To the editor:
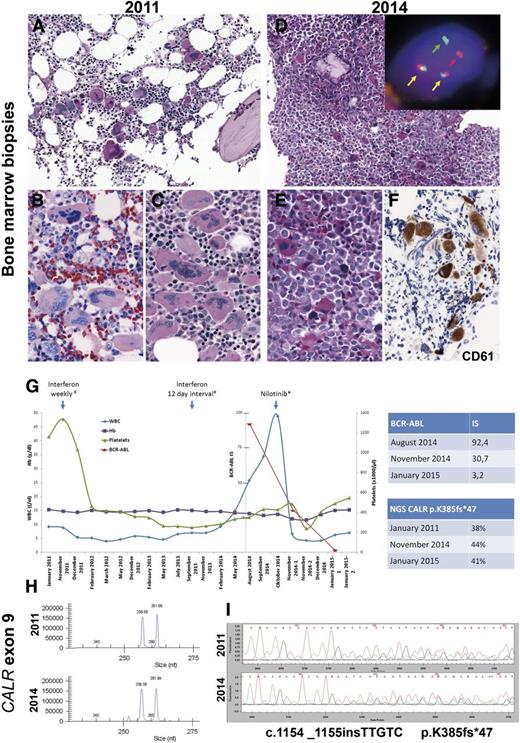
The understanding of the genetic basis of the myeloproliferative neoplasms has increased tremendously in the last few years.1 In essential thrombocythemia (ET), 50% to 65% of the patients carry the JAK2 V617F mutation, whereas only ∼5% of patients carry MPL exon 10 mutations. More recently, CALR exon 9 mutations were identified in ∼20% to 25% of ET patients.2,3 CALR mutations have been found almost exclusively in ET and primary myelofibrosis, suggesting that CALR mutations primarily affect the biology of megakaryocytes. Interestingly, CALR-mutant ET seems to be a distinctive entity within myeloid neoplasms with distinguishing clinical features. It affects relatively young individuals and is characterized by markedly elevated platelet count but relatively low thrombotic risk and no progression to polycythemia vera.4 Here we report a case of BCR-ABL+ chronic myeloid leukemia (CML) presenting 3 years after the diagnosis of a JAK2–MPL– ET, both lesions carrying the same CALR mutation. The case occurred in a 26-year-old man who was noted in 2011 to have new-onset thrombocytosis (platelets [PLTs], 1159 × 109/L) with normal hemoglobin and white blood cell (WBC) count. The bone marrow (BM) biopsy was normocellular with a normal myeloid:erythroid ratio but an increased amount of megakaryocytes without fibrosis. The megakaryocytes showed a predominance of hyperlobated “staghorn” forms often present in loose clusters (Figure 1A-C). Molecular studies at the time of diagnosis showed wild-type JAK2 and MPL genes. A diagnosis of JAK2–MPL– ET was rendered. The patient was treated with interferon 135 μg once per week and then with a 12-day interval with good response (Figure 1G).
Morphologic and molecular findings in a case of CALR-mutated essential thrombocytemia that evolved into a CALR-mutated BCR-ABL+ chronic myeloid leukemia. (A-C) Bone marrow biopsy in 2011. (A) Normocellular BM with increased amount of large hyperlobated “staghorn” megakaryocytes (PAS stain). (B-C) Higher magnification showing the loose clusters of megakaryocytes with mainly staghorn forms (B: Giemsa stain; C: periodic acid Schiff stain). (D-F) BM biopsy in 2014. (D) Hypercellular BM with increased myeloid:erythroid ratio and increased amount of small hypolobated dwarf megakaryocytes (PAS stain). Insert: Interphase fluorescent in situ hybridization analysis using BCR-ABL dual color, dual fusion translocation probe (Zytotomed, Zytolight BCR-ABL) shows one red signal (red arrow), one green signal (green arrow), and two red/green fusion signals (yellow arrow) indicative of a t(9;22)(q34;q11). (E) Higher magnification showing the atypical small hypolobated megakaryocytes in a background of left-shifted granulopoiesis (periodic acid Schiff stain). (F) CD61 (Dako; Glostrup, Denmark) staining highlights the small hypolobated megakaryocytes. (G) Graphic depiction of the peripheral blood counts from January 2011 to January 2015. The patient was treated originally with interferon 135 µg. In October 2014, he was switched to nilotinib 150 mg (2-0-2). The top table shows the quantitative reverse transcription polymerase chain reaction (qRT-PCR) results of the BCR-ABL fusion transcript in international standard (IS). The bottom table shows the next-generation sequencing results of the allele burden of CALR mutant. (H) Fragment length analysis of CALR exon 9 hotspot region shows concurrent amplification of the wild-type allele resulting in a 257-bp fragment and a mutated allele of 262 bp in both BM biopsies. (I) Sanger sequencing of the CALR exon 9 hotspot region confirmed an identical frameshift mutation in both biopsies (c.1154_1155insTTGTC, p.K385fs*47). Fluorescent in situ hybridization images were acquired with a ×100/1.40 oil immersion objective in a Zeiss Axio fluorescence microscope (Zeiss) equipped with the appropriate filters sets and an Axio CAM MRm camera (Zeiss) and were documented and processed by using the Axio Vision Rel 4.8 software (Zeiss). Immunohistochemical analysis was performed on an automated immunostainer (Ventana Medical Systems, Tucson, AZ), following the manufacturer's protocols. Fragment analysis of CALR exon 9 hotspot region was performed by using Phusion Hot Start DNA polymerase (Finnzymes) with adequate amplification conditions and D4-fluorescent dye primer modification (Sigma-Aldrich).2 The products were separated by capillary electrophoresis on the GenomeLab GeXP Genetic Analysis System and analyzed with GenomeLab GeXP 10.2 software (Beckman Coulter, Krefeld, Germany). Sequencing of CALR exon 9 hotspot region was performed by using M13-tailed primers (forward: 5′-CTGGTCCTGGTCCTGATGTC-3′; reverse: 5′-GGGGACATCTTCCTCCTCAT-3′) and Phusion Hot Start DNA polymerase with adequate amplification conditions, followed by dye terminator cycle sequencing (Quick Start Master Mix) using M13 primers and capillary electrophoresis on the GenomeLab GeXP Genetic Analysis System 10.2 software. Next-generation sequencing was applied for mutational screening of CALR (exon 9). By using a 2-step