Key Points
Vδ1 and Vδ2 T cells promptly reconstitute in children given haploidentical stem cell transplantation depleted of αβ+ T and CD19+ B cells.
Vδ1 cells are expanded in patients experiencing cytomegalovirus reactivation; ZOL potentiates Vδ2 killing against leukemia blasts.
Abstract
We prospectively assessed functional and phenotypic characteristics of γδ T lymphocytes up to 7 months after HLA-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) depleted of αβ+ T cells and CD19+ B cells in 27 children with either malignant or nonmalignant disorders. We demonstrate that (1) γδ T cells are the predominant T-cell population in patients during the first weeks after transplantation, being mainly, albeit not only, derived from cells infused with the graft and expanding in vivo; (2) central-memory cells predominated very early posttransplantation for both Vδ1 and Vδ2 subsets; (3) Vδ1 cells are specifically expanded in patients experiencing cytomegalovirus reactivation and are more cytotoxic compared with those of children who did not experience reactivation; (4) these subsets display a cytotoxic phenotype and degranulate when challenged with primary acute myeloid and lymphoid leukemia blasts; and (5) Vδ2 cells are expanded in vitro after exposure to zoledronic acid (ZOL) and efficiently lyse primary lymphoid and myeloid blasts. This is the first detailed characterization of γδ T cells emerging in peripheral blood of children after CD19+ B-cell and αβ+ T-cell–depleted haplo-HSCT. Our results can be instrumental to the development of clinical trials using ZOL for improving γδ T-cell killing capacity against leukemia cells. This trial was registered at www.clinicaltrials.gov as #NCT01810120.
Introduction
Hematopoietic stem cell transplantation from an HLA-haploidentical relative (haplo-HSCT) offers an immediate transplant treatment virtually to any patient in need of an allograft but lacking a suitable matched donor. A major breakthrough in the history of successful haplo-HSCT was the demonstration that efficient T-cell depletion of the allograft can prevent both acute and chronic graft-versus-host disease (GVHD), even when the donor was a relative differing from the recipient for an entire HLA haplotype.1-4 The therapeutic effect of haplo-HSCT is largely dependent on the graft-versus-leukemia effect exerted by natural killer (NK) cells, which contribute to eradicate leukemia cells surviving the preparative regimen.5-8 Unfortunately, in the haplo-HSCT setting performed through the infusion of positively selected CD34+ cells, the first emergence of fully functioning, killer immunoglobulin-like receptor (KIR)+ alloreactive NK cells from hematopoietic progenitors may require at least 6-8 weeks, and, thus, the benefit offered by their antileukemia effect is relatively delayed.9-12
A promising approach to circumvent such delay in NK-cell immune reconstitution is represented by the use of a recently developed method of graft manipulation based on depletion of the lymphocyte population responsible for GVHD, namely, T lymphocytes carrying the αβ chains of the T-cell receptor (TCR), coupled with B-cell depletion accomplished through the use of an anti-CD19 monoclonal antibody (mAb) to prevent the occurrence of Epstein-Barr virus–related posttransplant lymphoproliferative disorders.9,13-20 This novel approach allows to transfer to the recipient not only high numbers of CD34+ cells and mature donor NK cells, but also TCRγδ+ (from now on, referred to as γδ) T cells, which can exert their protective effect against both leukemia cell regrowth and life-threatening infections. In view of this immunologic rationale, we started a phase 1/2 clinical trial of haplo-HSCT in pediatric patients with either malignant or nonmalignant disorders using this approach of graft manipulation.20
γδ T cells combine conventional adaptive features with rapid, innate-like responses that place them in the initiation phase of immune reactions. In addition, γδ T cells recognize tumor cells without recourse to the classical major histocompatibility complex (MHC) presentation,21 with rare exceptions.22 Among circulating γδ T cells, there is a major subset bearing Vδ2 chain, always associated with Vγ9 (ie, Vγ9Vδ2), and a minor subset bearing Vδ1 chain. Vγ9Vδ2 cells recognize nonpeptide phosphoantigens and kill a wide variety of tumor cells including acute myeloid leukemia (AML) blasts, lymphoma cells, and putative cancer stem cells.21,23-30 Aminobisphosphonates, such as zoledronic acid (ZOL),31,32 activate and expand Vγ9Vδ2 T cells in vitro and sensitize tumor target cells to Vδ2-mediated lysis, their use thus representing an attracting approach for immunotherapeutic strategies against cancer.26,33-36 Vδ1 cells reside within epithelial tissues, especially at sites of cytomegalovirus (CMV) replication, and exert potent cytotoxic effects against acute lymphoblastic leukemia (ALL) or AML cells,37 chronic lymphocytic leukemia cells,38-40 and primary multiple myeloma cells.41
Overall, γδ T lymphocytes are important effector cells, especially in situations where the function of adaptive immunity is impaired, such as those characterizing early immune recovery after haplo-HSCT depleted of αβ T cells and CD19+ B cells. In light of this consideration, we started a prospective study aimed at characterizing, both phenotypically and functionally, γδ T cells recovering in the peripheral blood (PB) of children receiving this type of allograft.
Patients, materials, and methods
Patients and donors
Twenty-seven children, 15 of whom had acute leukemia, were enrolled in a phase 1/2 trial of allogeneic HSCT from an HLA partially matched family donor after TCRαβ/CD19 negative selection (www.clinicaltrials.gov, #NCT01810120). The composition of the graft infused into each child is reported in Table 1. The median number of CD34+ cells and αβ+ and of γδ+ T cells infused per kg of recipient body weight was 19.7 × 106 (range 9-34.7), 0.34 × 105 (range 0.06-2.3), and 7.85 × 106 (range 1.5-94.5), respectively. This clinical trial was approved by the Ethics Committee of the Ospedale Pediatrico Bambino Gesù (Rome, Italy). Ex vivo assays of immune cell phenotype and function were routinely performed until the seventh month after HSCT using 3 to 4 mL of PB. Samples were collected weekly until hospital discharge and monthly during routine follow-up visits at the outpatient clinic. Clinical characteristics of patients included in the study are reported in Table 2. Among the patients included in this study, 55.5% (15/27) experienced CMV reactivation. PB mononuclear cells (PBMCs) were also collected from 24 donors of the 27 patients (donors) and from 22 healthy adults after obtaining informed consent in accordance with the Declaration of Helsinki. In order to compare γδ T-cell reconstitution of our patients with that of children transplanted during past years using CD34+ cells from an HLA-haploidentical relative, we analyzed PBMCs of 9 children given this latter type of allograft collected and stored at 3 (n = 8) and 6 (n = 5) months after HSCT; 4 and 5 children of this subgroup did and did not experience CMV reactivation, respectively.
Graft characteristics
| Patients . | CD34+/kg (106) . | TCR-αβ/kg (106) . | TCR-γδ/kg (106) . | CD56+16+/kg (106) . | CD20+/kg (106) . |
|---|---|---|---|---|---|
| Pt#1 | 14.15 | 0.034 | 8.42 | 27.6 | 0.036 |
| Pt#2 | 20.3 | 0.047 | 7.4 | 64.17 | 0.009 |
| Pt#3 | 28.31 | 0.208 | 94.5 | 78.9 | 0.075 |
| Pt#4 | 9.14 | 0.026 | 3.9 | 54.1 | 0.015 |
| Pt#5 | 20.7 | 0.098 | 1.5 | 22.3 | 0.079 |
| Pt#6 | 13.3 | 0.026 | 2.15 | 13.7 | 0.024 |
| Pt#7 | 16.5 | 0.0995 | 26.8 | 32.2 | 0.15 |
| Pt#8 | 24.9 | 0.037 | 12.9 | 21.0 | 0.15 |
| Pt#9 | 18.9 | 0.042 | 14.77 | 53.7 | 0.055 |
| Pt#10 | 10.7 | 0.055 | 6.48 | 23.3 | 0.05 |
| Pt#11 | 21.6 | 0.09 | 5.8 | 38.2 | 0.02 |
| Pt#12 | 23.66 | 0.19 | 13.0 | 16.4 | 0.056 |
| Pt#13 | 13.7 | 0.023 | 4.9 | 24.6 | 0.06 |
| Pt#14 | 19.7 | 0.006 | 7.4 | 23.93 | 0.03 |
| Pt#15 | 21.4 | 0.006 | 9.1 | 42.9 | 0.012 |
| Pt#16 | 15.8 | 0.026 | 4.05 | 16.2 | 0.013 |
| Pt#17 | 19.86 | 0.004 | 3.12 | 60.4 | 0.04 |
| Pt#18 | 13.28 | 0.0996 | 4.8 | 12.3 | 0.02 |
| Pt#19 | 24.3 | 0.03 | 27.7 | 13.4 | 0.11 |
| Pt#20 | 34.7 | 0.23 | 7.85 | 41.0 | 0.21 |
| Pt#21 | 17.1 | 0.03 | 1.61 | 30.81 | 0.02 |
| Pt#22 | 30.5 | 0.02 | 15.97 | 176.82 | 0.01 |
| Pt#23 | 21.4 | 0.04 | 17.07 | 38.67 | 0.01 |
| Pt#24 | 22.2 | 0.02 | 6.49 | 51.92 | 0.00 |
| Pt#25 | 24.6 | 0.098 | 26.70 | 127 | 0.01 |
| Pt#26 | 14.1 | 0.097 | 6.98 | 24.6 | 0.003 |
| Pt#27 | 14.5 | 0.021 | 3.74 | 22.72 | 0.02 |
| Patients . | CD34+/kg (106) . | TCR-αβ/kg (106) . | TCR-γδ/kg (106) . | CD56+16+/kg (106) . | CD20+/kg (106) . |
|---|---|---|---|---|---|
| Pt#1 | 14.15 | 0.034 | 8.42 | 27.6 | 0.036 |
| Pt#2 | 20.3 | 0.047 | 7.4 | 64.17 | 0.009 |
| Pt#3 | 28.31 | 0.208 | 94.5 | 78.9 | 0.075 |
| Pt#4 | 9.14 | 0.026 | 3.9 | 54.1 | 0.015 |
| Pt#5 | 20.7 | 0.098 | 1.5 | 22.3 | 0.079 |
| Pt#6 | 13.3 | 0.026 | 2.15 | 13.7 | 0.024 |
| Pt#7 | 16.5 | 0.0995 | 26.8 | 32.2 | 0.15 |
| Pt#8 | 24.9 | 0.037 | 12.9 | 21.0 | 0.15 |
| Pt#9 | 18.9 | 0.042 | 14.77 | 53.7 | 0.055 |
| Pt#10 | 10.7 | 0.055 | 6.48 | 23.3 | 0.05 |
| Pt#11 | 21.6 | 0.09 | 5.8 | 38.2 | 0.02 |
| Pt#12 | 23.66 | 0.19 | 13.0 | 16.4 | 0.056 |
| Pt#13 | 13.7 | 0.023 | 4.9 | 24.6 | 0.06 |
| Pt#14 | 19.7 | 0.006 | 7.4 | 23.93 | 0.03 |
| Pt#15 | 21.4 | 0.006 | 9.1 | 42.9 | 0.012 |
| Pt#16 | 15.8 | 0.026 | 4.05 | 16.2 | 0.013 |
| Pt#17 | 19.86 | 0.004 | 3.12 | 60.4 | 0.04 |
| Pt#18 | 13.28 | 0.0996 | 4.8 | 12.3 | 0.02 |
| Pt#19 | 24.3 | 0.03 | 27.7 | 13.4 | 0.11 |
| Pt#20 | 34.7 | 0.23 | 7.85 | 41.0 | 0.21 |
| Pt#21 | 17.1 | 0.03 | 1.61 | 30.81 | 0.02 |
| Pt#22 | 30.5 | 0.02 | 15.97 | 176.82 | 0.01 |
| Pt#23 | 21.4 | 0.04 | 17.07 | 38.67 | 0.01 |
| Pt#24 | 22.2 | 0.02 | 6.49 | 51.92 | 0.00 |
| Pt#25 | 24.6 | 0.098 | 26.70 | 127 | 0.01 |
| Pt#26 | 14.1 | 0.097 | 6.98 | 24.6 | 0.003 |
| Pt#27 | 14.5 | 0.021 | 3.74 | 22.72 | 0.02 |
Patient characteristics
| Patients (gender) . | Age (y) . | Original disease . | Disease status at time of HSCT . | Conditioning regimen . | Duration of study monitoring (days from HSCT) . | CMV serological status (D/R) . | CMV reactivation (first day post-HSCT) . | Status . |
|---|---|---|---|---|---|---|---|---|
| Pt #1 (F) | 17 | BCP-ALL | Second CR | TBI/TT/L-PAM | 215 | +/− | — | Alive |
| Pt #2 (M) | 8 | BCP-ALL | Second CR | TBI/TT/CY | 214 | −/+ | — | Alive |
| Pt #3 (M) | 2 | SCID | NA | TREO/FLU | 512 | +/+ | + (9) | Alive |
| Pt #4 (F) | 11 | AML | First CR | TREO/TT/FLU | 200 | +/+ | + (30) | Alive |
| Pt #5 (M) | 10 | T-ALL | First CR | TBI/TT/FLU | 198 | +/− | + (17) | Alive |
| Pt #6 (F) | 13 | BCP-ALL | Second CR | TBI/TT/FLU | 193 | +/+ | + (27) | Alive |
| Pt #7 (F) | 10 | BCP-ALL | Second CR | TBI/TT/FLU | 216 | +/− | + (30) | Alive |
| Pt #8 (M) | 1 | SCID | NA | TREO/TT/FLU | 240 | +/− | + (13) | Alive |
| Pt #9 (M) | 6 | AML | First CR | BU/CY/L-PAM | 233 | +/+ | + (35) | Alive |
| Pt #10 (M) | 14 | AML | First CR | TBI/TT/FLU | 305 | +/+ | + (13) | Alive |
| Pt #11 (M) | 2 | SDS | NA | TREO/TT/FLU | 370 | +/+ | — | Alive |
| Pt #12 (M) | 7 | T-ALL | Second CR | TBI/TT/FLU | 130 | +/+ | — | Dead because of disease recurrence |
| Pt #13 (F) | 13 | BCP-ALL | Refractory disease | TBI/TT/FLU | 97 | +/− | — | Dead because of disease recurrence |
| Pt #14 (M) | 6 | AML | Refractory disease | TBI/TT/L-PAM | 55 | +/+ | + (37) | Dead because of disease recurrence |
| Pt #15 (M) | <1 | SCID | NA | TREO/FLU | 363 | +/+ | + (14) | Alive |
| Pt #16 (F) | 6 | Fanconi anemia | NA | TBI/CY/FLU | 140 | −/− | — | Alive |
| Pt #17 (M) | 3 | Hyper-IgE syndrome | NA | BU/TT/FLU | 91 | +/+ | — | Alive |
| Pt #18 (M) | 17 | Refractory cytopenia of childhood | Disease present | TREO/TT/FLU | 230 | +/+ | — | Alive |
| Pt #19 (F) | 7 | AML | First CR | BU/TT/FLU | 54 | +/+ | + (8) | Dead because of AdV infection |
| Pt #20 (F) | 1 | Osteopetrosis | NA | BU/FLU | 210 | −/− | — | Alive |
| Pt #21 (M) | 13 | Refractory cytopenia of childhood | Disease present | TREO/TT/FLU | 127 | +/+ | — | Alive |
| Pt #22 (F) | 5 | Fanconi anemia | NA | TBI/CY/FLU | 120 | +/+ | — | Alive |
| Pt #23 (F) | 6 | BCP-ALL | Third CR | TREO/TT/L-PAM | 98 | +/+ | + (28) | Dead because of disease recurrence |
| Pt #24 (F) | <1 | SCID | NA | TREO/FLU | 207 | +/+ | — | Alive |
| Pt #25 (M) | 2 | AML | First CR | BU/CY/L-PAM | 217 | +/+ | + (7) | Alive |
| Pt #26 (F) | <1 | ALL | First CR | BU/TT/FLU | 111 | +/+ | + (19) | Alive |
| Pt #27 (M) | 3 | Kostmann syndrome | NA | TREO/TT/FLU | 236 | +/+ | + (28) | Alive |
| Patients (gender) . | Age (y) . | Original disease . | Disease status at time of HSCT . | Conditioning regimen . | Duration of study monitoring (days from HSCT) . | CMV serological status (D/R) . | CMV reactivation (first day post-HSCT) . | Status . |
|---|---|---|---|---|---|---|---|---|
| Pt #1 (F) | 17 | BCP-ALL | Second CR | TBI/TT/L-PAM | 215 | +/− | — | Alive |
| Pt #2 (M) | 8 | BCP-ALL | Second CR | TBI/TT/CY | 214 | −/+ | — | Alive |
| Pt #3 (M) | 2 | SCID | NA | TREO/FLU | 512 | +/+ | + (9) | Alive |
| Pt #4 (F) | 11 | AML | First CR | TREO/TT/FLU | 200 | +/+ | + (30) | Alive |
| Pt #5 (M) | 10 | T-ALL | First CR | TBI/TT/FLU | 198 | +/− | + (17) | Alive |
| Pt #6 (F) | 13 | BCP-ALL | Second CR | TBI/TT/FLU | 193 | +/+ | + (27) | Alive |
| Pt #7 (F) | 10 | BCP-ALL | Second CR | TBI/TT/FLU | 216 | +/− | + (30) | Alive |
| Pt #8 (M) | 1 | SCID | NA | TREO/TT/FLU | 240 | +/− | + (13) | Alive |
| Pt #9 (M) | 6 | AML | First CR | BU/CY/L-PAM | 233 | +/+ | + (35) | Alive |
| Pt #10 (M) | 14 | AML | First CR | TBI/TT/FLU | 305 | +/+ | + (13) | Alive |
| Pt #11 (M) | 2 | SDS | NA | TREO/TT/FLU | 370 | +/+ | — | Alive |
| Pt #12 (M) | 7 | T-ALL | Second CR | TBI/TT/FLU | 130 | +/+ | — | Dead because of disease recurrence |
| Pt #13 (F) | 13 | BCP-ALL | Refractory disease | TBI/TT/FLU | 97 | +/− | — | Dead because of disease recurrence |
| Pt #14 (M) | 6 | AML | Refractory disease | TBI/TT/L-PAM | 55 | +/+ | + (37) | Dead because of disease recurrence |
| Pt #15 (M) | <1 | SCID | NA | TREO/FLU | 363 | +/+ | + (14) | Alive |
| Pt #16 (F) | 6 | Fanconi anemia | NA | TBI/CY/FLU | 140 | −/− | — | Alive |
| Pt #17 (M) | 3 | Hyper-IgE syndrome | NA | BU/TT/FLU | 91 | +/+ | — | Alive |
| Pt #18 (M) | 17 | Refractory cytopenia of childhood | Disease present | TREO/TT/FLU | 230 | +/+ | — | Alive |
| Pt #19 (F) | 7 | AML | First CR | BU/TT/FLU | 54 | +/+ | + (8) | Dead because of AdV infection |
| Pt #20 (F) | 1 | Osteopetrosis | NA | BU/FLU | 210 | −/− | — | Alive |
| Pt #21 (M) | 13 | Refractory cytopenia of childhood | Disease present | TREO/TT/FLU | 127 | +/+ | — | Alive |
| Pt #22 (F) | 5 | Fanconi anemia | NA | TBI/CY/FLU | 120 | +/+ | — | Alive |
| Pt #23 (F) | 6 | BCP-ALL | Third CR | TREO/TT/L-PAM | 98 | +/+ | + (28) | Dead because of disease recurrence |
| Pt #24 (F) | <1 | SCID | NA | TREO/FLU | 207 | +/+ | — | Alive |
| Pt #25 (M) | 2 | AML | First CR | BU/CY/L-PAM | 217 | +/+ | + (7) | Alive |
| Pt #26 (F) | <1 | ALL | First CR | BU/TT/FLU | 111 | +/+ | + (19) | Alive |
| Pt #27 (M) | 3 | Kostmann syndrome | NA | TREO/TT/FLU | 236 | +/+ | + (28) | Alive |
All patients were given rabbit anti-T cell globulin from day −5 to day −3 and rituximab (200 mg/m2 on day −1). No pharmacologic prophylaxis of GVHD was administered after transplantation.
AdV, adenovirus; BCP-ALL, B-cell precursor ALL; BU, busulfan; CR, complete remission; CY, cyclophosphamide; D, donor; F, female; FLU, fludarabine; L-PAM, melphalan; M, male; NA, not applicable; R, recipient; SCID, severe combined immunodeficiency; SDS, Shwachman-Diamond syndrome; T-ALL, T-cell ALL; TBI, total body irradiation; TREO, treosulfan; TT, thiotepa.
Phenotype of circulating γδ T cells reconstituting after haplo-HSCT
PBMCs were enriched by Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation. Mononuclear cells were labeled with a panel of mAbs (see supplemental Materials available on the Blood Web site). We acquired at least 105 events on Gallios flow cytometer (Beckman Coulter Brea, CA), or MACSQuant-analyzer (Miltenyi-Biotec, Bergisch Gladbach, Germany), and analyzed data using Kaluza analysis software (Beckman Coulter) or FlowJo Version 10 (TreeStar). Staining with different combinations of antibodies allowed us to identify the main γδ T cell subsets: naïve (identified as CD45RO−CD27+), central-memory (CM, CD45RO+CD27+ cells), effector-memory (EM, CD45RO+CD27−), and terminally differentiated (TD, CD45RO−CD27−) Vδ1 and Vδ2 cells. Interferon (IFN) γ production was evaluated after a 3-hour stimulation with calcium ionophore and phorbol-12-myristate-13-acetate.
Expansion of TCR Vγ9Vδ2 T cells
PBMCs obtained from patients 1- 8, 10-13, and 18, at different time points after HSCT, were resuspended in RPMI 1640 supplemented with l-glutamine, penicillin-streptomycin, nonessential amino acids (EuroClone, Milano, Italy), and 5% pooled human AB serum (obtained from Laboratorio Immunoematologia/Medicina Trasfusionale, Istituto Giannina Gaslini). To activate and expand Vγ9Vδ2 T cells, PBMCs were resuspended in complete medium and cultured with 5 μM ZOL (Enzo Life Sciences, Farmingdale, NY) and 50 U/mL interleukin (IL) 2 (Proleukin; Novartis, Basel, Switzerland). Proliferating T cells were maintained in IL-2-containing medium for 10 to 15 days. Cells were characterized for phenotype and cultures containing >90% Vδ2 cells were used for functional studies.
Cytotoxicity assay
A standard 4-hour 51Cr-release assay was performed as described,42 using either γδ T cells freshly purified from HSCT patients using immunomagnetic bead manipulation (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany) or ex vivo activated Vγ9Vδ2 cells (patients 1, 2, 5- 8, 10, and 11) as effector (E) cells; primary AML, T-ALL, or BCP-ALL blasts were used as targets (T).
Primary AML (n = 3), BCP-ALL (n = 4), and T-ALL (n = 4) blasts collected at diagnosis, either freshly isolated or from frozen aliquots, were cultured overnight with either 20 μM ZOL or medium alone. Cells were then washed extensively and labeled with 51Cr. These target cells were then cocultured with effectors in duplicate at different E:T ratios (from 20:1 to 1:1). In some experiments, Vγ9Vδ2 T cells were preincubated with anti-TCR Vγ9 (clone 7A5; Pierce Endogen, Rockford, IL) or anti–NK group 2D blocking mAbs (clone 149810; R&D Systems, Minneapolis, MN) before being added to 51Cr-labeled target cells. To inhibit phosphoantigen-mediated target cell recognition by Vγ9Vδ2 T cells, mevastatin (MEV, 25 μM; Sigma-Aldrich) was added to target cells 6 hours before, during ZOL pretreatment and during cytotoxicity assay. Results are expressed as percent specific lysis, according to the formula %lysis = 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Degranulation assay
Degranulation assay was performed by coculturing 105 effectors and 105 target cells with 3 μL anti-CD107a antibody in 96 V-bottom plates for 3 hours at 37°C.
Effectors were either PBMCs freshly obtained from patients at different time points after transplantation or activated Vγ9Vδ2 cells. Targets were primary AML (n = 4), T-ALL (n = 4), and BCP-ALL (n = 6) blasts, cultured overnight with either 20 μM ZOL or medium alone.
Thereafter, cells were collected, washed in phosphate buffered saline, and stained with anti-CD3, anti-panγδ, anti-Vδ1, anti-Vδ2, and anti-CD107a analyzed in gated Vδ1 or Vδ2 γδ T cells, by flow cytometry. At least 1.5 × 105 events were acquired.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (Software Inc., San Diego, CA). Data distributions were compared using either the Student t test, or the Mann-Whitney or Wilcoxon rank test, whenever appropriate. All statistical tests were 2-tailed. A P value <0.05 was considered to be statistically significant.
Results
Kinetics of γδ T-cell reconstitution in HLA-haplo-HSCT recipients
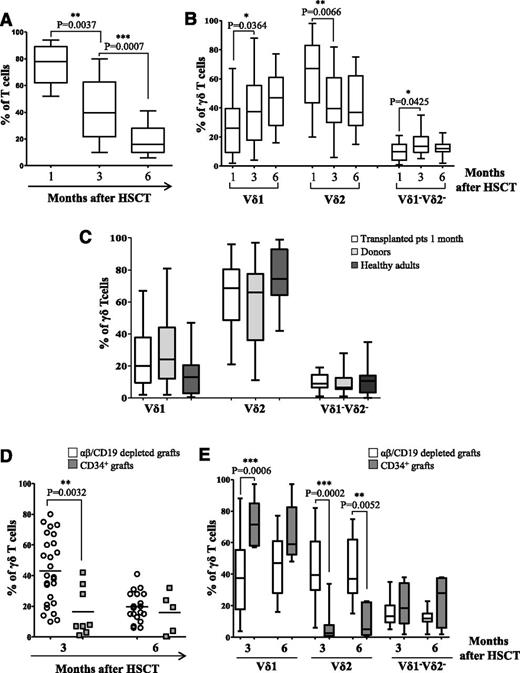
At time of evaluation of γδ T cells, analysis of chimerism on PBMCs using short tandem repeats showed only the presence of donor cells. Flow cytometry analyses performed on PB samples collected 2 to 3 weeks after HSCT showed that T lymphocytes were predominantly of the γδ T-cell lineage (mean 91.5% of gated CD3+ lymphocytes, range from 70% to 100%). Subsequently, the αβ T-cell population gradually increased, and the γδ T-cell population decreased over time (Figure 1A). The percentage of αβ T cells exceeded that of γδ T cells already 1 month after HSCT in 4 patients (14.8%), between 1 and 2 months in 8 patients (29.6%), and after 2 months in 12 patients (44.4%). The remaining 3 patients died before the percentage of αβ T cells exceeded that of γδ T cells. We found a correlation between the number of γδ infused and time of reconstitution of the αβ population (**P = .0098), because the more γδ T cells were infused, the later the percentage of αβ T cells exceeded that of γδ T cells. In addition, a significant increase in the Vδ1 and Vδ1−Vδ2− subsets, paralleled by a decrease of the Vδ2 cells, was observed between 1 and 3 months after HSCT (Figure 1B).
Phenotype of γδ T cells emerging in recipients of haplo-HSCT. Flow cytometry analyses of γδ (gated in CD3+ T cells) (A) and of Vδ1, Vδ2, and Vδ1−Vδ2− cells (gated in CD3+γδ+ T cells) (B) 1, 3, and 6 months after HSCT. (C) Percentage of Vδ1, Vδ2, and Vδ1−Vδ2− subsets was evaluated by flow cytometry in transplanted patients 1 month after HSCT, and compared with those found either in their donors or in healthy adults. Comparative analyses of γδ T cells (D) and Vδ1, Vδ2, and Vδ1−Vδ2− subsets (E) in patients transplanted with either αβ/CD19 depleted grafts or with CD34+ positively selected cells at 3 and 6 months after transplantation. Pooled results are shown. Whisker lines represent highest and lowest values; horizontal lines represent median values.
Phenotype of γδ T cells emerging in recipients of haplo-HSCT. Flow cytometry analyses of γδ (gated in CD3+ T cells) (A) and of Vδ1, Vδ2, and Vδ1−Vδ2− cells (gated in CD3+γδ+ T cells) (B) 1, 3, and 6 months after HSCT. (C) Percentage of Vδ1, Vδ2, and Vδ1−Vδ2− subsets was evaluated by flow cytometry in transplanted patients 1 month after HSCT, and compared with those found either in their donors or in healthy adults. Comparative analyses of γδ T cells (D) and Vδ1, Vδ2, and Vδ1−Vδ2− subsets (E) in patients transplanted with either αβ/CD19 depleted grafts or with CD34+ positively selected cells at 3 and 6 months after transplantation. Pooled results are shown. Whisker lines represent highest and lowest values; horizontal lines represent median values.
Detailed phenotypic characterization revealed that, in all individuals, γδ T cells included 3 different populations, which were mainly Vδ2, Vδ1, and, more marginally, Vδ1−Vδ2−. One month after haplo-HSCT, γδ T lymphocytes were mainly Vδ2 (mean 65%) and, to a lesser extent, Vδ1 (mean 25%) and Vδ1−Vδ2− (mean 9.6%, Figure 1B). The proportion of the different γδ T-cell populations in transplanted patients did not differ significantly from that present in the donors and in healthy adults (Figure 1C). When we compared the γδ T-cell reconstitution in our patients transplanted using αβ/CD19-depleted grafts with that of children that had been transplanted with positively selected CD34+ cells, we found that at month 3 after HSCT the former had a significant higher percentage of γδ T cells and of the Vδ2 subset, paralleled by a significant lower proportion of Vδ1 cells. Altogether, these findings highlight the peculiarity of γδ T-cell reconstitution in patients given haplo-HSCT depleted of αβ T cells and CD19+ B cells (Figure 1D-E).
Analysis of differentiation status of γδ T cells
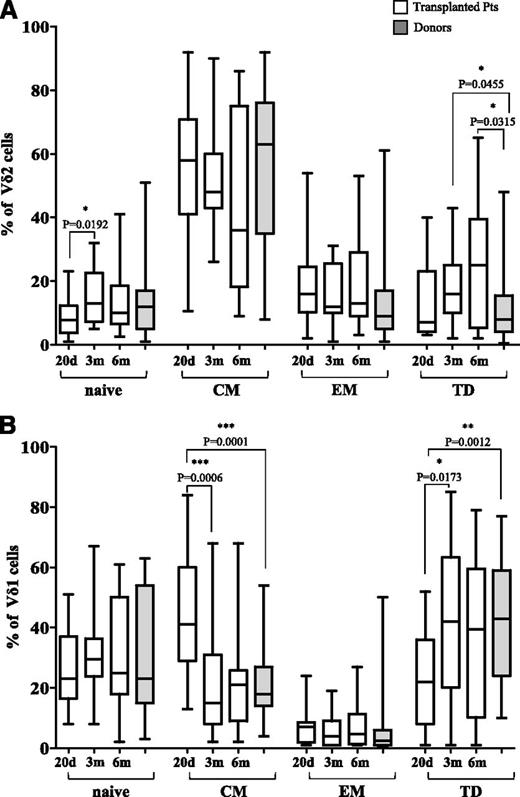
Analyses of Vδ2 and Vδ1 subsets in patients showed that, on day +20 after HSCT, 8.97% of Vδ2 lymphocytes were naïve, 54.38% were CM, 18.73% EM, and 13.26% TD (Figure 2A). The percentage of naïve Vδ2 cells, but not that of the other Vδ2 subsets, significantly increased between day +20 and 3 months after HSCT (Figure 2A). Comparison of Vδ2 subsets present in haplo-HSCT donors (gray plot) and in patients revealed no differences in naïve, CM, and EM subsets at day +20 and 3 and 6 months after transplantation, whereas a higher percentage of TD cells were observed in patients at 3 and 6 months after HSCT than in donors (Figure 2A).
Analysis of γδ differentiation subsets. Percentage of naïve, CM, EM, and TD subsets was evaluated by flow cytometry in gated CD3+γδ+Vδ2+T cells (A) or gated CD3+γδ+Vδ1+T cells (B) at 20 days (d) and 3 and 6 months (m) post-HSCT. Gray plots show results from haplo-HSCT donors. Pooled results are shown. Whisker lines represent highest and lowest values; horizontal lines represent median values.
Analysis of γδ differentiation subsets. Percentage of naïve, CM, EM, and TD subsets was evaluated by flow cytometry in gated CD3+γδ+Vδ2+T cells (A) or gated CD3+γδ+Vδ1+T cells (B) at 20 days (d) and 3 and 6 months (m) post-HSCT. Gray plots show results from haplo-HSCT donors. Pooled results are shown. Whisker lines represent highest and lowest values; horizontal lines represent median values.
In addition, 26% of Vδ1 cells were naïve, 45.34% CM, 7.03% EM, and 21.21% TD (Figure 2B). The proportions of CM Vδ1 cells significantly decreased and the TD Vδ1 subset increased between day +20 and 3 months after HSCT, whereas no significant changes were observed in naïve and EM subsets (Figure 2B). No significant changes were documented in any Vδ2 or Vδ1 subsets between 6 and 7 months after HSCT (data not shown). Comparison of Vδ1 subsets between haplo-HSCT donors and patients showed that the latter at day +20 after HSCT had a significantly higher percentage of the CM subset and a significantly lower proportion of TD subset (Figure 2B).
Peculiarities of γδ T cells in transplanted patients experiencing CMV reactivation
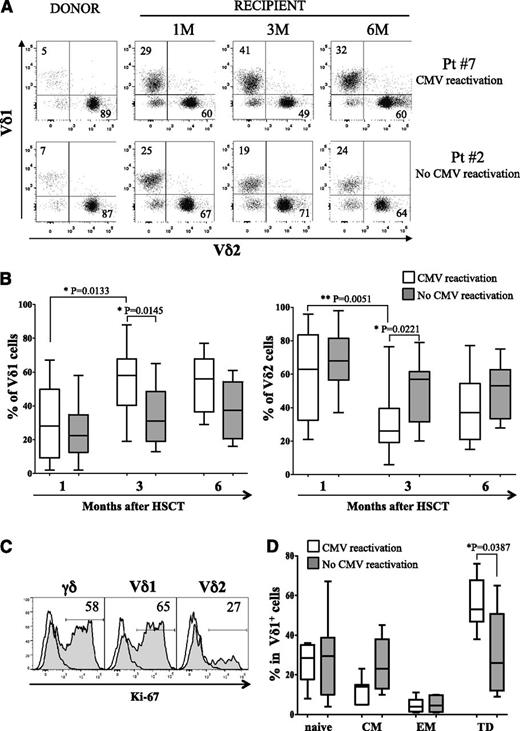
It was recently reported41,43 that CMV reactivation after allo-HSCT is associated with an expansion of Vδ2− γδ T cells, which are able to recognize both hematologic tumor cell lines and primary AML blasts. Thus, we investigated whether this observation holds true also for children given haplo-HSCT depleted of αβ+ T cells and CD19+ B cells. Figure 3A shows γδ T-cell subset distribution in 2 representative patients at various time points after HSCT compared with their respective donors: patient 7 who did experience CMV reactivation at day +30 and patient 2 who did not. A relative increase of the Vδ1 subset was clearly documented at the third month in patient 7, whereas subset composition did not change with time in patient 2. Patients experiencing CMV reactivation (n = 15) showed an expansion, between 1 and 3 months after HSCT, of the Vδ1 T-cell population (Figure 3B, left panel), which was more represented than the Vδ2 subset, which was seen decreasing at the same time point (Figure 3B, right panel). Indeed, this preferential expansion of Vδ1 subset could be confirmed by analysis of Ki-67 expression. In the representative patient 6, who was examined 39 days after CMV reactivation, the Vδ1 subset, corresponding to the majority of γδ T cells, was 65% Ki-67+; the Vδ2 subset was 27% Ki-67+ (Figure 3C). Ki-67 expression on NK or non-γδ T cells, as well as gating strategy and lymphocyte composition, is shown in supplemental Figure 1. Remarkably, a significant increase of the Vδ1 TD subset at 3 months after HSCT was observed in patients that experienced CMV as compared with those that did not (Figure 3D). It is noteworthy that expansion of Vδ1 cells was specifically related to CMV reactivation and not to other viral infections, including human herpes virus-6 as in patient 1 or AdV as in patient 2 (as shown in Figure 3A) and patient 13. These findings are in agreement with the results reported by Knight et al.41
Circulating γδ T cells in patients experiencing CMV reactivation. (A) Vδ1 and Vδ2 subsets (percentages are indicated) were identified in gated CD45+CD3+γδ+ cells by flow cytometry using PBMCs from 2 representative cases, evaluating both donors and patients 1, 3, and 6 months post-HSCT. Patient 7 did experience CMV reactivation, whereas patient 2 did not. (B) Comparative analysis of Vδ1 (left panel) and Vδ2 (right panel) T cells was performed in patients who did experience CMV reactivation (white plots) and in those who did not (gray plots). Vδ1 and Vδ2 cells were identified in gated CD45+CD3+γδ+ cells. (C) Intranuclear expression of Ki-67 (filled profiles) shows proliferating status in CD45+CD3+γδ+, CD45+CD3+γδ+Vδ1+, and CD45+CD3+γδ+Vδ2+ cells. Empty profiles represent staining with isotype control. (D) Percentage of naïve, CM, EM, and TD was evaluated by flow cytometry in gated CD45+CD3+γδ+Vδ1+ T cells collected from patients who did experience CMV reactivation (white plots) and in those who did not (gray plots) 3 months after HSCT. Pooled results are shown. Whisker lines represent the highest and lowest values; horizontal lines represent median values.
Circulating γδ T cells in patients experiencing CMV reactivation. (A) Vδ1 and Vδ2 subsets (percentages are indicated) were identified in gated CD45+CD3+γδ+ cells by flow cytometry using PBMCs from 2 representative cases, evaluating both donors and patients 1, 3, and 6 months post-HSCT. Patient 7 did experience CMV reactivation, whereas patient 2 did not. (B) Comparative analysis of Vδ1 (left panel) and Vδ2 (right panel) T cells was performed in patients who did experience CMV reactivation (white plots) and in those who did not (gray plots). Vδ1 and Vδ2 cells were identified in gated CD45+CD3+γδ+ cells. (C) Intranuclear expression of Ki-67 (filled profiles) shows proliferating status in CD45+CD3+γδ+, CD45+CD3+γδ+Vδ1+, and CD45+CD3+γδ+Vδ2+ cells. Empty profiles represent staining with isotype control. (D) Percentage of naïve, CM, EM, and TD was evaluated by flow cytometry in gated CD45+CD3+γδ+Vδ1+ T cells collected from patients who did experience CMV reactivation (white plots) and in those who did not (gray plots) 3 months after HSCT. Pooled results are shown. Whisker lines represent the highest and lowest values; horizontal lines represent median values.
It can also be mentioned that CMV serological status in the donors (20 positive vs 3 negative) did not significantly influence the relative proportions of the different naïve vs memory subsets among Vδ1 and Vδ2 cells (supplemental Figure 2).
γδ T cells from haplo-HSCT recipients expand in vitro upon exposure to ZOL, which also enhances killing of primary leukemia blasts
We investigated the effect of in vitro exposure to ZOL on γδ T cells collected from 13 patients at different time points from HSCT. As shown in supplemental Figure 4A, in the presence of ZOL and IL-2, γδ T cells consistently expanded in vitro, and the resultant population was Vγ9Vδ2 (supplemental Figure 4Aiv and supplemental Figure 3). As already reported in healthy subjects and in cancer patients,25-27,44,45 also in recipients of haplo-HSCT the ZOL-expanded Vγ9Vδ2 population had an EM/CM phenotype (supplemental Figure 4B).27,46
We next tested the cytotoxic activity of activated Vγ9Vδ2 T cells against primary acute leukemia cells of both myeloid and lymphoid origin.
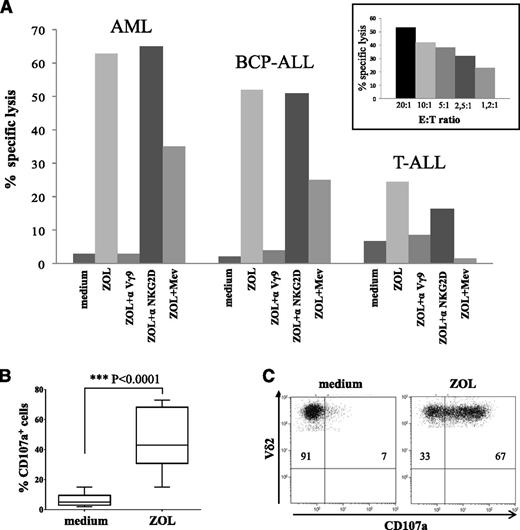
51Cr-release assay, performed with ZOL-expanded Vγ9Vδ2 cells from 8 patients, revealed that these cells lysed primary leukemia cells with low efficiency; however, their lytic capacity was strongly enhanced by sensitizing leukemic target cells with ZOL (1 representative experiment is shown in Figure 4A). Superimposable results were obtained using either primary myeloid or T-ALL and BCP-ALL cells as targets. The mean percentage of specific lysis at 20:1 E:T ratio was 65 ± 6% against AML, 68 ± 8% against BCP-ALL, and 33 ± 4% against T-ALL cells, when targets were pretreated with ZOL. By contrast, the mean percentage of specific lysis at 20:1 E:T ratio was <10% when target blasts were untreated. Spontaneous release did not exceed 20% of maximum release, suggesting that ZOL preincubation did not induce direct cytotoxicity of leukemia blasts. Cytotoxicity was unambiguously TCRγδ mediated, as proved by the inhibition of target cell lysis when Vγ9Vδ2 cells were pretreated with a Vγ9 blocking mAb (Figure 4A). By contrast, blocking of NKG2D did not modify γδ T-cell cytotoxicity (Figure 4A). In addition, incubation of target leukemia cells with MEV, which prevents ZOL-induced isopentenyl-pyrophosphate intracellular accumulation, during ZOL treatment, dampened Vγ9Vδ2 T-cell–mediated lysis (Figure 4A), indicating that cytotoxicity was dependent on phosphoantigen recognition.
Cytotoxic activity of γδ T cells after in vitro expansion with ZOL. (A) Cytotoxicity of Vγ9Vδ2 cells against AML, BCP-ALL, or T-ALL blasts at 20:1 E:T ratio is shown. One representative experiment using cells collected from patient 2 is reported. Vγ9Vδ2 cells were tested against leukemic blasts left untreated (medium), ZOL sensitized (ZOL), or treated with ZOL and MEV (ZOL+Mev). In some experiments, Vγ9Vδ2 cells were preincubated with an anti-TCR Vγ9 (ZOL+αVγ9) or an anti-NKG2D (ZOL+αNKG2D) mAb. Inset shows 1 representative experiment performed at different E:T ratios (from 20:1 to 1.2:1) using Vγ9Vδ2 cells collected from patient 2 against 1 AML sample treated with ZOL. (B) CD107a degranulation assay was performed using Vγ9Vδ2 cells (n = 10) against myeloid and lymphoid leukemia cells untreated (medium) or ZOL sensitized. Pooled results are shown. Whisker lines represent the highest and lowest values; horizontal lines represent median values. (C) One representative experiment with Vγ9Vδ2 T cells after in vitro expansion of PBMCs from patient 6. Double staining with anti-Vδ2 phycoerythrin and anti-CD107a allophycocyanin is shown on gated γδ+Vδ2+ T cells.
Cytotoxic activity of γδ T cells after in vitro expansion with ZOL. (A) Cytotoxicity of Vγ9Vδ2 cells against AML, BCP-ALL, or T-ALL blasts at 20:1 E:T ratio is shown. One representative experiment using cells collected from patient 2 is reported. Vγ9Vδ2 cells were tested against leukemic blasts left untreated (medium), ZOL sensitized (ZOL), or treated with ZOL and MEV (ZOL+Mev). In some experiments, Vγ9Vδ2 cells were preincubated with an anti-TCR Vγ9 (ZOL+αVγ9) or an anti-NKG2D (ZOL+αNKG2D) mAb. Inset shows 1 representative experiment performed at different E:T ratios (from 20:1 to 1.2:1) using Vγ9Vδ2 cells collected from patient 2 against 1 AML sample treated with ZOL. (B) CD107a degranulation assay was performed using Vγ9Vδ2 cells (n = 10) against myeloid and lymphoid leukemia cells untreated (medium) or ZOL sensitized. Pooled results are shown. Whisker lines represent the highest and lowest values; horizontal lines represent median values. (C) One representative experiment with Vγ9Vδ2 T cells after in vitro expansion of PBMCs from patient 6. Double staining with anti-Vδ2 phycoerythrin and anti-CD107a allophycocyanin is shown on gated γδ+Vδ2+ T cells.
Finally, killing of primary leukemic blasts by ZOL-activated Vγ9Vδ2 T cells was found to depend on granule-mediated mechanisms, as revealed by induction of surface expression of CD107a on Vγ9Vδ2 T lymphocytes cocultured with ZOL-pretreated myeloid or lymphoid leukemia cells (Figure 4B-C), in keeping with that observed in the 51Cr-release assay.
γδ T cells freshly isolated from transplanted patients degranulate in coculture with primary leukemia cells
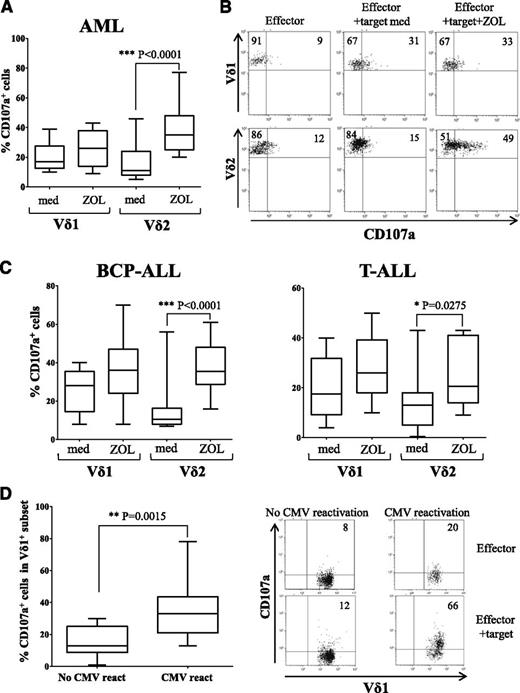
We investigated the expression levels of cytotoxic molecules in γδ T cells freshly collected from transplanted patients at different time points after haplo-HSCT. Circulating γδ T cells collected from haplo-HSCT expressed perforin, granzyme-B, and, upon calcium ionophore/phorbol-12-myristate-13-acetate, also IFN-γ, indicating that these cells are fully functional to lyse target cells (supplemental Figure 5). The non-γδ T- cell population that expressed perforin (supplemental Figure 6), and IFN-γ (supplemental Figure 7) are mainly NK cells and a proportion of αβ T lymphocytes. Next, we tested whether γδ T cells degranulate upon challenge with primary leukemia blasts, through the analysis of CD107a surface expression. CD107a is a lysosomal protein that colocalizes with perforin in lytic granules of cytotoxic cells, and its surface expression reflects exocytosis of lytic granules, thus representing a surrogate assay for cytotoxicity.47 For this purpose, AML or ALL blasts, either untreated or treated with ZOL, were cocultured with PBMCs freshly isolated from patients. The percentage of γδ T cells in patients’ PBMCs ranged from 0.5% to 5%. In these experimental conditions, both Vδ1 and Vδ2 cells cultured with AML blasts expressed CD107a on the cell surface (mean percentage of CD107+ cells in gated Vδ1 subset = 20.2, mean percentage of CD107+ cells in gated Vδ2 subset = 15.3). As shown in Figure 5A, pretreatment of AML cells with ZOL markedly increased the proportion of CD107a+ cells in gated Vδ2 (mean percentage = 37), but not in Vδ1 cells (mean percentage = 25.8). These findings indicate that ZOL-induced phosphoantigens on target leukemia cells are selectively recognized by fresh Vδ2 cells, consistent with results obtained with activated Vγ9Vδ2 (as discussed previously). One representative experiment is shown in Figure 5B. Similar results were obtained testing BCP-ALL and T-ALL cells as targets (Figure 5C). In particular, using untreated BCP-ALL as targets, the mean percentage of CD107+ cells in gated Vδ1 subset was 25.8%, whereas that detected in gated Vδ2 cells was 16.2%. When BCP-ALL blasts were pretreated with ZOL, the mean percentage of CD107+ cells in gated Vδ1 cells was 35.3, whereas that in gated Vδ2 cells was 37.2. Likewise, upon incubation with T-ALL blasts, the mean percentage of CD107+ cells in gated Vδ1 subset was 19.9, and that in Vδ2 cells was 13.3; when T-ALL blasts were pretreated with ZOL, the mean percentage of CD107+ cells in gated Vδ1 subset was 28.1, whereas that in Vδ2 cells was 24.3.
Cytotoxicity of circulating γδ T cells. (A) CD107a expression in gated γδ+Vδ1+ and γδ+Vδ2+ T cells of PBMCs from patients cultured with AML blasts untreated (medium) or ZOL sensitized was investigated by flow cytometry. (B) One representative experiment using PBMCs obtained from patient 7. (C) Flow cytometric expression of CD107a in gated γδ+Vδ1+ and Vδ2+ T cells in patients’ PBMCs upon coculture with either BCP-ALL or T-ALL blasts, either untreated (medium) or ZOL sensitized. (D) CD107a expression in gated CD3+γδ+Vδ1+ cells from PBMCs collected from patients who did experience CMV reactivation (n = 10) and those who did not (n = 10) was evaluated. Pooled results are shown. Whisker lines represent the highest and lowest values; horizontal lines represent median values. One representative experiment performed with PBMCs from patients 18 and 9 is shown in dot plots in the right.
Cytotoxicity of circulating γδ T cells. (A) CD107a expression in gated γδ+Vδ1+ and γδ+Vδ2+ T cells of PBMCs from patients cultured with AML blasts untreated (medium) or ZOL sensitized was investigated by flow cytometry. (B) One representative experiment using PBMCs obtained from patient 7. (C) Flow cytometric expression of CD107a in gated γδ+Vδ1+ and Vδ2+ T cells in patients’ PBMCs upon coculture with either BCP-ALL or T-ALL blasts, either untreated (medium) or ZOL sensitized. (D) CD107a expression in gated CD3+γδ+Vδ1+ cells from PBMCs collected from patients who did experience CMV reactivation (n = 10) and those who did not (n = 10) was evaluated. Pooled results are shown. Whisker lines represent the highest and lowest values; horizontal lines represent median values. One representative experiment performed with PBMCs from patients 18 and 9 is shown in dot plots in the right.
Finally, we investigated whether the cytotoxic activity against primary acute leukemia blasts of the Vδ1 population from patients that experienced CMV reactivation differed from that of Vδ1 cells obtained from patients that did not reactivate CMV infection. We found that CD107a expression was significantly higher in Vδ1 subset from patients that experienced CMV reactivation (Figure 5D), suggesting that this γδ T-cell subset was not only expanded, but also functionally activated by CMV infection, in keeping with the phenotypic profile data.
Discussion
In recent years, many studies have highlighted the importance of human γδ T cells in antiviral immunity and antitumor surveillance.21 These lymphocytes are important effector cells21,48-50 that (1) cross talk with other immune cells, including NK lymphocytes, αβ+ T cells and dendritic cells21 ; (2) recognize tumor cells in a MHC-independent manner; (3) participate in anti-CMV responses, particularly when conventional adaptive immunity is insufficient to clear the viral infection; and (4) do not initiate GVHD, because they do not recognize antigens presented on MHC. These features are of particular importance in the context of haplo-HSCT depleted of αβ+ T and CD19+ B cells because the only mature immune-competent effector cells transferred with the graft are NK and γδ T cells.
We focused our study on the kinetics and function of reconstituting peripheral γδ T cells in pediatric patients receiving this type of graft, with particular regard to their ability to kill primary acute leukemia cells.
We demonstrated that both Vδ1 and Vδ2 γδ T cells promptly reconstitute in children given haplo-HSCT depleted of αβ T and CD19 B cells, with a prevalence of the Vδ2 subset, and that these γδ T-cell subsets express a phenotype similar to that found in the haplo-HSCT donors. It is important to note that a significant increase of the naïve Vδ2 subset was observed to occur from day +20 to day +90 after haplo-HSCT, thus suggesting that the γδ T cells circulating in transplanted patients were not only mature cells derived from the graft, but also γδ T cells differentiating from donor hematopoietic stem cells in patients. The cytotoxic potential of reconstituted γδ T cells is supported by the expression of granzyme B, perforin, and IFN-γ as well as by functional studies. However, strong differences were observed in γδ T cells recovering in patients that experienced CMV reactivation as compared with those found in patients who did not reactivate CMV infection. In fact, we found in patients with CMV reactivation a significant expansion of Vδ1 T-cell subset with cytotoxic TD phenotype. This finding is in keeping with previously published data in recipients of unmanipulated, HLA-matched allo-HSCT or in healthy individuals in which CMV infection was associated with marked in vivo expansion of CMV-reactive Vδ2− γδ T cells.41,43,51-53 More importantly, Scheper and coworkers reported that these cells can cross-react with tumor cells of hematopoietic origin, thus explaining the protecting effect of CMV reactivation on the risk of leukemia relapse.43 It is noteworthy that CMV reactivation induces a similar activation/rapid maturation of NK cells in adult and pediatric patients given either intrabone or IV cord blood transplantation,54,55 indicating that a parallel CMV-induced activation of γδ and NK cells may be relevant in haplo-HSCT and is associated with a decreased risk of relapse.56
In our study, we unambiguously demonstrated that the Vδ1 T-cell subset was cytotoxic against primary ALL and AML cells, and that such activity was greater in Vδ1 lymphocytes obtained from patients who experienced CMV reactivation. However, the specific ligands on leukemia cells recognized by Vδ1 cells remain to be identified. In this respect, a prominent role in recognition of tumor cells by Vδ1 cells is played by activating receptors such as NKG2D,57 natural cytotoxicity receptors such as NKp30,38 and DNAX accessory molecule-1,58 in addition to TCR-dependent pathways.
We also demonstrated that Vδ2 cells reconstituting in patients after haplo-HSCT may be efficiently expanded in vitro by ZOL, giving rise to an EM lymphocyte population cytotoxic against primary AML and ALL blasts. Such activity was clearly dependent on the level of phosphoantigens expressed by leukemia cells and on TCR Vγ9 recognition, as demonstrated by the inhibition of leukemia cell lysis upon treatment of target cells with MEV or with an anti-Vγ9 blocking mAb, respectively.
Taken together, our results on circulating γδ T cells that reconstitute in patients receiving haplo-HSCT depleted of αβ+ T lymphocytes and CD19+ B cells, have a potential translational relevance, because they pave the way to novel therapeutic approaches for patients affected by acute leukemia. In particular, we provide a biological rationale for the development of clinical trials based on in vivo administration of ZOL in the posttransplantation period, with the aim of improving γδ T-cell cytotoxic capacity against leukemia cells. In this regard, it has to be noted that most immunotherapeutic approaches using γδ T cells and ZOL were conducted in patients with solid tumor, whereas only 3 studies were conducted in patients with hematologic disorders.35,59,60 Two of the latter were performed by combining low-dose IL-2 together with ZOL to activate autologous γδ T cells in patients with various hematologic disorders, who had not received an allograft.35,60 Treatment was generally well tolerated, and clinical responses, including partial remission and disease stabilization, were observed in both trials.
In summary, our study provides the first detailed characterization of reconstitution of γδ T-cell subsets in children given haplo-HSCT depleted of αβ+ T and CD19+ B cells. γδ T-cell recovery is significantly influenced by CMV reactivation, this biological finding potentially contributing to explain the reported protective effect of viral reactivation on the risk of AML relapse. Whether the favorable effect played by ZOL exposure of both γδ T cells recovering after transplantation and blasts escaping the cytotoxic effect of conditioning regimen translates into a clinical benefit for patients with leukemia remains to be proved in properly designed clinical trials.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from AIRC (IG-13018 [I.A.], Special Grant “5xmille”-9962 [F. Locatelli, L.M., and A.M.], “My first AIRC” grant 15925 [A.B.]); Ministero della Salute (RF-2010-2308270 [I.A.], RF-2010-2316606 [F. Locatelli, L.M., and D. Pende], Grant “5xmille” 2011 [D. Pende], Ricerca Corrente [V.P. and F. Locatelli]); and Ministero dell’Istruzione, Università e Ricerca (Grant Progetto di Rilevante Interesse Nazionale, PRIN [F. Locatelli]). C.C. is a Fondazione Veronesi fellow. Thanks to Dr. Sergio Rutella for critical reading of the manuscript.
Authorship
Contribution: I.A. designed the study, analyzed and interpreted data, and wrote the manuscript; A.B. performed transplantation, contributed to study design, collected data, and followed patients; I.P. expanded γδ T cells in vitro and performed cytotoxic assays; A.Z. performed phenotypic and functional studies in all patients and purified γδ T cells; D. Pende provided samples, analyzed and interpreted data, and revised the manuscript; C.C. set up the panel and combination of antibodies to use throughout the study and performed staining for cytotoxic molecules; R.M. and F. Loiacono performed phenotypic analysis of donors and evaluated Ki-67 expression; G.B. performed phenotypic analyses; M.E.B., D. Pagliara, and B.L. performed transplantation and collected data; A.M., L.M., and V.P. supervised the study and revised the manuscript; and F. Locatelli designed the study, interpreted data, performed transplantation, and wrote the manuscript.
Conflict-of-interest disclosure: A.M. is a cofounder and shareholder of Innate-Pharma (Marseille, France). The remaining authors declare no competing financial interests.
Correspondence: Irma Airoldi, Laboratorio di Oncologia, Istituto Giannina Gaslini, Via G. Gaslini 1, 16147 Genova, Italy; e-mail: irmaairoldi@ospedale-gaslini.ge.it.
References
Author notes
I.A. and A.B. contributed equally to this study.
L.M. and F.L. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal