Key Points
Developmental defects and impairment in lymphopoiesis in Mysm1-knockout mice are the result of p53 activation.
Loss of hematopoietic stem cell function and bone marrow failure in Mysm1-knockout mice are the result of p53 activation.
Abstract
MYSM1 is a chromatin-binding transcriptional cofactor that deubiquitinates histone H2A. Studies of Mysm1-deficient mice have shown that it is essential for hematopoietic stem cell (HSC) function and lymphopoiesis. Human carriers of a rare MYSM1-inactivating mutation display similar lymphopoietic deficiencies. However, the mechanism by which MYSM1 regulates hematopoietic homeostasis remains unclear. Here, we show that Mysm1-deficiency results in p53 protein elevation in many hematopoietic cell types. p53 is a central regulator of cellular stress responses and HSC homeostasis. We thus generated double-knockout mice to assess a potential genetic interaction between Mysm1 and p53 in hematopoiesis. Mysm1−/−p53−/− mouse characterization showed a full rescue of Mysm1−/− developmental and hematopoietic defects. This included restoration of lymphopoiesis, and HSC numbers and functions. These results establish p53 activation as the driving mechanism for hematopoietic abnormalities in Mysm1 deficiency. Our findings may advance the understanding of p53 regulation in hematopoiesis and implicate MYSM1 as a potential p53 cofactor.
Introduction
Myb-like SWIRM and MPN domains (MYSM1) is a chromatin-interacting protein previously shown to act as a transcriptional regulator through histone H2A (H2AK119ub) deubiquitination.1 Mysm1 deficiency in mice (Mysm1−/−) results in partial embryonic lethality, growth retardation, and severe hematopoietic defects, including lymphopenia and anemia.2-4 Human patients with rare homozygous MYSM1 E390* mutations develop bone marrow failure with similar lymphopenic features.5 Mysm1-deficient mice have impaired hematopoietic stem cell (HSC) function,3,6 which is associated with an increase in p53 protein levels and a reduction in Gfi1 locus expression in hematopoietic stem and progenitor cells (HSPCs). Forced expression of Gfi1 only partially enhanced the functional capacity of Mysm1−/− HSCs by restoring quiescence, which implies a broader molecular mechanism.6 MYSM1 was also shown to regulate the differentiation of multiple hematopoietic lineages by activating Ebf1, Id2, or Flt3 transcription.2,4,7 Nevertheless, the significance of p53 elevation in Mysm1−/− hematopoiesis has not been addressed.
p53 is a central regulator of cellular stress responses.8 It primarily acts through transcriptional regulation as a sequence-specific DNA-binding protein that triggers cell cycle arrest, cellular senescence, and apoptosis. Recent studies have revealed specialized functions of p53 in HSCs, not only in responses to exogenous stress, but also under homeostatic conditions.9,10 p53 regulates HSC quiescence and self-renewal,11,12 antioxidant and DNA repair functions,13-16 as well as HSC differentiation checkpoints.17,18 The p53 pathway is also emerging as a critical mediator of pathology in human bone marrow failure syndromes, including Fanconi anemia,19,20 5q− syndrome,21,22 and Diamond-Blackfan anemia.23 Despite the widely recognized role of p53 in HSC maintenance and human disease, our understanding of HSPC-specific mechanisms regulating p53 activity remains limited.
Here, we use a genetic approach to address the functional interactions between MYSM1 and p53. We demonstrate that loss of HSC functions and most other aspects of the Mysm1−/− phenotype are the result of aberrant p53 activation.
Study design
Mice
Mysm1tm1a(KOMP)WTSI mice were previously described3 and have over 100-fold reduced expression of Mysm1 transcript in a homozygous state (referred to here as Mysm1−/−). The p53 knockout was obtained from The Jackson Laboratory (B6.129S2-Trp53tm1Tyj/J). Mice were maintained under specific pathogen-free conditions; all experiments were in accordance with the Canadian Council on Animal Care and approved by the McGill Animal Care Committee.
Competitive bone marrow transplantation
Recipient B6.SJL-Ptprca Pepcb/Boy mice (JAX002014, congenic for CD45.1) were irradiated twice with 4.5 Gy 3 hours apart in an RS2000 irradiator (Rad Source). The mice were injected with 2.5 × 106 bone marrow cells from the following donor mice in a 1:1 ratio: B6.SJL Mysm1+/+p53+/+ mixed with C57BL/6 Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/−. The mice were kept on neomycin in drinking water (2 g/L; BioShop) for 3 weeks and analyzed at 24 weeks.
Flow cytometry
Protocols are provided in supplemental Methods (available on the Blood Web site).
Results and discussion
Loss of p53 rescues lymphopoiesis in Mysm1−/− mice
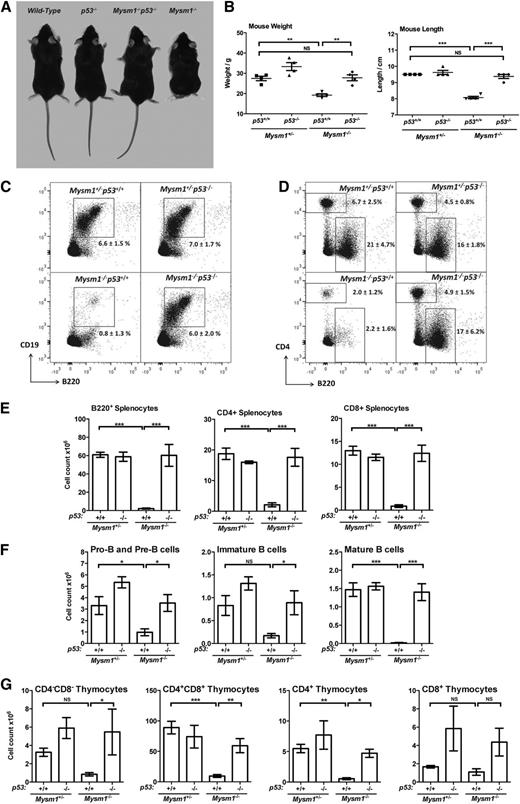
Multiple lineages of Mysm1−/− hematopoietic cells have elevated levels of p53 protein3 (supplemental Figure 1). To evaluate the role of p53 as a mediator of the Mysm1−/− phenotype, a double-knockout line was generated. In contrast to Mysm1−/−p53+/+ mice that are growth-retarded with morphologic abnormalities,2,3 Mysm1−/−p53−/− littermates were normal in size and appearance (Figure 1A-B, supplemental Figure 2). Flow cytometry showed that p53 deletion also fully restored lymphocyte development in Mysm1−/− mice (Figure 1C-G). Mysm1−/−p53−/− mice had normal numbers of B cells in the bone marrow and spleen, including pro- and pre-, immature, transitional, and follicular B-cell subsets. T-cell development was also restored to normal levels in the thymus and spleen. Mysm1−/−p53+/− mice showed an intermediate phenotype, which indicates a dose-dependent effect of p53 activity on Mysm1−/− hematopoiesis (data not shown). Overall, the rescue of developmental and hematopoietic abnormalities in Mysm1−/−p53−/− mice identifies p53 as the common mediator of Mysm1−/− phenotypes.
p53 mediates the developmental abnormalities and impaired lymphocyte differentiation in the Mysm1−/− mice. Data from mice of the following genotypes is presented: Mysm1+/−p53+/+, Mysm1+/−p53−/−, Mysm1−/−p53−/−, and Mysm1−/−p53+/+. Mysm1+/− mice were shown to be phenotypically indistinguishable from wild-type throughout our previous studies, and in this case the comparison against Mysm1+/− allowed the experimental mice to be bred as age-matched littermates. (A) Representative photograph of the mice, showing that loss of p53 rescues the growth retardation, as well as the tail and hind-limb abnormalities of the Mysm1−/− mice. (B) Mouse lengths and weights. (C) Representative flow cytometry plots of mouse bone marrow, stained for B220 and CD19, and gated on live cells. Average percentage of cells within the B220+CD19+ B-cell lineage gate is shown. (D) Representative flow cytometry plots of the mouse spleen, stained for B220 and CD4, and gated on live cells. Average percentages of cells within the B220+ B-cell gate and CD4+ T-helper cell gate are shown. (E) Numbers of B- and T-lineage cells in the spleen of the mice; cells gated as B220+, CD4+, or CD8+. (F) Numbers of pro-B and pre-B cells (B220+IgM−IgD−), immature B cells (B220+IgM+IgD−), and mature B cells (B220+IgM+IgD+) in the bone marrow of the mice. (G) Numbers of double-negative (CD4−CD8−), double-positive (CD4+CD8+), and CD4 and CD8 single-positive thymocytes in the mice. Bars show means ± SEM; *P < .05, **P < .01, ***P < .001; all data are from 4 to 5 mice per group, and are representative of 3 independent experiments. NS, nonsignificant using analysis of variance with the Bonferroni post-hoc test; SEM, standard error of the mean.
p53 mediates the developmental abnormalities and impaired lymphocyte differentiation in the Mysm1−/− mice. Data from mice of the following genotypes is presented: Mysm1+/−p53+/+, Mysm1+/−p53−/−, Mysm1−/−p53−/−, and Mysm1−/−p53+/+. Mysm1+/− mice were shown to be phenotypically indistinguishable from wild-type throughout our previous studies, and in this case the comparison against Mysm1+/− allowed the experimental mice to be bred as age-matched littermates. (A) Representative photograph of the mice, showing that loss of p53 rescues the growth retardation, as well as the tail and hind-limb abnormalities of the Mysm1−/− mice. (B) Mouse lengths and weights. (C) Representative flow cytometry plots of mouse bone marrow, stained for B220 and CD19, and gated on live cells. Average percentage of cells within the B220+CD19+ B-cell lineage gate is shown. (D) Representative flow cytometry plots of the mouse spleen, stained for B220 and CD4, and gated on live cells. Average percentages of cells within the B220+ B-cell gate and CD4+ T-helper cell gate are shown. (E) Numbers of B- and T-lineage cells in the spleen of the mice; cells gated as B220+, CD4+, or CD8+. (F) Numbers of pro-B and pre-B cells (B220+IgM−IgD−), immature B cells (B220+IgM+IgD−), and mature B cells (B220+IgM+IgD+) in the bone marrow of the mice. (G) Numbers of double-negative (CD4−CD8−), double-positive (CD4+CD8+), and CD4 and CD8 single-positive thymocytes in the mice. Bars show means ± SEM; *P < .05, **P < .01, ***P < .001; all data are from 4 to 5 mice per group, and are representative of 3 independent experiments. NS, nonsignificant using analysis of variance with the Bonferroni post-hoc test; SEM, standard error of the mean.
Aged Mysm1−/− × p53−/− mice were analyzed to rule out a transient rescue. The analysis compared Mysm1−/−p53+/− mice against control Mysm1−/− and p53+/− groups due to the early lethality of p53−/− animals.24 Surprisingly, we observed that Mysm1−/− mice succumb to tumors around 6 to 9 months of age, and these are primarily CD8+ thymic lymphomas resembling the tumors in p53−/− mice (supplemental Figure 3A-D).24 Nevertheless, a significant lifespan extension was observed in Mysm1−/−p53+/− mice relative to Mysm1−/− littermates (supplemental Figure 3E), which is likely linked to an overall improvement in immune homeostasis and immune mechanisms delaying cancer-associated lethality.25
p53 hyperactivity mediates Mysm1−/− HSC defects
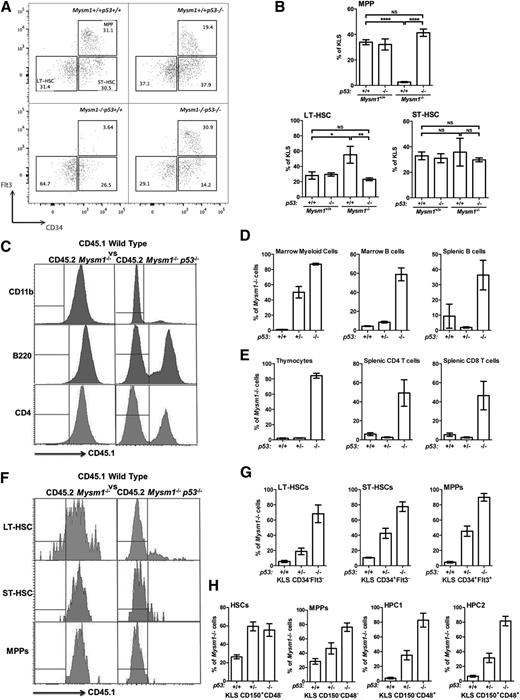
Mysm1 loss results in a severe depletion of hematopoietic progenitor cells3,6 including multipotent progenitors (MPPs), lymphoid progenitors, and myeloid progenitors. The cellular-deficiency phenotype was rescued in Mysm1−/−p53−/− animals (Figure 2A-B, supplemental Figures 4-5A) and colony-forming unit (CFU) assays validated a full rescue of progenitor functions in Mysm1−/−p53−/− mice (supplemental Figure 5B).
Loss of p53 rescues Mysm1−/− HSPC numbers and functions. (A) Flow cytometry plots gated on live KLS bone marrow cells and showing the expression of Flt3 and CD34. Stem and progenitor cell gates and population frequencies of representative samples from each group are shown. (B) Lin−cKit+Sca1+CD34−Flt3− LT-HSCs, Lin−cKit+Sca1+CD34+Flt3− ST-HSCs, and Lin−cKit+Sca1+CD34+Flt3+ MPPs as a percentage of KLS cells in the bone marrow of the mice. All data are from 4 to 5 mice per group, and are representative of 2 independent experiments. Absolute cell numbers are provided in supplemental Figure 4. (C-H) Competitive bone marrow transplantations, with the total bone marrow from Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/− mice mixed in a 1:1 ratio with CD45.1-marked wild-type bone marrow and injected into separate groups of lethally irradiated recipients. The recipient mice were analyzed for the relative contribution of Mysm1-deficient (CD45.2) and wild-type (CD45.1) cells to hematopoiesis at 24 weeks after the reconstitution. (C) Flow cytometry histograms of the bone marrow (top panel) and spleen (middle and bottom panels) of the chimeras gated on CD11b+ myeloid lineage cells, B220+ B cells, and CD4+ T cells, respectively. Histogram gates indicate CD45.1− (Mysm1−/−) and CD45.1+ (wild-type) donor cells. (D-E) Percentage contribution of Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/− cells to (D) myeloid lineage cells (CD11b+) in the recipient bone marrow, B-lineage cells (B220+) in the recipient bone marrow and spleen, and (E) total thymocytes and splenic CD4 and CD8 T cells. (F) Flow cytometry histograms of the bone marrow of the chimeras gated on LT-HSCs (Lin−cKit+Sca1+CD34−Flt3−), ST-HSCs (Lin−cKit+Sca1+CD34+Flt3−), and MPPs (Lin−cKit+Sca1+CD34+Flt3+). Histogram gates indicate CD45.1− (Mysm1−/−) and CD45.1+ (wild-type) donor cells. (G-H) Percentage contribution of Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/− cells to the stem cell and progenitor cell populations in the recipients’ bone marrow, gated based on (G) KLS, CD34, Flt3 or (H) KLS, CD150, CD48 expression. Bars show means ± SEM; *P < .05, **P < .01, ***P < .001 using analysis of variance with the Bonferroni post-hoc test. KLS, Lin−cKit+Sca1+; LT-HSC, long-term HSC; NS, nonsignificant; SEM, standard error of the mean; ST-HSC, short-term HSC.
Loss of p53 rescues Mysm1−/− HSPC numbers and functions. (A) Flow cytometry plots gated on live KLS bone marrow cells and showing the expression of Flt3 and CD34. Stem and progenitor cell gates and population frequencies of representative samples from each group are shown. (B) Lin−cKit+Sca1+CD34−Flt3− LT-HSCs, Lin−cKit+Sca1+CD34+Flt3− ST-HSCs, and Lin−cKit+Sca1+CD34+Flt3+ MPPs as a percentage of KLS cells in the bone marrow of the mice. All data are from 4 to 5 mice per group, and are representative of 2 independent experiments. Absolute cell numbers are provided in supplemental Figure 4. (C-H) Competitive bone marrow transplantations, with the total bone marrow from Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/− mice mixed in a 1:1 ratio with CD45.1-marked wild-type bone marrow and injected into separate groups of lethally irradiated recipients. The recipient mice were analyzed for the relative contribution of Mysm1-deficient (CD45.2) and wild-type (CD45.1) cells to hematopoiesis at 24 weeks after the reconstitution. (C) Flow cytometry histograms of the bone marrow (top panel) and spleen (middle and bottom panels) of the chimeras gated on CD11b+ myeloid lineage cells, B220+ B cells, and CD4+ T cells, respectively. Histogram gates indicate CD45.1− (Mysm1−/−) and CD45.1+ (wild-type) donor cells. (D-E) Percentage contribution of Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/− cells to (D) myeloid lineage cells (CD11b+) in the recipient bone marrow, B-lineage cells (B220+) in the recipient bone marrow and spleen, and (E) total thymocytes and splenic CD4 and CD8 T cells. (F) Flow cytometry histograms of the bone marrow of the chimeras gated on LT-HSCs (Lin−cKit+Sca1+CD34−Flt3−), ST-HSCs (Lin−cKit+Sca1+CD34+Flt3−), and MPPs (Lin−cKit+Sca1+CD34+Flt3+). Histogram gates indicate CD45.1− (Mysm1−/−) and CD45.1+ (wild-type) donor cells. (G-H) Percentage contribution of Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/− cells to the stem cell and progenitor cell populations in the recipients’ bone marrow, gated based on (G) KLS, CD34, Flt3 or (H) KLS, CD150, CD48 expression. Bars show means ± SEM; *P < .05, **P < .01, ***P < .001 using analysis of variance with the Bonferroni post-hoc test. KLS, Lin−cKit+Sca1+; LT-HSC, long-term HSC; NS, nonsignificant; SEM, standard error of the mean; ST-HSC, short-term HSC.
We conducted competitive bone marrow transplantations to test the role of p53 in the loss of long-term functional capacity of Mysm1−/− HSCs. Bone marrow from CD45.2 Mysm1−/−p53+/+, Mysm1−/−p53+/−, or Mysm1−/−p53−/− mice was mixed in a 1:1 ratio with CD45.1-marked wild-type bone marrow and injected into lethally irradiated CD45.1 recipients. The relative contribution of Mysm1-deficient (CD45.2) and wild-type (CD45.1) cells to hematopoiesis was assessed at 8 weeks and 24 weeks. Consistent with previous reports,3,6 Mysm1−/−p53+/+ progenitors were unable to compete with wild-type cells, which indicates a severe loss of HSPC function (Figure 2C-H, supplemental Figure 5C-D). However, inactivation of p53 in the Mysm1−/−p53−/− donors rescued this defect, and even gave Mysm1−/− cells a competitive advantage over wild-type cells. Importantly, Mysm1−/−p53−/− but not control Mysm1−/−p53+/+ cells contributed to the HSC and MPP cell pools in the recipient mice (Figure 2F-H), which demonstrated that p53 inactivation also rescued the self-renewal and differentiation defects of Mysm1-deficient HSCs.
Although these findings implicate p53 as a mediator of Mysm1−/− HSPC defects, the mechanisms leading to p53 activation need to be addressed. One possibility is an indirect mechanism, with p53 activation triggered by the oxidative stress in Mysm1−/− HSPCs.3 However, antioxidant treatment of Mysm1−/− mice did not result in any phenotypic improvement (supplemental Figures 6-7), which suggests that oxidative stress is not the causative factor for p53 activation. This does not preclude the potential role of other p53-activating stress signals that could result from Mysm1 deficiency. Alternatively, because both MYSM1 and p53 are transcriptional regulators, they may be directly coregulating the transcriptional stress response and differentiation programs of HSPCs. Notably, both proteins were independently reported to regulate the expression of the Gfi1 locus.6,11 Therefore, analysis of the cross-talk between MYSM1 and p53 may provide important insights into p53-driven HSPC transcriptional programs with important clinical implications.
The p53 pathway is a critical mediator of pathology in human bone marrow failure syndromes.19-23 Our data suggest that the hematopoietic dysfunction in human MYSM1-deficient patients5 may also be mediated by p53-dependent mechanisms. Increased incidence of lymphoma in our Mysm1−/− mice (supplemental Figure 3) further suggests that the human patients may be similarly predisposed to cancer. The complex relationship between MYSM1 and p53 in carcinogenesis and tumor suppression remains to be explored, and will aid in the optimal application of bone marrow transplantation or chemotherapy in this condition.
Overall, we demonstrate that p53 activation is the common mechanism mediating the diverse hematopoietic and immune-deficiency phenotypes in Mysm1−/− mice. The rescue of Mysm1−/− HSC activity upon loss of p53 further indicates an essential functional interaction between MYSM1 and p53 in regulating stem cell maintenance and differentiation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Tanya Koch, Geneviève Perreault, Patricia D’Arcy, and other staff of McGill Comparative Medicine and Animal Resources Centre (CMARC) for their assistance with colony maintenance, and Su-Jin Park and Shiyang Shen for genotyping.
This work was supported by the Canadian Institutes of Health Research (CIHR; grant 123403 [A.N.]), the Canadian Foundation for Innovation (CFI; grant 29838 [A.N.]), and Startup Funds from the McGill University Faculty of Medicine. A.N. is a Canada Research Chair Tier 2 in Hematopoiesis and Lymphocyte Differentiation (grant 950-228977). M.P. was supported by the Wellcome Trust [079643/Z/06/Z]. D.L. was supported by a postdoctoral fellowship from Fonds de Recherche du Québec–Santé (FRSQ).
Authorship
Contribution: J.I.B. and A.N. performed the experiments; J.C.P. assisted with some experiments; A.N., J.I.B., and D.L. wrote the paper; and A.N., D.L., R.G.J., P.G., and M.P. supervised the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anastasia Nijnik, McGill University, 368 Bellini Life Sciences Complex, 3649 Promenade Sir William Osler, H3G 0B1 Montreal, QC, Canada; e-mail: anastasiya.nyzhnyk@mcgill.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal