that the NF-E2 and β1-tubulin expression increased 120-fold and 75-fold, respectively, in the Aurka deleted megakaryocytes (Figure 6E). These results indicate that loss of Aurka leads to an increased CD41+ population with greater DNA content and enhanced expression of megakaryocyte genes.
Ex vivo deletion of Aurka increases CD41 and CD42 expression, DNA content, and apoptosis of megakaryocytes. (A-C) Deletion of Aurka by expression of Cre in Aurkafl/fl cells (Aurka−/−) significantly increased (A) CD41+CD42+ cells, (B) DNA content of CD41+ cells, and (C) apoptosis of the CD41+ population relative to Aurkafl/fl cells without Cre (WT). (Left) Representative flow plots. (Right) Bar graphs of means ± SD; n = 4. (D) Flow cytometry was used to measure the size of the CD41+ cells after deletion; significant increases in cell size were observed across the different ploidy classes. (E) Quantitative reverse transcriptase-PCR determination of expression changes in the master megakaryocyte regulator NF-E2 and β1-tubulin following Aurka deletion. Mean fold increases ± SD are shown. ***P < .001; *P < .05.
Ex vivo deletion of Aurka increases CD41 and CD42 expression, DNA content, and apoptosis of megakaryocytes. (A-C) Deletion of Aurka by expression of Cre in Aurkafl/fl cells (Aurka−/−) significantly increased (A) CD41+CD42+ cells, (B) DNA content of CD41+ cells, and (C) apoptosis of the CD41+ population relative to Aurkafl/fl cells without Cre (WT). (Left) Representative flow plots. (Right) Bar graphs of means ± SD; n = 4. (D) Flow cytometry was used to measure the size of the CD41+ cells after deletion; significant increases in cell size were observed across the different ploidy classes. (E) Quantitative reverse transcriptase-PCR determination of expression changes in the master megakaryocyte regulator NF-E2 and β1-tubulin following Aurka deletion. Mean fold increases ± SD are shown. ***P < .001; *P < .05.
We recently used a high-throughput small molecule screen coupled with an shRNA screen of the kinome to identify compounds and kinases that mediate the switch a normal proliferative cell cycle to the endomitotic cell cycle. Kinases that regulate this switch include AURKA and AURKB, ROCK1, CDK1, CDK2, and PLK1.2 We validated that inhibition or knockdown of each kinase led to increased megakaryocyte polyploidization. However, not all the compounds that induced polyploidization also facilitated terminal megakaryocyte maturation. Indeed, treatment of SET2 cells with the AURKA inhibitor MLN8237 or the cell cycle inhibitors cytochalasin D and latrunculin B led to increased polyploidization; however, only MLN8237 promoted CD41 expression, indicative of megakaryocyte maturation (Figure 7). Thus, inhibition of AURKA specifically has the dual effect of enforcing polyploidization concomitant with promoting megakaryocyte differentiation.
MLN8237 selectively induces CD41 expression concomitant with polyploidization. SET2 cells were treated with MLN8237, the cell cycle inhibitors cytochalasin D (Cyto. D), or latrunculin B (Lat. B) for 72 hours and analyzed for (A) DNA content and (B) CD41 expression by flow cytometry. Bar graphs of mean ± SD; n = 3 (MLN8237) and n = 2 (cytochalasin D and latrunculin B). ***P < .001; **P < .01.
MLN8237 selectively induces CD41 expression concomitant with polyploidization. SET2 cells were treated with MLN8237, the cell cycle inhibitors cytochalasin D (Cyto. D), or latrunculin B (Lat. B) for 72 hours and analyzed for (A) DNA content and (B) CD41 expression by flow cytometry. Bar graphs of mean ± SD; n = 3 (MLN8237) and n = 2 (cytochalasin D and latrunculin B). ***P < .001; **P < .01.
Discussion
The discovery that AURKA inhibitors enforce differentiation of normal and malignant megakaryocytes has raised the question of how AURKA participates in hematopoiesis. Here we report that hematopoietic expression of Aurka is essential for survival of adult animals and is indispensable for the proliferation and/or survival of hematopoietic stem and progenitor cells. Similar results have been seen with hematopoietic deletion of other cell cycle regulators. For example, loss of the chromosome passenger protein survivin leads to rapid bone marrow failure and lethality,17 and simultaneous loss of all 3 D-type cyclins caused death with a similar time course.18 Thus, it is likely that the mortality observed in the Aurka hematopoietic-null animals is a consequence of bone marrow failure, which is associated with profound anemia and thrombocytopenia. We also discovered that the hematopoietic defects in the Aurka-deficient mice are cell autonomous. Of note, a previous study using 4-hydroxytamoxifen-inducible Cre driver to excise AURKA reported a ∼35% mortality rate and a modest reduction in blood counts.12 The difference in severity of phenotypes reported for Aurka loss in the bone marrow likely stems from differences in the efficiency of Aurka deletion between the 2 models.
In contrast to the broad requirement for Aurka observed in most blood cells, we noted that megakaryocytes escaped growth arrest and apoptosis. Furthermore, we saw that the depleted megakaryocytes were not merely spared from mitotic catastrophe, but continued to undergo polyploidization as evidenced by BrdU incorporation. Aurka-null megakaryocytes also displayed increased size and strikingly elevated expression of NF-E2 and β1-tubulin compared with the control cells. These observed effects of loss of Aurka activity on megakaryocytes do not simply stem from inhibition of the proliferative cell cycle, as cell cycle inhibitors known to induce polyploidy failed to induce differentiation of the megakaryocyte lineage. These observations suggest that polyploidization and terminal megakaryocyte differentiation are not inextricably linked. Indeed, evidence against this model has been provided by studies of the role of cyclin D1 in megakaryopoiesis: overexpression of cyclin D1 and cdk4 potently rescued polyploidization of Gata1-deficient cells but did not rescue maturation.19 Rather, the megakaryocyte maturation program appears to temporally couple specific cell cycle regulators to differentiation processes. One model for this coordinated differentiation is that Aurka may control the activity of the key megakaryocyte transcription factor NF-E2 through phosphorylation of a consensus AURKA site within the protein.20 We hypothesize that phosphorylation of NF-E2 restricts its ability to upregulate expression of critical differentiation target genes; this activity may be restored as the level of unphosphorylated NF-E2 declines concomitant with the decrease in Aurka expression. In an apparent contradiction to this model, the platelet counts in the Aurka homozygous floxed mice were markedly diminished on excision. This observation suggests that loss of Aurka does not allow for complete maturation, potentially due to excess expression of certain late stage genes, preferential progression through the endomitotic cell cycle, or lack of a supportive bone marrow environment. We note that, although AURKA inhibitors promote polyploidization and expression of megakaryocyte markers in vivo and in vitro, they also fail to promote platelet production.2
Intriguingly, by analyzing ex vivo cultures of megakaryocyte differentiation, we found that Aurka protein levels decrease with megakaryocyte differentiation during ex vivo culture. This observation adds nuance to a previous report, which used immunofluorescence to show that AURKA is present in polyploid megakaryocytes.6 We speculate that an overall reduction in AURKA activity may accompany megakaryocyte differentiation, although low amounts of AURKA may persist at centrosomes. Based on our findings, we propose that decreasing activity of AURKA promotes polyploidization. Given the function of AURKA in cell cycle regulation, 1 possible mechanism under investigation is that spindle checkpoint activation and G2 arrest caused by AURKA inhibition leads to cell cycle re-entry and endoreduplication in cells that are predisposed to become polyploid.21
AURKA’s sibling, AURKB, has been well studied in megakaryopoiesis, with differing reports of expression during polyploidization.3,4,6,22 A recent study concluded that Aurkb activity is not formally required for megakaryocyte polyploidization.3 Specifically, addition of an AURKB inhibitor AZD1152-HQPA did not impair endomitosis but led to mitotic failure in anaphase of dividing cells. Together, these findings and our work confirm that postproliferative megakaryocytes are not subject to control by the essential aurora kinases and are able to survive and undergo repeated rounds of DNA replication without cell division. Therefore, the endomitotic cell cycle appears to fundamentally differ from the proliferative cell cycle in more ways than defects in the contractile ring and cleavage furrow formation and regression.23,24 Indeed chromosome segregation patterns have been found to be distinct in low vs high ploidy megakaryocytes.25 Discovering the mechanism of cell cycle regulation during megakaryocyte polyploidization may therefore provide insight, not only into maturation of this lineage, but into the evasion of cell cycle control of aneuploid cancer cells as well. Furthermore, these studies shed light onto the link between cell cycle regulation and terminal cell differentiation processes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rachael Schultz for technical assistance, Benjamin Thompson for critical reading of the manuscript, and Terry van Dyke for the Aurka floxed mice.
This work was supported by National Institutes of Health, National Heart, Lung and Blood Institute grant HL112792, the National Center for Research Resources, National Center for Advancing Translational Sciences grant TL1R000108, and the Samuel Waxman Cancer Research Foundation.
Authorship
Contribution: B.G., G.K., M.J.S., and Q.J.W. designed and performed the experiments, analyzed the data, and wrote the manuscript; and J.D.C. designed the experiments, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John D. Crispino, Northwestern University, Division of Hematology/Oncology, 303 East Superior St, Lurie 5-113, Chicago, IL 60611; e-mail: j-crispino@northwestern.edu.