PCR, design amplicons were generated that included sequencing adaptors for Roche 454 sequencing and an individual multiplex identifier (MID tag) to allow multiplexing. After amplicon pooling, the library was purified (QIAquick PCR purification kit; Qiagen, Hilden, Germany) followed by agencourt AMPure XP (Beckman Coulter) and quantified by using the Quant-iT PicoGreen Kit (Invitrogen, Carlsbad, CA). Following the emulsion-based PCR amplification (GS Junior emPCR kit), clonally amplified beads were enriched according to the manufacturer’s recommendations and quantified on a CASY cell counter (Roche). The 454 sequencing data were generated on a GS Junior using the GS Junior Titanium Sequencing Kit (Roche). The expected coverage was ×1000. Data analysis was performed by using JSI Sequence Pilot, SEQNext (JSI Medical Systems GmbH, Kippenheim, Germany). Hb, hemoglobin.
Morphologic and molecular findings in a case of CALR-mutated essential thrombocytemia that evolved into a CALR-mutated BCR-ABL+ chronic myeloid leukemia. (A-C) Bone marrow biopsy in 2011. (A) Normocellular BM with increased amount of large hyperlobated “staghorn” megakaryocytes (PAS stain). (B-C) Higher magnification showing the loose clusters of megakaryocytes with mainly staghorn forms (B: Giemsa stain; C: periodic acid Schiff stain). (D-F) BM biopsy in 2014. (D) Hypercellular BM with increased myeloid:erythroid ratio and increased amount of small hypolobated dwarf megakaryocytes (PAS stain). Insert: Interphase fluorescent in situ hybridization analysis using BCR-ABL dual color, dual fusion translocation probe (Zytotomed, Zytolight BCR-ABL) shows one red signal (red arrow), one green signal (green arrow), and two red/green fusion signals (yellow arrow) indicative of a t(9;22)(q34;q11). (E) Higher magnification showing the atypical small hypolobated megakaryocytes in a background of left-shifted granulopoiesis (periodic acid Schiff stain). (F) CD61 (Dako; Glostrup, Denmark) staining highlights the small hypolobated megakaryocytes. (G) Graphic depiction of the peripheral blood counts from January 2011 to January 2015. The patient was treated originally with interferon 135 µg. In October 2014, he was switched to nilotinib 150 mg (2-0-2). The top table shows the quantitative reverse transcription polymerase chain reaction (qRT-PCR) results of the BCR-ABL fusion transcript in international standard (IS). The bottom table shows the next-generation sequencing results of the allele burden of CALR mutant. (H) Fragment length analysis of CALR exon 9 hotspot region shows concurrent amplification of the wild-type allele resulting in a 257-bp fragment and a mutated allele of 262 bp in both BM biopsies. (I) Sanger sequencing of the CALR exon 9 hotspot region confirmed an identical frameshift mutation in both biopsies (c.1154_1155insTTGTC, p.K385fs*47). Fluorescent in situ hybridization images were acquired with a ×100/1.40 oil immersion objective in a Zeiss Axio fluorescence microscope (Zeiss) equipped with the appropriate filters sets and an Axio CAM MRm camera (Zeiss) and were documented and processed by using the Axio Vision Rel 4.8 software (Zeiss). Immunohistochemical analysis was performed on an automated immunostainer (Ventana Medical Systems, Tucson, AZ), following the manufacturer's protocols. Fragment analysis of CALR exon 9 hotspot region was performed by using Phusion Hot Start DNA polymerase (Finnzymes) with adequate amplification conditions and D4-fluorescent dye primer modification (Sigma-Aldrich).2 The products were separated by capillary electrophoresis on the GenomeLab GeXP Genetic Analysis System and analyzed with GenomeLab GeXP 10.2 software (Beckman Coulter, Krefeld, Germany). Sequencing of CALR exon 9 hotspot region was performed by using M13-tailed primers (forward: 5′-CTGGTCCTGGTCCTGATGTC-3′; reverse: 5′-GGGGACATCTTCCTCCTCAT-3′) and Phusion Hot Start DNA polymerase with adequate amplification conditions, followed by dye terminator cycle sequencing (Quick Start Master Mix) using M13 primers and capillary electrophoresis on the GenomeLab GeXP Genetic Analysis System 10.2 software. Next-generation sequencing was applied for mutational screening of CALR (exon 9). By using a 2-step PCR, design amplicons were generated that included sequencing adaptors for Roche 454 sequencing and an individual multiplex identifier (MID tag) to allow multiplexing. After amplicon pooling, the library was purified (QIAquick PCR purification kit; Qiagen, Hilden, Germany) followed by agencourt AMPure XP (Beckman Coulter) and quantified by using the Quant-iT PicoGreen Kit (Invitrogen, Carlsbad, CA). Following the emulsion-based PCR amplification (GS Junior emPCR kit), clonally amplified beads were enriched according to the manufacturer’s recommendations and quantified on a CASY cell counter (Roche). The 454 sequencing data were generated on a GS Junior using the GS Junior Titanium Sequencing Kit (Roche). The expected coverage was ×1000. Data analysis was performed by using JSI Sequence Pilot, SEQNext (JSI Medical Systems GmbH, Kippenheim, Germany). Hb, hemoglobin.
