Key Points
Closed head trauma sequentially releases tPA followed by uPA from injured brain.
Increased uPA is responsible for delayed intracerebral hemorrhage, which is prevented by a tPA variant that inhibits uPA activity.
Abstract
Persistent intracerebral hemorrhage (ICH) is a major cause of death and disability after traumatic brain injury (TBI) for which no medical treatment is available. Delayed bleeding is often ascribed to consumptive coagulopathy initiated by exposed brain tissue factor. We examined an alternative hypothesis, namely, that marked release of tissue-type plasminogen activator (tPA) followed by delayed synthesis and release of urokinase plasminogen activator (uPA) from injured brain leads to posttraumatic bleeding by causing premature clot lysis. Using a murine model of severe TBI, we found that ICH is reduced in tPA−/− and uPA−/− mice but increased in PAI-1−/− mice compared with wild-type (WT) mice. tPA−/−, but not uPA−/−, mice developed a systemic coagulopathy post-TBI. Tranexamic acid inhibited ICH expansion in uPA−/−mice but not in tPA−/− mice. Catalytically inactive tPA-S481A inhibited plasminogen activation by tPA and uPA, attenuated ICH, lowered plasma d-dimers, lessened thrombocytopenia, and improved neurologic outcome in WT, tPA−/−, and uPA−/− mice. ICH expansion was also inhibited by tPA-S481A in WT mice anticoagulated with warfarin. These data demonstrate that protracted endogenous fibrinolysis induced by TBI is primarily responsible for persistent ICH and post-TBI coagulopathy in this model and offer a novel approach to interrupt bleeding.
Introduction
Traumatic brain injury (TBI) is a leading cause of death and a major cause of sustained disability in young adults.1,2 Delayed intracerebral hemorrhage (ICH) is a strong predictor of morbidity and mortality.3-5 Serial computed tomography scans reveal expansion of ICH in ∼50% of patients post-TBI,6-8 but intervention based on the current presumptive pathophysiology is often futile.
It generally accepted that post-TBI bleeding results from a consumptive coagulopathy caused by exposure of the injured brain to tissue factor, followed by secondary fibrinolysis.3,9,10 In support of this concept, high levels of d-dimers, prolongation of clotting times, thrombocytopenia, and increased plasmin/anti-plasmin complexes correlate with increased mortality.7,11,12 However, measures that attenuate the consumptive coagulopathy fail to reduce mortality and/or disability.9,10 Thus, important gaps in our understanding of the pathophysiology and management of post-TBI bleeding persist13,14 and there is continued need to examine other possible causes.
One potential explanation for the dichotomy between the correlation of consumptive coagulopathy with poor outcome and the futility of its management may be that TBI also initiates fibrinolysis by releasing tissue-type plasminogen activator (tPA)15 and urokinase plasminogen activator (uPA)16 from contusional brain tissue. Plasminogen activators (PAs) play important roles in neurotransmission,17-19 postinjury neuronal apoptosis,20,21 and regulation of cerebrovascular reactivity,15,16,22 but their contributions to ICH have received less attention.
We hypothesized that a rapid, protracted, and striking increase in the local concentration of endogenous PAs is primarily responsible for exuberant local fibrinolysis at sites of trauma, leading to premature clot lysis and expansion of ICH. Our studies in tPA−/−, uPA−/−, and PAI-1−/− mice show that both PAs contribute to enhanced ICH after TBI. A catalytically inactive tPA variant that blocks both tPA and uPA catalytic activities attenuated ICH, prevented the development of systemic coagulopathy, and improved neurologic outcome in a murine model of severe TBI. The effectiveness of this variant provides new insights into the pathogenesis and, potentially, treatment of delayed ICH after TBI.
Methods
Generation of PAs
Wild-type (WT)-tPA, tPA-S481A, and uPA were cloned, purified, and characterized as previously described.23 Briefly, complementary DNAs encoding mature human PAs were cloned into the pMT/BiP/V5-HisA plasmid (Invitrogen, Carlsbad, CA). To generate the catalytically inactive tPA (S481A) variant, the mutation was introduced in WT-tPA by polymerase chain reaction using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA), and the complete sequence was verified. The protein contains 2 extra amino acids (RS- at the NH2 terminus), resulting from introduction of the Bgl II cloning site. Proteins were expressed in the S2 Drosophila Expression System (Invitrogen) according to the manufacturer’s protocol and purified by antibody affinity chromatography using anti-PA coupled to cyanogen bromide–activated Sepharose. The final products migrated as single bands on sodium dodecyl sulfate–polyacrylamide gel electrophoresis at the expected sizes. Proteins were stored at −70°C or lyophilized before use.
Binding of WT and variant tPA to fibrin
Proteins were radiolabeled with 125I, and binding to fibrin was measured as reported.24 To determine specific binding, fibrin was incubated with increasing concentrations of radiolabeled ligands alone or in the presence of 10-fold molar excess unlabeled ligand.24 In other experiments, 125I-WT-tPA was incubated with fibrin in the presence of increasing concentrations of unlabeled tPA-S481A prior to measuring binding.
Binding of WT and variant tPA to plasminogen
tPA-S481A was incubated with plasminogen (100 nM each) at 4°C alone or in the presence of 1 µM uPA and in the presence or absence of soluble fibrin prepared as previously reported.25 The absence of plasmin was confirmed using a small plasmin chromogenic substrate as described below. Complexes were precipitated with goat polyclonal anti-plasminogen and detected by western blot analysis with goat anti-mouse immunoglobulin G, as reported.26
Lysis of plasma clots
Lysis of plasma clots was measured27 by adding phosphate buffered saline (PBS) containing WT-tPA or uPA (10 nM each) to the clot surface for 2 hours at 37°C in the absence or presence of tPA-S481A (50 nM). Clots were then washed with PBS, incubated overnight with 0.2% trypan blue, rinsed with PBS, and photographed. Photographs were scanned using a Hoefer GS 300 densitometer (Amersham Pharmacia Biotech, Piscataway, NJ). Sizes of the lytic zones were calculated using the National Institutes of Health Image program. In other experiments, clot lysis was performed using cerebrospinal fluid (CSF; 20 µL) collected from 4 to 6 mice per sample28,29 obtained 2 hours after TBI or sham injury.
Clot lysis monitored by thromboelastography
Clots were prepared from whole human blood by adding kaolin. Lysis initiated by adding WT-tPA or WT-uPA (10 nM each) alone or in the presence of increasing concentrations of tPA-S481A was measured using a TEG 5000 thromboelastograph 30 (Haemonetics, Braintree, MA).
Chromogenic assay of plasminogen activation
Chromogenic activity was measured as previously described.22 WT-tPA (50 nM) or WT-uPA (5 nM) was incubated with plasminogen (500 nM) and the chromogenic substrate of plasmin S-2251 (Innovative Research, Novi, MI) (100 µM) alone or in the presence of tPA-S481A (100 nM) or tranexamic acid (1 µM). Generation of plasmin was assessed by change in optical density (OD) at 405 nm.22
Closed head injury
All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committees of the Perelman School of Medicine at the University of Pennsylvania and the Hebrew University, Jerusalem, Israel. Male WT, tPA−/−, uPA−/−, or PAI-1−/− mice (12 weeks old) on a C57BL/6J background were fed regular chow, allowed free access to drinking water, and kept under controlled temperature and lighting. Closed head injury (CHI) was induced using a weight-drop device, as described in detail elsewhere.28,31 Saline containing tPA-S481A (1 mg/kg) or saline alone was injected IV immediately after or 2 hours after CHI. There were no significant differences in mean arterial blood pressure (75 ± 4.6 mm Hg),22 oxygen saturation (pO2: 94.0 ± 3.3), or pH (7.39 ± 0.16) between treated and untreated animals post-CHI.
Measurement of ICH
MRI studies
MRI studies were performed immediately after CHI28 using a 4.7-T Bruker BioSpec spectrometer. Mice were anesthetized using 1% to 1.5% isoflurane vaporized in a 70%/30% nitrous oxide/oxygen gas mixture. An active, detuned 12-mm surface coil was placed over the skull and centered over the midline of the brain. The T2*-weighted images were taken by gradient echo sequence with a field of view of 1.8 × 1.8 cm and a spatial resolution of 256 × 128 converted to 256 × 256 and a repetition time of 147 milliseconds, echo time of 10 milliseconds, and 12 averages. The T2*-weighted images detects hemorrhage because the sequence is highly sensitive to deoxyhemoglobin, which is paramagnetic.
Extravasation of hemoglobin
The ipsilateral and contralateral hemispheres were removed after extensive transcardial perfusion. OD was measured after homogenization, sonication, and centrifugation (see the supplemental Data, available on the Blood Web site for more details).
Neurobehavioral evaluation
Neurologic function was evaluated 24 hours after CHI using a neurologic severity score28,31 that was modified to emphasize motor functions33 (see the supplemental Data for additional details). All neurologic evaluations were performed by 1 investigator who was blinded to the prior experimental conditions. Neurologic function was graded on a scale of 0 to 18 (normal score, 0; maximal deficit, 18).
Expression of uPA and tPA in brain parenchyma and CSF
Whole brains were removed from naïve WT mice at prespecified times post-CHI. uPA and tPA were detected using western blot analysis23 ; expression of β-actin was used as loading control. The CSF combined from 4 to 6 mice per sample28,29 was taken from sham or injured mice at the indicated time points after CHI, and tPA and uPA were measured by enzyme-linked immunosorbent assay.34,35 In other experiments, CSF fibrinolytic activity was measured using plasma-derived blood clots.27 Blood was taken from sham-injured mice or 2 hours after CHI. Whole blood platelet counts were measured (Sysmex, Kobe, Japan). Plasma d-dimers were measured by enzyme-linked immunosorbent assay (Diagnostic Products, Los Angeles, CA).36
Effect of iron-induced thrombus formation
Six-week-old WT male mice were given an intraperitoneal injection (60 mg/kg)37 of iron dextran (Sigma-Aldrich) diluted in normal saline every 3 to 5 days for a total of 10 doses over 6 weeks. Mice were randomized to receive deferoxamine (200 mg/kg)38 (Sigma-Aldrich) or saline intraperitoneally 1 day after each injection of iron dextran. Mice were then subjected to CHI, and intracerebral bleeding was assessed.
Statistical analysis
The statistical significance of experimental differences was analyzed using 1-way analysis of variance with Newman-Keuls post hoc test28 or Student t test, as indicated.
Results
Inhibition of clot lysis by tPA variants
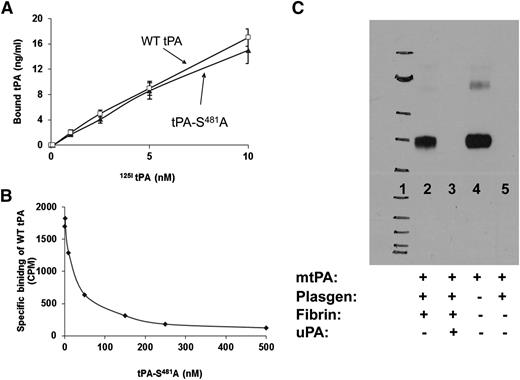
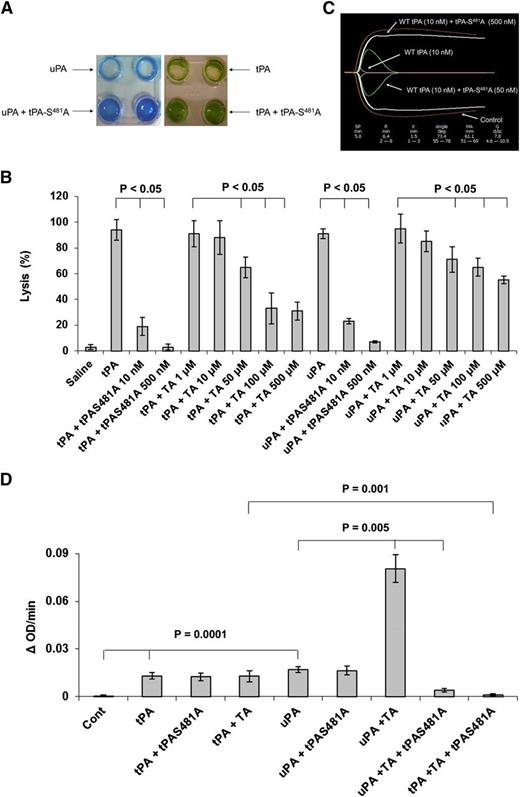
We first examined the effect of catalytically inactive tPA-S481A on clot lysis. tPA-S481A bound to fibrin with the same affinity as WT-tPA (Figure 1A) and inhibited the binding of WT-tPA to fibrin in a concentration-dependent manner (Figure 1B). tPA-S481A bound to plasminogen only in the presence of fibrin, and binding of the variant was inhibited by uPA (Figure 1C). This finding suggests that tPA-S481A might inhibit proteolytic activation of plasminogen by uPA in the presence of fibrin. Fibrin induced conformational changes in plasminogen that accelerated plasminogen activation by uPA (see supplemental Figure 1). As predicted, tPA-S481A inhibited lysis of human plasma clots by WT-uPA and inhibited lysis by WT-tPA, assessed both macroscopically26 (Figure 2A-B) and by quantifying lysis of clots formed from fresh human blood using thromboelastography30 (Figure 2C). tPA-S481A was a more potent inhibitor of fibrinolysis than tranexamic acid when tested at its maximal inhibitory concentration (Figure 2B). tPA-S481A had no effect on plasminogen activation initiated by WT-tPA or uPA in the absence of fibrin assessed with a chromogenic assay that uses a small peptide as a plasmin substrate39 (Figure 2D). In contrast to its antifibrinolytic activity, and as anticipated on the basis of prior studies,40,41 tranexamic acid stimulated the activation of plasminogen by uPA40 but not by tPA (Figure 2D).
Interaction of tPA-S481A with fibrin and plasminogen. (A) The specific binding of catalytically inactive human tPA-S481A and WT-tPA (Bound tPA) to fibrin was measured.24 tPA-S481A and WT-tPA bind to fibrin with comparable affinity. Data represent results from 1 of 3 independent experiments. (B) tPA-S481A inhibits the binding of 125I-WT-tPA (5 nM) to fibrin.24 Data represent results from 1 of 3 independent experiments. (C) Binding of tPA-S481A to plasminogen. tPA-S481A was incubated with plasminogen (Plasgen; 100 nM each) and fibrin in the absence (lane 2) or presence (lane 3) of 1 µM uPA and in the absence of fibrin (lane 5). Lane 4 shows the positive control (80 nM human tPA-S481A). Complexes were immunoprecipitated with anti-plasminogen antibody and detected with anti-tPA antibody.26 tPA-S481A binds to plasminogen, forming a stable complex (lane 2) in the presence, but not in the absence, of fibrin, as shown in lane 5, where tPA-S481A and plasminogen were incubated alone. Binding of tPA-S481A is inhibited by uPA (lane 3). Lane 1 shows the molecular weight markers. Data represent results from 1 of 3 independent experiments. mtPA, mutant tPA; plasgen, plasminogen.
Interaction of tPA-S481A with fibrin and plasminogen. (A) The specific binding of catalytically inactive human tPA-S481A and WT-tPA (Bound tPA) to fibrin was measured.24 tPA-S481A and WT-tPA bind to fibrin with comparable affinity. Data represent results from 1 of 3 independent experiments. (B) tPA-S481A inhibits the binding of 125I-WT-tPA (5 nM) to fibrin.24 Data represent results from 1 of 3 independent experiments. (C) Binding of tPA-S481A to plasminogen. tPA-S481A was incubated with plasminogen (Plasgen; 100 nM each) and fibrin in the absence (lane 2) or presence (lane 3) of 1 µM uPA and in the absence of fibrin (lane 5). Lane 4 shows the positive control (80 nM human tPA-S481A). Complexes were immunoprecipitated with anti-plasminogen antibody and detected with anti-tPA antibody.26 tPA-S481A binds to plasminogen, forming a stable complex (lane 2) in the presence, but not in the absence, of fibrin, as shown in lane 5, where tPA-S481A and plasminogen were incubated alone. Binding of tPA-S481A is inhibited by uPA (lane 3). Lane 1 shows the molecular weight markers. Data represent results from 1 of 3 independent experiments. mtPA, mutant tPA; plasgen, plasminogen.
Inhibition of plasminogen activation by tPA variant depends on the conformation of plasminogen. (A) tPA-S481A inhibits lysis of plasma clots. Plasma clots were lysed by WT-tPA (10 nM) or WT-uPA (10 nM)24 in the absence or presence of tPA-S481A (50 nM). (B) Clot lysis by WT-tPA and WT-uPA was performed as in panel A in the absence or presence of the indicated concentrations of tPA-S481A or tranexamic acid (TA). The data are expressed as percent lysis compared to WT-tPA and WT-uPA alone. The mean ± SD of 3 experiments is shown. (C) Lysis of clots prepared from fresh whole human blood by WT-tPA (10 nM) was monitored by thromboelastography30 in the absence (Control) or presence of 50 nM or 500 nM tPA-S481A. Data represent results from 1 of 3 independent experiments. (D) Effect of tPA variants on PA activity of tPA and uPA in the absence of fibrin. Plasminogen (500 nM) was incubated alone (Cont) or with WT-tPA (5 nM) or uPA (5 nM) in the absence or presence of tPA-S481A (100 nM) or tranexamic acid (1 µM) plus a plasmin chromogenic substrate (100 µM) (TA). The OD at 405 nm was measured continuously over the next 20 minutes.22 In another set of experiments, tranexamic acid was added together with tPA-S481A (100 nM). The data are expressed as the change in OD per minute. The figure shows that tPA-S481A had no effect on plasminogen activation by tPA or uPA in the absence of fibrin and that the effect of tranexamic acid mimics the stimulatory effect of fibrin on plasminogen activation by uPA and inhibition by the tPA variant. Comparisons between groups were analyzed using the Student t test. The mean ± SD of 3 experiments is shown. SD, standard deviation.
Inhibition of plasminogen activation by tPA variant depends on the conformation of plasminogen. (A) tPA-S481A inhibits lysis of plasma clots. Plasma clots were lysed by WT-tPA (10 nM) or WT-uPA (10 nM)24 in the absence or presence of tPA-S481A (50 nM). (B) Clot lysis by WT-tPA and WT-uPA was performed as in panel A in the absence or presence of the indicated concentrations of tPA-S481A or tranexamic acid (TA). The data are expressed as percent lysis compared to WT-tPA and WT-uPA alone. The mean ± SD of 3 experiments is shown. (C) Lysis of clots prepared from fresh whole human blood by WT-tPA (10 nM) was monitored by thromboelastography30 in the absence (Control) or presence of 50 nM or 500 nM tPA-S481A. Data represent results from 1 of 3 independent experiments. (D) Effect of tPA variants on PA activity of tPA and uPA in the absence of fibrin. Plasminogen (500 nM) was incubated alone (Cont) or with WT-tPA (5 nM) or uPA (5 nM) in the absence or presence of tPA-S481A (100 nM) or tranexamic acid (1 µM) plus a plasmin chromogenic substrate (100 µM) (TA). The OD at 405 nm was measured continuously over the next 20 minutes.22 In another set of experiments, tranexamic acid was added together with tPA-S481A (100 nM). The data are expressed as the change in OD per minute. The figure shows that tPA-S481A had no effect on plasminogen activation by tPA or uPA in the absence of fibrin and that the effect of tranexamic acid mimics the stimulatory effect of fibrin on plasminogen activation by uPA and inhibition by the tPA variant. Comparisons between groups were analyzed using the Student t test. The mean ± SD of 3 experiments is shown. SD, standard deviation.
These outcomes support the concept that the conformation changes in plasminogen imparted by fibrin or tranexamic acid not only make it more susceptible to cleavage by uPA (supplemental Figure 1) but also are required for binding of tPA-S481A and inhibition of fibrinolysis caused by WT-tPA and uPA. As a further test of this hypothesis, plasminogen activation by WT-tPA and uPA was assessed in the presence of tPA-S481A together with tranexamic acid, which mimics the effect of fibrin on plasminogen structure and activation42 (supplemental Figure 1). In the presence of tranexamic acid, tPA-S481A inhibited the activation of plasminogen by uPA and tPA (Figure 2D).
Together, these data suggest that in settings where fibrin is limiting, as in whole blood and brain parenchyma, lysine analogs such as tranexamic acid may be deleterious because they stimulate activation of plasminogen by uPA. In contrast, tPA-S481A is inert under these conditions. Therefore, we examined the possibility that the tPA variant would exert less plasmin-dependent off-target toxicity after TBI.
Inhibition of endogenous PA activity induced by TBI attenuates delayed ICH and changes in coagulation parameters
The closed head injury (CHI) model of TBI28 induces extensive time-dependent ICH evident on MRI and by extravasation of hemoglobin into the parenchyma (Figure 3A-C and supplemental Figure 3). We observed a marked increase in tPA and uPA in the CSF 2 hours after CHI (Figure 3D), accompanied by an increase in CSF fibrinolytic activity (Figure 3E-F).
CHI in mice induces time-dependent expansion of ICH. ICH induced by CHI was evaluated by MRI and by measuring extravasation of hemoglobin. (A) The hemorrhagic area was evaluated 24 hours post-CHI by T2*-weighted images in untreated WT mice (Control), mice given tPA-S481A (1 mg/kg) either immediately after CHI (Treated) or 2 hours later (Treated 2 hrs). Images from 2 representative mice from each group (n = 5 each) are shown in the top and bottom panels. The hemorrhagic areas are delineated by circles. (B) Reduction in hemorrhage by tPA variant. WT mice were given saline (Cont) or tPA-S481A (1 mg/kg) immediately after or 2 hours post-CHI, and the extravasation of hemoglobin into a lysate of whole brain was quantified by spectrophotometry34 2, 8, or 24 hours later. The mean ± SD of 3 experiments performed in 5 to 7 mice is shown. (C) Comparative effect of inhibitors of plasminogen activation on hemorrhage 2 hours posttrauma. Immediately after CHI, WT mice were given saline (Cont), WT-tPA (5 mg/kg) or tPA-S481A (1 mg/kg), WT-tPA (5 mg/kg) plus tPA-S481A (5 mg/kg), tranexamic acid (TA; 150 mg/kg), aprotinin (Ap; 1 mg/kg), TA plus Ap, WT-tPA plus TA, WT-tPA plus Ap, or WT-tPA plus TA plus Ap, as indicated. Extravasation of hemoglobin into a lysate of whole brain was quantified by spectrophotometry34 2 hours later. The mean ± SD of 3 experiments performed in 5 to 7 mice is shown. (D) Trauma induces tPA and uPA from brain parenchyma. The concentrations of tPA and uPA in the CSF of WT mice were measured by enzyme-linked immunosorbent assay before (Sham) or 2 hours after (Post) CHI. The mean ± SD of 3 experiments performed in 5 to 7 mice is shown. (E) Trauma induces fibrinolytic activity. The fibrinolytic activity of CSF was measured 2 hours post-CHI. Upper line: CSF (5 µL or 10 µL) obtained 2 hours after CHI from untreated mice was placed on top of plasma-derived clots as in Figure 2A. Lower line: CSF obtained 2 hours after CHI from mice treated with tPA-S481A (1 mg/kg) immediately post-CHI. Data represent results from 1 of 3 independent experiments. (F) tPA variant inhibits fibrinolytic activity posttrauma. The fibrinolytic activity of CSF taken 2 hours after CHI from untreated mice or mice given tPA-S481A (1 mg/kg) immediately posttrauma was quantified as in Figure 2B. The mean ± 2 SD of 3 experiments performed in 4 to 8 mice is shown. The statistical significance of experimental differences in this set of experiments and in those that follow was analyzed using 1-way analysis of variance with Newman-Keuls post hoc test.33
CHI in mice induces time-dependent expansion of ICH. ICH induced by CHI was evaluated by MRI and by measuring extravasation of hemoglobin. (A) The hemorrhagic area was evaluated 24 hours post-CHI by T2*-weighted images in untreated WT mice (Control), mice given tPA-S481A (1 mg/kg) either immediately after CHI (Treated) or 2 hours later (Treated 2 hrs). Images from 2 representative mice from each group (n = 5 each) are shown in the top and bottom panels. The hemorrhagic areas are delineated by circles. (B) Reduction in hemorrhage by tPA variant. WT mice were given saline (Cont) or tPA-S481A (1 mg/kg) immediately after or 2 hours post-CHI, and the extravasation of hemoglobin into a lysate of whole brain was quantified by spectrophotometry34 2, 8, or 24 hours later. The mean ± SD of 3 experiments performed in 5 to 7 mice is shown. (C) Comparative effect of inhibitors of plasminogen activation on hemorrhage 2 hours posttrauma. Immediately after CHI, WT mice were given saline (Cont), WT-tPA (5 mg/kg) or tPA-S481A (1 mg/kg), WT-tPA (5 mg/kg) plus tPA-S481A (5 mg/kg), tranexamic acid (TA; 150 mg/kg), aprotinin (Ap; 1 mg/kg), TA plus Ap, WT-tPA plus TA, WT-tPA plus Ap, or WT-tPA plus TA plus Ap, as indicated. Extravasation of hemoglobin into a lysate of whole brain was quantified by spectrophotometry34 2 hours later. The mean ± SD of 3 experiments performed in 5 to 7 mice is shown. (D) Trauma induces tPA and uPA from brain parenchyma. The concentrations of tPA and uPA in the CSF of WT mice were measured by enzyme-linked immunosorbent assay before (Sham) or 2 hours after (Post) CHI. The mean ± SD of 3 experiments performed in 5 to 7 mice is shown. (E) Trauma induces fibrinolytic activity. The fibrinolytic activity of CSF was measured 2 hours post-CHI. Upper line: CSF (5 µL or 10 µL) obtained 2 hours after CHI from untreated mice was placed on top of plasma-derived clots as in Figure 2A. Lower line: CSF obtained 2 hours after CHI from mice treated with tPA-S481A (1 mg/kg) immediately post-CHI. Data represent results from 1 of 3 independent experiments. (F) tPA variant inhibits fibrinolytic activity posttrauma. The fibrinolytic activity of CSF taken 2 hours after CHI from untreated mice or mice given tPA-S481A (1 mg/kg) immediately posttrauma was quantified as in Figure 2B. The mean ± 2 SD of 3 experiments performed in 4 to 8 mice is shown. The statistical significance of experimental differences in this set of experiments and in those that follow was analyzed using 1-way analysis of variance with Newman-Keuls post hoc test.33
IV administration of tPA-S481A (1 mg/kg) immediately after or 2 hours after CHI inhibited endogenous CSF fibrinolytic activity (Figure 3E-F) and attenuated the expansion of ICH (P < .01; Figure 3A-C). In contrast, injection of WT-tPA (5 mg/kg) immediately after CHI, the dose needed to induce fibrinolysis in mice,28 increased the extent of ICH in WT mice (Figure 3C), an effect that was inhibited by injecting equimolar concentrations of tPA-S481A (Figure 3C). This outcome affirms that tPA-S481A is active under in vivo conditions. The tPA variant was a more potent inhibitor of ICH than optimal concentrations of tranexamic acid or aprotinin (Figure 3C).
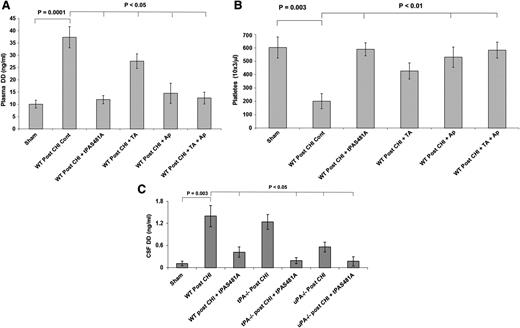
To establish the source of the tPA and uPA in the CSF after CHI, we measured contemporaneous levels in plasma. CHI had minimal effect on plasma levels of either enzyme compared to sham-injured mice, in contrast to the increases in tPA and uPA in the CSF (Figure 3D), strongly suggesting that both PAs come predominantly from injured brain parenchyma. We cannot entirely exclude the possibility that the differences in tPA and uPA concentrations in the CSF compared to plasma result in part from delayed clearance, although we did not observe such differences in clearance after TBI in rats (data not shown). CHI was followed by a significant increase in plasma d-dimers (Figure 4A, P < .0001) and a fall in platelet counts (Figure 4B, P < .003). tPA-S481A given immediately after CHI attenuated the increase in d-dimers significantly (Figure 4A, P < .05) and prevented the development of thrombocytopenia (Figure 4B, P < .01). To assess whether fibrin is formed and lysed in the brain parenchyma as a result of injury, we measured d-dimers in the CSF. d-dimers in the CSF increased post-TBI in WT mice (Figure 4C) but to a lesser extent than in the blood (Figure 4A). tPA-S481A attenuated the rise in CSF d-dimers in WT mice post-TBI (Figure 4C).
tPA-S481A blocks development of a systemic coagulopathy. Immediately after CHI, mice were given saline (Cont) or saline containing tPA-S481A (1 mg/kg), tranexamic acid (TA; 150 mg/kg), aprotinin (Ap; 1 mg/kg), or TA plus Ap. tPA-S481A inhibited formation of d-dimers (DD) (A) and development of thrombocytopenia (B) post-CHI. (C) tPA-S481A attenuated the post-TBI rise in CSF d-dimers. d-dimers in the CSF increased post TBI in WT mice but to a lesser extent than in the plasma (compare panels C and A). Plasma and CSF d-dimers and platelet counts were measured 2 hours later. The mean ± 2 SD of 3 experiments performed in 6 to 8 mice is shown.
tPA-S481A blocks development of a systemic coagulopathy. Immediately after CHI, mice were given saline (Cont) or saline containing tPA-S481A (1 mg/kg), tranexamic acid (TA; 150 mg/kg), aprotinin (Ap; 1 mg/kg), or TA plus Ap. tPA-S481A inhibited formation of d-dimers (DD) (A) and development of thrombocytopenia (B) post-CHI. (C) tPA-S481A attenuated the post-TBI rise in CSF d-dimers. d-dimers in the CSF increased post TBI in WT mice but to a lesser extent than in the plasma (compare panels C and A). Plasma and CSF d-dimers and platelet counts were measured 2 hours later. The mean ± 2 SD of 3 experiments performed in 6 to 8 mice is shown.
Blocking endogenous PA activity provides neuroprotection post-TBI
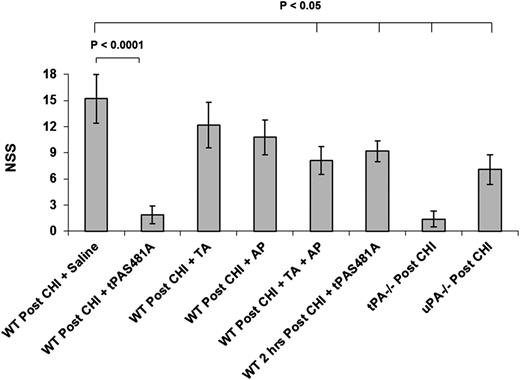
CHI induces severe neurologic deficits.28,43 Therefore, we next asked whether inhibiting endogenous PA activity would improve neurologic recovery post-TBI. Injection of tPA-S481A either immediately after or 2 hours after injury significantly improved neurologic recovery (Figure 5).
tPA-S481A attenuates neurologic deterioration post CHI. CHI was induced in WT, tPA−/−, and uPA−/− mice as in Figure 3. Saline (Cont) or saline containing tPA-S481A (1 mg/kg), tranexamic acid (TA; 150 mg/kg), aprotinin (AP; 1 mg/kg), or TA plus AP was given immediately or 2 hours (as indicated) after CHI. Neurologic evaluation was performed 24 hours post-CHI. The mean ± 2 SD of 3 experiments performed in 6 to 8 mice is shown. NSS, neurologic severity score.
tPA-S481A attenuates neurologic deterioration post CHI. CHI was induced in WT, tPA−/−, and uPA−/− mice as in Figure 3. Saline (Cont) or saline containing tPA-S481A (1 mg/kg), tranexamic acid (TA; 150 mg/kg), aprotinin (AP; 1 mg/kg), or TA plus AP was given immediately or 2 hours (as indicated) after CHI. Neurologic evaluation was performed 24 hours post-CHI. The mean ± 2 SD of 3 experiments performed in 6 to 8 mice is shown. NSS, neurologic severity score.
Contribution of tPA, uPA, and PAI-1 to post-TBI ICH and brain damage
Brain damage caused by focal cerebral ischemia is not followed by extensive delayed ICH in most models, tPA−/−, but not uPA−/−, mice show less brain damage post-TBI than WT mice.44 This difference indicates that endogenous tPA plays the predominant role in brain damage in the absence of significant delayed ICH. In contrast, both tPA−/− and uPA−/− mice showed better neurologic outcomes than WT mice in the model of CHI studied here (Figure 5), suggesting that endogenous uPA also makes an important contribution to posttraumatic ICH and subsequent brain damage.
To test this hypothesis in greater detail, CHI was induced in WT, tPA−/−, uPA−/−, and PAI-1−/− mice. tPA−/− and uPA−/− mice developed significantly (P < .01) less ICH than WT mice subjected to the same injury (Figure 6A), concomitant with less fibrinolytic activity in the CSF post-TBI (Figure 6B). The injury phenotype in both types of knockout mice was “rescued” by exogenous WT-tPA or WT-uPA (5 mg/kg each), and in both cases, co-injection of an equimolar dose of tPA-S481A inhibited the expansion of posttraumatic ICH induced by exogenous PA (Figure 6A). Furthermore, tPA-S481A inhibited the progression of ICH in tPA−/− mice. In contrast, tranexamic acid decreased ICH in uPA−/− mice but increased bleeding in tPA−/− mice (Figure 6A). These findings are in line with the known difference in the effect of tranexamic acid on plasminogen activation by tPA and uPA40,41,45,46 (supplemental Figure 1D and Figure 2D). As an independent approach, we induced CHI in PAI-1−/− mice. PAI-1−/− mice developed more extensive ICH than WT mice (Figure 6A), which was also attenuated by tPA-S481A.
tPA, uPA, and PAI-1 participate in the development of ICH, generation of d-dimers, and platelet counts post CHI. (A) Effect of endogenous tPA, uPA, and PAI-1 on ICH. CHI was induced in WT, tPA−/−, uPA−/−, and PAI-1−/− mice, followed immediately by an IV injection of WT-tPA or WT-uPA either alone (5 mg/kg each) or together with tPA-S481A (5 mg/kg). Two hours later, brain hemoglobin was determined as in Figure 3B. The mean ± SD of 3 experiments performed in 6 to 8 mice is shown. (B) Effect of endogenous tPA and uPA on fibrinolytic activity. Two hours post CHI, the fibrinolytic activity in CSF taken from WT, tPA−/−, and uPA−/− mice was measured as in Figure 3F. The mean ± SD of 3 experiments performed in 6 to 8 mice is shown. (C-D) Effect of endogenous tPA, uPA, and PAI-1 on d-dimers and thrombocytopenia. The experiment was performed as in panel A with the exception that some animals were given tPA-S481A (1 mg/kg each), TA (10 mg/kg), or Ap (1 mg/kg) or TA plus Ap. Plasma d-dimers (DD) (C) and platelet counts (D) were measured as in Figures 4A and 4B, respectively. The mean ± SD of 3 experiments performed in 6 to 8 mice is shown. (E) tPA-S481A inhibited expansion of ICH in mice treated with warfarin (WR). CHI was induced in WT, tPA−/−, and uPA−/− mice treated with WR to an INR of 2.5 ± 0.4 at the time of trauma. Saline or tPA-S481A (1 mg/kg) was given immediately after CHI, and brain hemoglobin was measured 2 or 8 hours after the injury as in Figure 3B. (F) The prothrombotic effect of iron on delayed ICH. CHI was induced in WT mice given iron dextran (IR; 60 mg/kg) alone or together with deferoxamine (Def; 200 mg/kg). Brain hemoglobin was measured 2 to 24 hours postinjury. The mean ± 2 SD of 3 experiments performed in 7 to 9 mice is shown. INR, international normalized ratio.
tPA, uPA, and PAI-1 participate in the development of ICH, generation of d-dimers, and platelet counts post CHI. (A) Effect of endogenous tPA, uPA, and PAI-1 on ICH. CHI was induced in WT, tPA−/−, uPA−/−, and PAI-1−/− mice, followed immediately by an IV injection of WT-tPA or WT-uPA either alone (5 mg/kg each) or together with tPA-S481A (5 mg/kg). Two hours later, brain hemoglobin was determined as in Figure 3B. The mean ± SD of 3 experiments performed in 6 to 8 mice is shown. (B) Effect of endogenous tPA and uPA on fibrinolytic activity. Two hours post CHI, the fibrinolytic activity in CSF taken from WT, tPA−/−, and uPA−/− mice was measured as in Figure 3F. The mean ± SD of 3 experiments performed in 6 to 8 mice is shown. (C-D) Effect of endogenous tPA, uPA, and PAI-1 on d-dimers and thrombocytopenia. The experiment was performed as in panel A with the exception that some animals were given tPA-S481A (1 mg/kg each), TA (10 mg/kg), or Ap (1 mg/kg) or TA plus Ap. Plasma d-dimers (DD) (C) and platelet counts (D) were measured as in Figures 4A and 4B, respectively. The mean ± SD of 3 experiments performed in 6 to 8 mice is shown. (E) tPA-S481A inhibited expansion of ICH in mice treated with warfarin (WR). CHI was induced in WT, tPA−/−, and uPA−/− mice treated with WR to an INR of 2.5 ± 0.4 at the time of trauma. Saline or tPA-S481A (1 mg/kg) was given immediately after CHI, and brain hemoglobin was measured 2 or 8 hours after the injury as in Figure 3B. (F) The prothrombotic effect of iron on delayed ICH. CHI was induced in WT mice given iron dextran (IR; 60 mg/kg) alone or together with deferoxamine (Def; 200 mg/kg). Brain hemoglobin was measured 2 to 24 hours postinjury. The mean ± 2 SD of 3 experiments performed in 7 to 9 mice is shown. INR, international normalized ratio.
Enhanced primary fibrinolysis by uPA is the predominant cause of post-TBI coagulopathy
The data reported to this point suggest that the induction of primary fibrinolysis by trauma, rather than exposure of tissue factor and the subsequent activation of coagulation, is the principal cause of persistent ICH and post-TBI coagulopathy in this model.3,9,10 To pursue this hypothesis using independent experimental approaches, we examined the relationship between the intensity of posttraumatic endogenous fibrinolysis and activation of coagulation. We measured 2 global parameters of coagulation after TBI in WT, uPA−/−, tPA−/−, and PAI−/− mice, namely d-dimers as a marker of sequential thrombin- and plasmin-mediated cleavage of fibrin(ogen) and platelet counts. tPA−/− mice developed elevated d-dimers and a significant fall in platelet count (P < .003) after CHI, comparable to findings in WT mice (Figure 6C-D). PAI-1−/− mice developed even higher levels of d-dimers (Figure 6C) and a more dramatic fall in platelet count (P < .0002) than did WT mice (Figure 6D), both of which were attenuated by tPA-S481A. In contrast, uPA−/− mice, which showed a reduction in ICH comparable to tPA−/− mice (Figure 6A) and comparable endogenous fibrinolytic activity (Figure 6B), developed only a modest increase in plasma d-dimers (Figure 6C) and did not develop thrombocytopenia (Figure 6D). Together, these data support several conclusions. First, there is direct correlation between endogenous fibrinolysis post-TBI and the extent of ICH. Second, increased endogenous uPA is the primary driver of the coagulopathy that follows severe TBI. Third, the coagulopathy itself did not have significant effect on the progression of intracerebral bleeding. Thus, the coagulopathy appears to be the consequence, not the cause, of delayed ICH.
We next pursued the finding that tPA−/− mice developed thrombocytopenia and elevated plasma d-dimers comparable to WT mice (Figure 6C-D), yet were largely protected from ICH (Figure 6A). We examined the effect of CHI on the plasma concentration of fibrinogen and the INR in a separate cohort of WT, tPA−/−, and uPA−/− animals. The INR after CHI was <1.3 ± 0.21 in tPA−/−, uPA−/−, and WT mice. There was no significant depletion of fibrinogen or a difference in fibrinogen concentration among the 3 cohorts (data not shown), providing additional support for the contention that the post-TBI coagulopathy is not of sufficient severity to induce delayed ICH. As an additional independent approach, we examined the effect of inducing a systemic coagulopathy with warfarin prior to CHI. Anticoagulated mice had a mean INR of 2.5 ± 0.43 at the time of CHI. Although warfarin increased the size of ICH in WT mice (P < .002), it did not significantly affect ICH in tPA−/− or uPA−/− mice (P > .14 each; Figure 6E). Moreover, tPA-S481A almost totally inhibited posttraumatic ICH in WT mice anticoagulated with warfarin (P < .005; Figure 6E).
The prothrombotic effect of iron attenuates delayed ICH
We then asked whether mimicking the stimulatory effect of tissue factor on thrombosis would exacerbate or attenuate ICH. We took advantage of the observation that moderate iron loading accelerates thrombus formation after arterial injury.37 We gave mice iron dextran for 6 weeks alone or along with the iron chelator deferoxamine,38 followed by CHI. The extent of ICH was examined 2, 8, and 24 hours later by measuring extravasation of hemoglobin. Post-TBI ICH was decreased in mice loaded with iron dextran, and the beneficial effect was abolished by deferoxamine (Figure 6F).
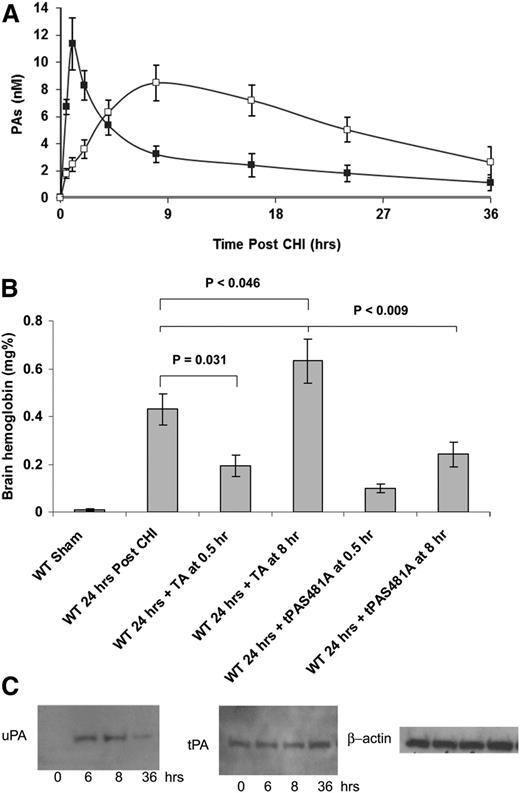
Enhanced uPA activity by tranexamic acid exacerbates delayed bleeding
The data presented in Figure 6A show that tranexamic acid increased ICH in tPA−/− mice. To investigate this outcome in greater detail, we measured time-dependent changes in the concentrations of tPA and uPA in the CSF after CHI and their effect on the response to tranexamic acid. tPA increased immediately post-CHI and then fell to 50% of its maximal concentration by 4 hours, whereas the increase in uPA was delayed and protracted (Figure 7A). Tranexamic acid also showed a time-dependent effect on post-TBI ICH. Thirty minutes post-CHI, a time when the levels of uPA in the CSF are low and the levels of tPA are high, tranexamic acid decreased ICH (Figure 7B). In contrast, tranexamic acid significantly (P = .026) increased ICH when given 8 hours post-CHI, a time when uPA has reached its peak and tPA levels have fallen (Figure 7B). tPA-S481A, which inhibits both PAs (see supplemental Figure 2), attenuated ICH at both time points (Figure 7B).
tPA and uPA participate in the delayed bleeding post-CHI. (A) Time course of tPA and uPA release post-CHI. The concentrations of tPA (filled squares) and uPA (empty squares) in the CSF of WT mice were measured by enzyme-linked immunosorbent assay before or at different time points after CHI. The mean ± SE of 3 experiments is shown (n = 3-7). (B) uPA mediates the delayed ICH in untreated mice. CHI was induced in WT mice. Thirty minutes or 8 hours postinjury, mice were given tPA-S481A (1 mg/kg) or tranexamic acid (TA; 150 mg/kg). Twenty-four hours after CHI, brain hemoglobin was determined as in Figure 3B. The mean ± SD of 3 experiments performed is shown (n = 5-7). (C) The expression of uPA (upper row) and tPA (lower row) was measured in the brain parenchyma of WT mice without (time 0) or at specified times after CHI by western blot analysis. The lower panel shows expression of β-actin as a loading control. Data represent results from 1 of 3 independent experiments.
tPA and uPA participate in the delayed bleeding post-CHI. (A) Time course of tPA and uPA release post-CHI. The concentrations of tPA (filled squares) and uPA (empty squares) in the CSF of WT mice were measured by enzyme-linked immunosorbent assay before or at different time points after CHI. The mean ± SE of 3 experiments is shown (n = 3-7). (B) uPA mediates the delayed ICH in untreated mice. CHI was induced in WT mice. Thirty minutes or 8 hours postinjury, mice were given tPA-S481A (1 mg/kg) or tranexamic acid (TA; 150 mg/kg). Twenty-four hours after CHI, brain hemoglobin was determined as in Figure 3B. The mean ± SD of 3 experiments performed is shown (n = 5-7). (C) The expression of uPA (upper row) and tPA (lower row) was measured in the brain parenchyma of WT mice without (time 0) or at specified times after CHI by western blot analysis. The lower panel shows expression of β-actin as a loading control. Data represent results from 1 of 3 independent experiments.
Time course of tPA and uPA expression in the injured brain parenchyma
tPA is synthesized by neurons and other cell types within the central nervous system.50 In neurons, tPA is stored in granules and is released by several agonists, serves as a neurotransmitter, is critical for learning, and potentiates seizure activity at high concentrations.47,48 tPA is also released by brain injury caused by trauma and stroke. CSF tPA increased soon after TBI, whereas the increase of uPA was delayed (Figure 7A), suggesting postinjury de novo synthesis. To assess the source of CSF PAs, we measured the expression of tPA and uPA in the brain parenchyma before and after CHI using western blot analysis. CHI induced a significant time-dependent increase in uPA expression and a much more modest increase in tPA (36 hours post-CHI) (Figure 7C), consistent with de novo synthesis of uPA and release of preformed, stored tPA.
Discussion
We examined the relatively unexplored but clinically important process of delayed intracerebral bleeding that follows TBI, an event that identifies a window of opportunity for intervention. It is generally thought that tissue factor, abundant in brain tissue, is exposed to blood after traumatic injury. It is postulated that this exposure induces a procoagulant state that may help stem bleeding but may also cause a consumptive coagulopathy and secondary fibrinolysis that leads to delayed ICH. However, our data and those of others show that delayed bleeding often occurs in the absence of clinically relevant thrombocytopenia49 or a clinically significant prolongation of clotting times,3 suggesting that perturbations in other aspects of intracerebral hemostasis may predominate.
To study the contribution of fibrinolysis, we employed a model of TBI that leads to significant delayed ICH and intravascular coagulation, exemplified by thrombocytopenia and an increase in d-dimers, simulating the clinical condition. CHI increased tPA and uPA in the CSF, reflecting parenchymal release, which exacerbated intracerebral bleeding. Inhibiting primary fibrinolysis with tPA-S481A, tranexamic acid, or aprotinin prevented delayed ICH in WT mice, reduced circulating d-dimers, and attenuated thrombocytopenia. The importance of primary fibrinolysis is further supported by the finding that warfarin did not cause significant ICH in tPA−/− and uPA−/− mice in contrast to WT mice, by the increase in ICH seen in PAI-1−/− mice, and by the finding that tPA481A provided protection even in the setting of an overt coagulopathy induced by warfarin.
The outcomes in uPA−/− and tPA−/− mice support the contention that neither the levels of d-dimer nor the development of thrombocytopenia correlated with the severity of intracranial bleeding. ICH after trauma is almost identical in uPA−/− and tPA−/− mice (Figure 6A,E), although there were marked differences in platelet counts and plasma d-dimers (Figure 6C-D). Moreover, tPA−/− mice developed far less intracerebral bleeding than WT mice (Figure 6A), although the levels of d-dimers and platelet counts were similar (Figure 6C-D). Comparing outcomes in uPA−/− and tPA−/− mice suggests that endogenous uPA is the major contributor to delayed intracerebral bleeding post-TBI, a finding in line with the well-known fibrin-independent activation of plasminogen by uPA but not tPA (Figure 2D and supplemental Figure 1). Additional support for this conclusion comes from the finding that tranexamic acid had greater antifibrinolytic activity and prevented development of a systemic coagulopathy more completely in uPA−/− than in tPA−/− mice (Figure 6C-D), where it actually exacerbated ICH (Figure 6A). This outcome implies that tranexamic acid is effective in preventing tPA-mediated fibrinolysis but has the opposite effect on uPA, as anticipated.470,45,46
This discrepancy in the effect of tranexamic acid is likely due to its capacity to stimulate fibrin-independent activation of plasminogen by uPA (Figure 2D and supplemental Figure 2), which generates intravascular plasmin. This, in turn, directly or indirectly leads to activation/inactivation and clearance of platelets and coagulation proteins. These results, together with the delayed and protracted increase of uPA in the CSF post-TBI (Figure 7A) and accompanied by the transformation of tranexamic acid from inhibiting to enhancing hemorrhage (Figure 7B) due a switch in its fibrinolytic activities, could also explain the clinical observation that the administration of tranexamic acid >3 hours after general trauma increased death due to bleeding and did not improve the outcome of TBI.50
In summary, these data suggest that the induction of primary fibrinolysis by TBI is primarily responsible for delayed ICH in this model. Tranexamic acid loses its effectiveness because it inhibits tPA-mediated, but not uPA-mediated, fibrinolysis. Inhibiting both PAs using a catalytically inactive tPA variant prevents and interrupts post-TBI fibrinolysis and improves neurologic outcome in this murine model. The finding that the tPA variant attenuated ICH and its sequelae, even when given 8 hours after TBI, provides new insights into the mechanism and possible prevention of progressive posttraumatic intracerebral bleeding. Additional studies are needed to more fully assess the potential clinical efficacy and safety of this approach.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke grant R21 NS080014 and National Heart, Lung, and Blood Institute grants PO1 HL076406 and HL82545; and the Israeli Science Foundation grant 930/04.
Authorship
Contribution: N.H., R.A.F., R.A., M.H., S.A., M.B., and E.M. performed the research and helped to analyze the data and design additional studies; R.A. performed and analyzed the magnetic resonance imaging study in the context of all other findings; S.Y. synthesized and characterized the tPA mutants; D.B.C. analyzed the data and helped to write the paper; and A.A.-R.H. conceived the study, designed the research protocols, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Abd Al-Roof Higazi, Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania, 513A Stellar-Chance, 422 Curie Blvd, Philadelphia, PA 19104; e-mail: higazi@mail.med.upenn.edu.
References
Author notes
N.H. and R.A.F. contributed equally to this work.