In May 2014, the patient was noted to have new-onset leukocytosis (WBC, 14.8 × 109/L) with normal PLT count. Three months later, the patient’s WBC count increased to 25.7 × 109/L. Molecular studies revealed a BCR-ABL (e14a2) fusion transcript in peripheral blood (PB). A BM biopsy revealed hypercellularity with an elevated myeloid:erythroid ratio. Megakaryocytes were predominantly small and hypolobated (Figure 1D-E). Immunohistochemical analysis with CD61 highlighted the increased number of dwarf megakaryocytes (Figure 1F). Fluorescent in situ hybridization analysis using a BCR-ABL dual color, dual fusion translocation probe (Zytolight, Zytomed) confirmed a BCR-ABL fusion in 99 of 100 cells, indicative of the t(9:22)(q34;q11.2) translocation (Figure 1D, insert). Retrospectively, molecular studies and fluorescent in situ hybridization analysis for the BCR-ABL fusion transcript were performed in the 2011 BM biopsy, both of which rendered negative results. Fragment length analysis with subsequent Sanger sequencing of CALR exon 9 gene demonstrated an identical 5-bp insertion (mutation type 2; c.1154_1155insTTGTC, p.K385fs*47) in both biopsies (Figure 1H-I). Next-generation sequencing performed in purified PB granulocytes revealed a mutant CALR allele burden of 44%, which is in agreement with the heterozygous mutation found with fragment length analysis. To rule out the possibility of a CALR germ line mutation, a hair shaft probe was analyzed that revealed a CALR wild-type sequence confirming the presence of a somatic mutation in the hematopoietic cells.5,6 The interferon therapy was switched to the tyrosine kinase inhibitor nilotinib (150 mg). After 3 months, the patient has had a good hematologic response but has not achieved complete molecular response (BCR-ABL International Standard 3.23), whereas PLT counts have steadily increased, and no change in the CALR allele burden has been observed (Figure 1G).
This is the first description of a CALR exon 9 mutated ET with characteristic clinical findings (young adult with very high PTL count at presentation) that acquired a t(9;22)(q34;q11.2) translocation and changed the morphology from ET to CML. The high allele burden of mutant CALR concurrent with BCR-ABL translocation in almost 100% of the BM cells is a strong argument in favor of a common clone that harbors both genetic alterations. Our findings suggest that a subclone of the preexisting CALR-mutated heterozygous clone acquired a BCR-ABL translocation, conferring an additional growth advantage to double-mutant progenitors and shifted the morphology from ET to CML. Treatment with nilotinib is causing disappearance of the double-mutant clone and favoring re-emergence of the CALR-mutant–only clone manifested by the constant allele burden of mutant CALR with increased PLTs in PB, returning to the original phenotype. Similar findings have been described in cases with simultaneous BCR-ABL translocation and JAK2 V617F mutations.7-9 Our case highlights how the clinical and morphologic appearance of myeloproliferative neoplasms is governed by their mutational profile.
Authorship
Acknowledgments: The authors thank the technical staff of the molecular and immunohistochemical laboratories at the Institute of Pathology, University Hospital, Tübingen, Germany.
This work was supported in part by Sonderforschungsbereich 685 (L.Q.-M., F.F., and I.B.).
Contribution: I.B., B.M., and J.S. performed molecular analysis and analyzed data; P.K. contributed vital patient information; T.H. and O.W. performed genetic analysis; L.Q.-M. and F.F. designed the study, performed the histologic analysis, analyzed data, and wrote the paper.
F.F. and L.Q.-M. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leticia Quintanilla-Martinez, Institute of Pathology, University Hospital Tübingen, Liebermeisterstrasse 8, 72076 Tübingen, Germany; e-mail: leticia.quintanilla-fend@med.uni-tuebingen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal