Key Points
Chinese patients with newly diagnosed CML-CP achieved higher rates of MMR with nilotinib vs imatinib.
Nilotinib was well tolerated, and no new safety signals were observed.
Abstract
Treatment with a tyrosine kinase inhibitor (TKI) targeting BCR-ABL1 is currently the standard of care for patients with chronic myeloid leukemia (CML) in chronic phase (CML-CP). In this study, we present results of the ENESTchina (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–China) that was conducted to investigate nilotinib 300 mg twice daily vs imatinib 400 mg once daily in a Chinese population. ENESTchina met its primary end point with a statistically significant higher rate of major molecular response (MMR; BCR-ABL1 ≤0.1% on the International Scale) at 12 months in the nilotinib arm vs the imatinib arm (52.2% vs 27.8%; P < .0001), and MMR rates remained higher with nilotinib vs imatinib throughout the follow-up period. Rates of complete cytogenetic response (0% Philadelphia chromosome–positive [Ph+] metaphases by standard cytogenetics) were comparable and ≥80% by 24 months in both arms. The estimated rate of freedom from progression to accelerated phase/blast crisis at 24 months was 95.4% in each arm. The safety profiles of both drugs were similar to those from previous studies. In conclusion, rates of MMR at 12 months were superior with nilotinib vs imatinib in Chinese patients with newly diagnosed Ph+ CML-CP. This trial was registered at www.clinicaltrials.gov as #NCT01275196.
Introduction
Nilotinib (Tasigna; Novartis Pharmaceuticals Corporation) and imatinib (Gleevec; Novartis Pharmaceuticals Corporation) are BCR-ABL1 tyrosine kinase inhibitors (TKIs) approved for the treatment of patients with newly diagnosed Philadelphia chromosome–positive (Ph+) chronic myeloid leukemia (CML) in chronic phase (CML-CP).1,2 The superior efficacy of nilotinib over imatinib as frontline therapy for CML-CP was first demonstrated in the international phase 3 study Evaluating Nilotinib Efficacy and Safety in Clinical Trials–Newly Diagnosed Patients (ENESTnd). ENESTnd met its primary end point with significantly higher rates of major molecular response (MMR; defined as BCR-ABL1 ≤0.1% on the International Scale [IS; BCR-ABL1IS]) at 12 months in each nilotinib arm (300 mg twice daily [44%] and 400 mg twice daily [43%]) vs the imatinib arm (22%; P < .001 for each comparison).3 Throughout 5 years of follow-up in ENESTnd, nilotinib demonstrated earlier and deeper molecular responses than did imatinib, with higher rates of response and a lower risk of progression to accelerated phase/blast crisis (AP/BC) at all time points.3-7
Because the efficacy and safety of TKIs may vary depending on ethnic background or genetic factors, focused investigations within well-defined patient populations are crucial in order to better understand the relative benefits and risks of each treatment option for individual patients. ENESTchina (Evaluating Nilotinib Efficacy and Safety in Clinical Trials–China) is a phase 3 study evaluating the efficacy and safety of nilotinib 300 mg twice daily vs imatinib 400 mg once daily in Chinese patients with newly diagnosed Ph+ CML-CP.
Materials and methods
Patients, treatments, and study design
Adult patients of Chinese ethnicity with Ph+ CML-CP within 6 months of diagnosis and with an Eastern Cooperative Oncology Group performance status ≤2 were eligible. Prior treatment with hydroxyurea, anagrelide, ≤3 months of recombinant interferon α (rIFNɑ), and/or ≤2 weeks of imatinib were allowed. The following patients were excluded:
those previously treated with a TKI other than imatinib for any duration,
those with known cytopathologically confirmed central nervous system infiltration,
those with impaired cardiac function (defined as complete left bundle branch block, long QT syndrome, known family history of long QT syndrome, history or presence of clinically significant ventricular or atrial tachyarrhythmia, clinically significant resting bradycardia, QTcF >450 ms, history of clinically documented myocardial infarction or unstable angina within the past year, or other clinically significant heart disease),
those with impaired gastrointestinal function,
those with a history of chronic pancreatitis,
those who had acute pancreatitis within the past year,
those with a history of significant congenital or acquired bleeding disorder unrelated to cancer,
those with another primary malignancy (unless not currently clinically significant and not requiring active intervention),
those with other severe or uncontrolled medical conditions,
those who had major surgery within 4 weeks of day 1 or who had not recovered from prior surgery,
those who received treatment with other investigational agents within 30 days of day 1,
those with a documented T315I mutation,
those who were pregnant or breastfeeding, or
those with acute or chronic liver, pancreatic, or severe renal disease considered unrelated to disease.
Patients receiving strong cytochrome P450 3A4 (CYP3A4) inhibitors or inducers and those receiving medications with the potential to prolong the QT interval were not eligible unless the medications were discontinued prior to starting study treatment.
Patients were randomized 1:1 to nilotinib 300 mg twice daily or imatinib 400 mg once daily. Randomization was stratified by Sokal risk score at diagnosis (per investigator assessment) and prior rIFNɑ therapy. Crossover between treatment arms was not permitted. Nilotinib dose escalation was not permitted. The imatinib dose could be escalated to 600 mg per day in patients with suboptimal response or treatment failure. The study was conducted according to the ethical principles of the Declaration of Helsinki. The study protocol and all amendments were reviewed by an ethics committee or institutional review board at each center. Written informed consent was obtained from each patient before screening.
The primary analysis was conducted after all patients completed 12 months (1 month = 28 days) of treatment or discontinued early. The 24-month analysis was conducted after all patients completed 24 months of treatment or discontinued early.
End points and definitions
The primary end point was the rate of MMR at 12 months. Secondary end points included rates of MMR at and by scheduled time points, rate of complete cytogenetic response (CCyR; defined as 0% Ph+ metaphases by standard cytogenetics) by scheduled time points, time to MMR, rate of durable MMR at 24 months, time to progression to AP/BC, event-free survival (EFS), overall survival (OS), and safety.
Time to progression to AP/BC was defined as the time from randomization until progression to AP/BC or CML-related death, whichever occurred first. Progression to AP/BC was defined as any of the following: ≥15% blasts in peripheral blood or bone marrow aspirate, ≥30% blasts plus promyelocytes in peripheral blood or bone marrow aspirate, ≥20% basophils in the peripheral blood, <100 × 109/L platelets within 30 days of discontinuation due to disease progression, or appearance of extramedullary involvement other than hepatosplenomegaly proven by biopsy or cytology. All progression events occurring on core treatment or after discontinuation were included in the analysis of progressions on study. EFS was defined as the time from randomization until progression to AP/BC, death from any cause, loss of partial cytogenetic response (PCyR; defined as >0%-35% Ph+ metaphases by standard cytogenetics), loss of CCyR, or loss of complete hematologic response (CHR). Only on-treatment events were considered in the EFS analysis. OS was defined as the time from randomization until death from any cause at any time, including during follow-up after discontinuation of treatment. Patients who discontinued the study drug were followed for disease progression, stem cell transplant, and survival every 3 months for up to 2 calendar years and every 6 months during the third year.
Definitions of suboptimal response and treatment failure were derived from the 2009 European LeukemiaNet (ELN) criteria.8 Suboptimal response was defined as no PCyR at 6 months, no CCyR at 12 months, no MMR at 18 months, or confirmed loss of MMR (loss of MMR in 2 assessments or in association with loss of CHR or loss of CCyR). Treatment failure was defined as no CHR at 3 months, >95% Ph+ at 6 months, no PCyR at 12 months, no CCyR at 18 months, loss of response (CHR, CCyR, or PCyR), or development of clonal chromosomal abnormalities or BCR-ABL1 mutations.
Assessments
Molecular responses were assessed at a central laboratory (KingMed, Guangzhou, China) by real-time quantitative reverse transcriptase polymerase chain reaction using a kit from MolecularMD and standardized to the IS. Molecular responses were assessed at baseline, at the end of month 1, at the end of every third month through month 36 (assessments at months 27 and 33 were not required for patients in MMR), and at the end of study or on early discontinuation. Evaluation of MMR required a minimum ABL copy number of 3000. Molecular remission 4 (MR4) (BCR-ABL1IS ≤0.01%) and MR4.5 (BCR-ABL1IS ≤0.0032%) were initially included as secondary end points but were removed after it was determined that the laboratory used for this study could not reproducibly evaluate these deep levels of response.
Standard bone marrow cytogenetic assessments (≥20 metaphases) were performed locally within 6 weeks prior to randomization; at the end of months 6, 12, 24, and 36; and at the end of study or on early discontinuation. For patients without CCyR by 12 months or without MMR by 24 months, additional cytogenetic assessments were scheduled for the end of months 18 and 30, respectively.
Mutational analysis was performed at a central laboratory (MolecularMD) for all patients at baseline. In patients without mutations detected at baseline, subsequent mutational analyses were performed if any of the following occurred during the first year on study: failure to achieve MMR at 12 months, confirmed loss of MMR, at least a fivefold increase in BCR-ABL1 transcript levels, progression to AP/BC, or treatment discontinuation for any reason. Mutational analyses were not performed beyond month 12.
Statistical analysis
To detect an odds ratio of ≥2.333 (based on the observed results from ENESTnd3 ) for the primary end point with a power of ≥85%, enrollment of 127 patients per arm was required. The primary end point was tested using a 2-sided Cochran-Mantel-Haenszel test stratified by Sokal risk group and prior rIFNɑ at a significance level of 0.05. For all secondary efficacy end points, P values provided are nominal and no multiplicity adjustments were made; therefore, statistical interpretation should be made with caution.
Response rates were provided at and/or by specific time points. Rates of response by a time point considered all patients who achieved a response at or before the indicated time point as responders. Patients with atypical BCR-ABL1 transcripts at baseline were considered nonresponders for MMR. For MMR and CCyR, patients without molecular or cytogenetic assessments, respectively, at or by the indicated time point were considered nonresponders. Response rates were compared between arms using a Cochran-Mantel-Haenszel test stratified by Sokal risk group and prior rIFNɑ. The Pearson-Clopper method was used to calculate 95% confidence intervals (CIs) for response rates. The Wald method was used to calculate 95% CIs for the difference in response rates between arms. Rate of MMR was also presented as a cumulative incidence graph with an increasing step function based on the time (after randomization) at which each new responder was recorded, thus reflecting the best recorded response rate. Time to progression to AP/BC, EFS, and OS were analyzed using the Kaplan-Meier method and were compared between arms using a stratified log-rank test on the basis of randomization strata. Efficacy analyses included all randomized patients; safety analyses included all patients who received ≥1 dose of study drug.
Results
Patients and treatments
A total of 267 patients were randomized (nilotinib, n = 134; imatinib, n = 133) at 13 sites in China between April 15, 2011 and July 28, 2011. The safety set consisted of 265 patients who received ≥1 dose of study drug (nilotinib, n = 133; imatinib, n = 132). Two patients (1 patient randomized to each arm) who were randomized but never treated were excluded from the safety set; all other patients received the treatment to which they were randomized (Figure 1). Patient characteristics at baseline were similar in each arm (Table 1). The median age was 41 years in the nilotinib arm and 39 years in the imatinib arm. Approximately half of the patients had low Sokal risk scores, one-third had intermediate Sokal risk scores, and 16% had high Sokal risk scores.
Baseline patient characteristics
| . | Nilotinib, n = 134 . | Imatinib, n = 133 . |
|---|---|---|
| Median age (range), y | 41 (18-76) | 39 (19-74) |
| Median time since diagnosis (range), d | 39.5 (7-177) | 38.0 (5-194)* |
| Sex | ||
| Male | 91 (67.9) | 81 (60.9) |
| Female | 43 (32.1) | 52 (39.1) |
| Atypical transcripts | 1 (0.7) | 1 (0.8) |
| Sokal risk group† | ||
| Low | 69 (51.5) | 69 (51.9) |
| Intermediate | 44 (32.8) | 43 (32.3) |
| High | 21 (15.7) | 21 (15.8) |
| Prior rIFNɑ therapy | ||
| Yes | 11 (8.2) | 11 (8.3) |
| No | 123 (91.8) | 122 (91.7) |
| Other prior therapy | ||
| Hydroxyurea | 129 (96.3) | 126 (94.7) |
| Imatinib‡ | 6 (4.5) | 7 (5.3) |
| Cardiovascular risk factors at baseline§ | ||
| Hypertension | 9 (6.8) | 10 (7.6) |
| Hyperlipidemia | 1 (0.8) | 2 (1.5) |
| Diabetes mellitus | 4 (3.0) | 5 (3.8) |
| Prior cardiovascular event | 3 (2.3) | 0 |
| . | Nilotinib, n = 134 . | Imatinib, n = 133 . |
|---|---|---|
| Median age (range), y | 41 (18-76) | 39 (19-74) |
| Median time since diagnosis (range), d | 39.5 (7-177) | 38.0 (5-194)* |
| Sex | ||
| Male | 91 (67.9) | 81 (60.9) |
| Female | 43 (32.1) | 52 (39.1) |
| Atypical transcripts | 1 (0.7) | 1 (0.8) |
| Sokal risk group† | ||
| Low | 69 (51.5) | 69 (51.9) |
| Intermediate | 44 (32.8) | 43 (32.3) |
| High | 21 (15.7) | 21 (15.8) |
| Prior rIFNɑ therapy | ||
| Yes | 11 (8.2) | 11 (8.3) |
| No | 123 (91.8) | 122 (91.7) |
| Other prior therapy | ||
| Hydroxyurea | 129 (96.3) | 126 (94.7) |
| Imatinib‡ | 6 (4.5) | 7 (5.3) |
| Cardiovascular risk factors at baseline§ | ||
| Hypertension | 9 (6.8) | 10 (7.6) |
| Hyperlipidemia | 1 (0.8) | 2 (1.5) |
| Diabetes mellitus | 4 (3.0) | 5 (3.8) |
| Prior cardiovascular event | 3 (2.3) | 0 |
Values are n (%) unless otherwise indicated.
Two patients in the imatinib arm were enrolled >6 months after initial diagnosis.
Sokal risk scores calculated per evaluations performed at diagnosis (prior to receipt of any CML treatment).
No patient was previously treated with imatinib for >2 weeks.
In the safety population (nilotinib, n = 133; imatinib, n = 132).
As of the 24-month data cutoff (May 28, 2013), 86.6% and 88.7% of patients in the nilotinib and imatinib arms, respectively, remained on treatment. Approximately 96% of patients in each arm were either on treatment or in follow-up for survival and progression status after discontinuation of treatment (Table 2). The most common reasons for discontinuation of study drug were disease progression (nilotinib, n = 4; imatinib, n = 6) and treatment failure (nilotinib, n = 6; imatinib, n = 3). The median time on treatment was 22.3 months in the nilotinib arm and 22.6 months in the imatinib arm. In the nilotinib arm, 113 (85.0%) patients received an actual nilotinib dose intensity of >400 to ≤600 mg per day, 18 (13.5%) received >200 to ≤400 mg per day, and 2 (1.5%) received ≤200 mg per day; the median actual dose intensity was 579.4 mg per day (25th-75th percentile, 507.0-597.8 mg per day). In the imatinib arm, 100 (75.8%) patients received an actual imatinib dose intensity of >200 to ≤400 mg per day, 32 (24.2%) received >400 to ≤600 mg per day, and no patient received ≤200 mg per day; the median actual dose intensity was 399.4 mg per day (25th-75th percentile, 380.8-400.0 mg per day).
Patient disposition
| . | Nilotinib, n = 134 . | Imatinib, n = 133 . |
|---|---|---|
| Remaining on study* | 129 (96.3) | 128 (96.2) |
| Remaining on treatment | 116 (86.6) | 118 (88.7) |
| Discontinued treatment† | 18 (13.4) | 15 (11.3) |
| Treatment failure‡ | 6 (4.5) | 3 (2.3) |
| Disease progression§ | 4 (3.0) | 6 (4.5) |
| Adverse events | 4 (3.0) | 2 (1.5) |
| Consent withdrawal|| | 4 (3.0) | 1 (0.8) |
| Lost to follow-up | 0 | 1 (0.8) |
| Protocol deviation | 0 | 1 (0.8) |
| Suboptimal response‡ | 0 | 1 (0.8) |
| . | Nilotinib, n = 134 . | Imatinib, n = 133 . |
|---|---|---|
| Remaining on study* | 129 (96.3) | 128 (96.2) |
| Remaining on treatment | 116 (86.6) | 118 (88.7) |
| Discontinued treatment† | 18 (13.4) | 15 (11.3) |
| Treatment failure‡ | 6 (4.5) | 3 (2.3) |
| Disease progression§ | 4 (3.0) | 6 (4.5) |
| Adverse events | 4 (3.0) | 2 (1.5) |
| Consent withdrawal|| | 4 (3.0) | 1 (0.8) |
| Lost to follow-up | 0 | 1 (0.8) |
| Protocol deviation | 0 | 1 (0.8) |
| Suboptimal response‡ | 0 | 1 (0.8) |
Values are n (%).
Patients on treatment or in follow-up (safety or survival) as of cutoff date.
Includes 1 patient in each arm who did not receive study drug.
Per investigator assessment (derived from modified 2009 European LeukemiaNet recommendations8 ).
As a reason for discontinuation, disease progression was defined per investigator judgment, as reported on the end of treatment case report form. Two additional patients in the nilotinib arm progressed to AP/BC on study but did not discontinue treatment due to disease progression (1 patient progressed during follow-up after treatment discontinuation; 1 patient remained on treatment despite progression [protocol deviation]).
At the time of discontinuation due to withdrawal of consent, 1 patient in the nilotinib arm had adverse events related to biochemical abnormalities (grade 1/2 elevations in bilirubin, alanine aminotransferase, and aspartate aminotransferase); the other 4 patients who discontinued due to withdrawal of consent did not have adverse events reported around the time of discontinuation.
Efficacy
The study met its primary end point with a statistically significantly (P < .0001) higher rate of MMR at 12 months in the nilotinib arm (52.2%; 95% CI, 43.4%-60.9%) vs the imatinib arm (27.8%; 95% CI, 20.4%-36.3%). Rates of MMR at 12 months were higher on nilotinib vs imatinib across all Sokal risk groups and regardless of prior rIFNɑ (Table 3).
MMR at 12 months (primary end point)
| . | Nilotinib . | Imatinib . | Nilotinib vs imatinib response rate difference, % (95% CI)* . |
|---|---|---|---|
| Overall | 134 | 133 | — |
| Patients with response | 70 (52.2) | 37 (27.8) | 24.4 (13.0-35.8) |
| P vs imatinib | <.0001 | — | |
| Low Sokal risk† | 69 | 69 | — |
| Patients with response‡ | 44 (63.8) | 22 (31.9) | 31.9 (16.1-47.7) |
| Intermediate Sokal risk† | 44 | 43 | — |
| Patients with response‡ | 18 (40.9) | 13 (30.2) | 10.7 (9.3-30.7) |
| High Sokal risk† | 21 | 21 | — |
| Patients with response‡ | 8 (38.1) | 2 (9.5) | 28.6 (4.3-52.8) |
| No prior rIFNɑ† | 123 | 122 | — |
| Patients with response‡ | 64 (52.0) | 33 (27.0) | 25.0 (13.1-36.8) |
| Prior rIFNɑ† | 11 | 11 | — |
| Patients with response‡ | 6 (54.5) | 4 (36.4) | 18.2 (−22.7-59.1) |
| . | Nilotinib . | Imatinib . | Nilotinib vs imatinib response rate difference, % (95% CI)* . |
|---|---|---|---|
| Overall | 134 | 133 | — |
| Patients with response | 70 (52.2) | 37 (27.8) | 24.4 (13.0-35.8) |
| P vs imatinib | <.0001 | — | |
| Low Sokal risk† | 69 | 69 | — |
| Patients with response‡ | 44 (63.8) | 22 (31.9) | 31.9 (16.1-47.7) |
| Intermediate Sokal risk† | 44 | 43 | — |
| Patients with response‡ | 18 (40.9) | 13 (30.2) | 10.7 (9.3-30.7) |
| High Sokal risk† | 21 | 21 | — |
| Patients with response‡ | 8 (38.1) | 2 (9.5) | 28.6 (4.3-52.8) |
| No prior rIFNɑ† | 123 | 122 | — |
| Patients with response‡ | 64 (52.0) | 33 (27.0) | 25.0 (13.1-36.8) |
| Prior rIFNɑ† | 11 | 11 | — |
| Patients with response‡ | 6 (54.5) | 4 (36.4) | 18.2 (−22.7-59.1) |
Values are n or n (%) unless otherwise indicated.
95% CIs for the differences in MMR rates between treatment groups calculated using the Wald method.
Total number of patients in each subgroup.
Frequency of MMR at 12 months among patients in each subgroup.
The cumulative incidence of MMR was higher on nilotinib vs imatinib throughout 24 months of follow-up (Figure 2). Substantially more patients in the nilotinib arm (68 of 134) than in the imatinib arm (37 of 133) achieved durable MMR at 24 months (defined as MMR at 12 and 24 months with no confirmed loss of MMR between 12 and 24 months). Among patients who achieved MMR by the 24-month cutoff, the median time to MMR was 5.55 months in the nilotinib arm (25th-75th percentiles, 5.36-8.31 months) and 10.86 months in the imatinib arm (25th-75th percentiles, 5.55-16.53 months).
Cumulative incidence of MMR. * indicates patients with atypical transcripts at baseline or missing molecular assessments by the indicated time point were considered nonresponders. Absolute difference between rates of MMR in the nilotinib arm vs the imatinib arm: by 12 months, 25.1% (95% CI, 13.6%-36.6%); by 24 months, 15.3% (95% CI, 3.7%-26.9%). †, P value is nominal. ‡, Response rates consider each month to consist of one 28-day cycle.
Cumulative incidence of MMR. * indicates patients with atypical transcripts at baseline or missing molecular assessments by the indicated time point were considered nonresponders. Absolute difference between rates of MMR in the nilotinib arm vs the imatinib arm: by 12 months, 25.1% (95% CI, 13.6%-36.6%); by 24 months, 15.3% (95% CI, 3.7%-26.9%). †, P value is nominal. ‡, Response rates consider each month to consist of one 28-day cycle.
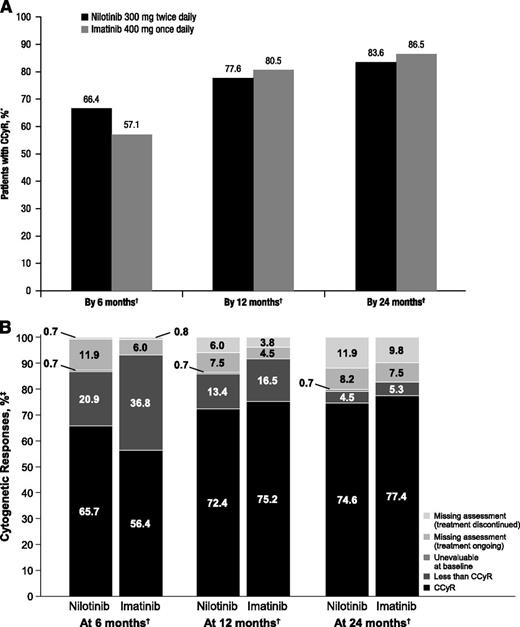
CCyR rates in both arms were high and comparable (Figure 3A). By 6 months, 66.4% (95% CI, 57.8%-74.3%) and 57.1% (95% CI, 48.3%-65.7%) of patients in the nilotinib and imatinib arms, respectively, achieved CCyR; these cumulative rates increased to 77.6% (95% CI, 69.6%-84.4%) and 80.5% (95% CI, 72.7%-86.8%), respectively, by 12 months and to 83.6% (95% CI, 76.2%-89.4%) and 86.5% (95% CI, 79.5%-91.8%), respectively, by 24 months. In both arms, most patients with cytogenetic assessments at 12 and 24 months were in CCyR (Figure 3B).
Rates of CCyR. * indicates that patients with missing cytogenetic assessments by the indicated time point were considered nonresponders. †, Response rates consider each month to consist of one 28-day cycle. ‡, Percentages may not add up to 100 due to rounding. (A) Cumulative incidence of CCyR by 6, 12, and 24 months. (B) Cytogenetic responses at 6, 12, and 24 months.
Rates of CCyR. * indicates that patients with missing cytogenetic assessments by the indicated time point were considered nonresponders. †, Response rates consider each month to consist of one 28-day cycle. ‡, Percentages may not add up to 100 due to rounding. (A) Cumulative incidence of CCyR by 6, 12, and 24 months. (B) Cytogenetic responses at 6, 12, and 24 months.
Among patients with typical transcripts at baseline and evaluable 3-month molecular assessments, the rate of BCR-ABL1IS ≤10% at 3 months was 82.0% (105 of 128) in the nilotinib arm and 66.7% (86 of 129) in the imatinib arm.
An M351T mutation was detected at baseline in 1 patient in the imatinib arm; no other baseline mutations were detected. Among patients without baseline mutations, fewer patients in the nilotinib arm (n = 26) vs in the imatinib arm (n = 53) had evaluable postbaseline mutational analyses by 12 months because lack or loss of response, which triggered postbaseline mutational analysis, occurred less frequently with nilotinib vs imatinib. Treatment-emergent mutations were detected in 6 and 3 patients in the nilotinib and imatinib arms, respectively. Of the 6 patients with treatment-emergent mutations in the nilotinib arm, 3 developed multiple mutations and 3 developed single mutations; 3, 1, and 2 patients were in the low, intermediate, and high Sokal risk groups, respectively. Treatment-emergent mutations detected in the nilotinib arm included Y253H (n = 3), F359V (n = 3), E255V (n = 1), T315I (n = 1), and F359I (n = 1). In the imatinib arm, 3 patients (2 in the low Sokal risk group and 1 in the high Sokal risk group) each developed single mutations, including Y253H, T315I, and E355G. In each arm, the patient with a treatment-emergent T315I mutation was in the high Sokal risk group.
By the 24-month data cutoff, 10 patients in the nilotinib arm and 8 patients in the imatinib arm experienced an EFS event. Rates of estimated EFS were similar between the 2 arms at 24 months: 91.7% (95% CI, 85.1%-95.5%) in the nilotinib arm and 93.8% (95% CI, 87.9%-96.8%) in the imatinib arm. Six patients in each arm progressed to AP/BC on study (based on objective hematology criteria, including progressions after discontinuation of treatment); the estimated rate of freedom from progression to AP/BC on study at 24 months was 95.4% for each arm. No deaths occurred on treatment in either arm. Two patients in each arm died >28 days after discontinuation of study treatment, including 1 death in each arm due to disease progression. In both arms, estimated OS at 24 months was 98.5%.
Safety
By the 24-month cutoff, 68.4% (91 of 133) and 59.8% (79 of 132) of patients in the nilotinib and imatinib arms, respectively, experienced adverse events (AEs) leading to dose interruption/adjustment. In both arms, the AE most commonly leading to dose interruption/adjustment was thrombocytopenia (nilotinib, 20.3%; imatinib, 17.4%). No nonlaboratory AE led to dose interruption/adjustment for >5% of patients in either arm. In the nilotinib arm, 4 patients discontinued due to AEs (bone marrow failure, cerebral hemorrhage, hyperbilirubinemia, and neutropenia [1 each]). In the imatinib arm, 2 patients discontinued due to AEs (recurrent non-Hodgkin lymphoma and thrombocytosis in the context of treatment failure [1 each]).
Most nonlaboratory AEs were grade 1/2 (Table 4). The most common nonlaboratory AE on nilotinib was rash. The most common nonlaboratory AE on imatinib was eyelid edema, which contributed to a higher total frequency of AEs related to fluid retention on imatinib (34.8%) than on nilotinib (7.5%). All fluid retention AEs in both arms were grade 1/2. Nine patients (6.8%) in each arm had an absolute QTcF interval >450 ms; no patient had an absolute QTcF interval >480 ms. In the nilotinib arm, 1 patient with a history of cerebral infarction experienced a grade 3 ischemic cerebrovascular event. No other ischemic cardiovascular events, including peripheral artery disease, occurred in either arm. A patient in the nilotinib arm with a prior history of diabetes mellitus had recurrent diabetes mellitus. Hypertension was reported in 1 nilotinib-treated patient vs no imatinib-treated patients.
Safety findings
| . | Nilotinib, n = 133 . | Imatinib, n = 132 . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Adverse events | ||||
| Rash | 47 (35.3) | 2 (1.5) | 17 (12.9) | 1 (0.8) |
| Myalgia | 16 (12.0) | 1 (0.8) | 8 (6.1) | 0 |
| Nasopharyngitis | 14 (10.5) | 0 | 22 (16.7) | 0 |
| Eyelid edema | 4 (3.0) | 0 | 23 (17.4) | 0 |
| Diarrhea | 4 (3.0) | 0 | 15 (11.4) | 1 (0.8) |
| Hematologic abnormalities | ||||
| Platelet count (direct) | 78 (58.6) | 34 (25.6) | 81 (61.4) | 40 (30.3) |
| Absolute neutrophils (segmented and bands) | 70 (52.6) | 28 (21.1) | 107 (81.1) | 29 (22.0) |
| Total white blood cells | 77 (57.9) | 13 (9.8) | 106 (80.3) | 23 (17.4) |
| Absolute lymphocytes | 91 (68.4) | 6 (4.5) | 108 (81.8) | 18 (13.6) |
| Hemoglobin | 51 (38.3) | 5 (3.8) | 70 (53.0) | 8 (6.1) |
| Biochemical abnormalities | ||||
| Bilirubin (total) | 116 (87.2) | 6 (4.5) | 25 (18.9) | 0 |
| Alanine aminotransferase | 77 (57.9) | 4 (3.0) | 47 (35.6) | 1 (0.8) |
| Phosphate (inorganic phosphorus) | 66 (49.6) | 6 (4.5) | 95 (72.0) | 7 (5.3) |
| Cholesterol (total) | 58 (43.6) | 0 | 11 (8.3) | 0 |
| Lipase (blood) | 55 (41.4) | 19 (14.3) | 59 (44.7) | 9 (6.8) |
| Glucose: hyper | 47 (35.3) | 2 (1.5) | 36 (27.3) | 0 |
| Calcium: hypo | 46 (34.6) | 1 (0.8) | 67 (50.8) | 1 (0.8) |
| Aspartate aminotransferase | 43 (32.3) | 0 | 27 (20.5) | 1 (0.8) |
| Sodium: hyper | 37 (27.8) | 0 | 32 (24.2) | 0 |
| Triglycerides | 34 (25.6) | 1 (0.8) | 35 (26.5) | 3 (2.3) |
| Magnesium: hyper | 31 (23.3) | 8 (6.0) | 27 (20.5) | 7 (5.3) |
| Potassium: hypo | 28 (21.1) | 2 (1.5) | 66 (50.0) | 3 (2.3) |
| Alkaline phosphatase, serum | 18 (13.5) | 0 | 35 (26.5) | 0 |
| . | Nilotinib, n = 133 . | Imatinib, n = 132 . | ||
|---|---|---|---|---|
| Any grade . | Grade 3/4 . | Any grade . | Grade 3/4 . | |
| Adverse events | ||||
| Rash | 47 (35.3) | 2 (1.5) | 17 (12.9) | 1 (0.8) |
| Myalgia | 16 (12.0) | 1 (0.8) | 8 (6.1) | 0 |
| Nasopharyngitis | 14 (10.5) | 0 | 22 (16.7) | 0 |
| Eyelid edema | 4 (3.0) | 0 | 23 (17.4) | 0 |
| Diarrhea | 4 (3.0) | 0 | 15 (11.4) | 1 (0.8) |
| Hematologic abnormalities | ||||
| Platelet count (direct) | 78 (58.6) | 34 (25.6) | 81 (61.4) | 40 (30.3) |
| Absolute neutrophils (segmented and bands) | 70 (52.6) | 28 (21.1) | 107 (81.1) | 29 (22.0) |
| Total white blood cells | 77 (57.9) | 13 (9.8) | 106 (80.3) | 23 (17.4) |
| Absolute lymphocytes | 91 (68.4) | 6 (4.5) | 108 (81.8) | 18 (13.6) |
| Hemoglobin | 51 (38.3) | 5 (3.8) | 70 (53.0) | 8 (6.1) |
| Biochemical abnormalities | ||||
| Bilirubin (total) | 116 (87.2) | 6 (4.5) | 25 (18.9) | 0 |
| Alanine aminotransferase | 77 (57.9) | 4 (3.0) | 47 (35.6) | 1 (0.8) |
| Phosphate (inorganic phosphorus) | 66 (49.6) | 6 (4.5) | 95 (72.0) | 7 (5.3) |
| Cholesterol (total) | 58 (43.6) | 0 | 11 (8.3) | 0 |
| Lipase (blood) | 55 (41.4) | 19 (14.3) | 59 (44.7) | 9 (6.8) |
| Glucose: hyper | 47 (35.3) | 2 (1.5) | 36 (27.3) | 0 |
| Calcium: hypo | 46 (34.6) | 1 (0.8) | 67 (50.8) | 1 (0.8) |
| Aspartate aminotransferase | 43 (32.3) | 0 | 27 (20.5) | 1 (0.8) |
| Sodium: hyper | 37 (27.8) | 0 | 32 (24.2) | 0 |
| Triglycerides | 34 (25.6) | 1 (0.8) | 35 (26.5) | 3 (2.3) |
| Magnesium: hyper | 31 (23.3) | 8 (6.0) | 27 (20.5) | 7 (5.3) |
| Potassium: hypo | 28 (21.1) | 2 (1.5) | 66 (50.0) | 3 (2.3) |
| Alkaline phosphatase, serum | 18 (13.5) | 0 | 35 (26.5) | 0 |
Values are n (%).
Nonlaboratory adverse events occurring in ≥10% of patients in either arm at any grade (regardless of relationship to study drug) and newly occurring or worsening laboratory abnormalities occurring in ≥25% of patients in either arm at any grade or ≥5% of patients in either arm at grade 3/4.
The observed newly occurring or worsening laboratory abnormalities were consistent with the known safety profile of each drug.3-5 Newly occurring or worsening glucose elevations of any grade were reported in 35.3% (47 of 133) and 27.3% (36 of 132) of patients in the nilotinib and imatinib arms, respectively, including in 2 patients in the nilotinib arm with grade 3 elevations and no grade 4 elevations. Both patients with grade 3 glucose elevations had a history of diabetes mellitus; one of these patients developed grade 4 lipase elevation at one point during the study, whereas the second patient did not have any other grade 3/4 biochemical abnormalities. Newly occurring or worsening total cholesterol elevations were reported in 43.6% (58 of 133) and 8.3% (11 of 132) of patients in the nilotinib and imatinib arms, respectively; all cholesterol elevations were grade 1/2. Lipid abnormalities were reported in 4.5% (6 of 133) of patients in the nilotinib arm (hypertriglyceridemia, n = 3; blood cholesterol increased, blood triglycerides increased, hypercholesterolemia, and hyperlipidemia, n = 1 each) and 2.3% (3 of 132) of patients in the imatinib arm (hypertriglyceridemia, n = 2; blood cholesterol increased and blood triglycerides increased, n = 1 each).
Discussion
Results from ENESTchina confirm the safety and efficacy of nilotinib, as previously demonstrated in ENESTnd,3-7 and extend findings to Chinese patients with newly diagnosed Ph+ CML-CP. Because genetic differences between ethnic populations can result in differing efficacy and safety profiles for any drug (eg, BIM deletion polymorphism, which is common in Asian populations and is associated with resistance to TKI therapy9,10 ), local trials focusing on specific patient populations, such as Chinese patients in ENESTchina, are a crucial step toward enabling physicians to make personalized treatment decisions.
ENESTchina met its primary efficacy end point with a statistically significantly higher rate of MMR at 12 months on nilotinib vs imatinib, providing the first prospective confirmation of the results of ENESTnd in a Chinese population. Compared with imatinib, nilotinib resulted in higher rates of MMR across all Sokal risk groups and regardless of prior rIFNɑ therapy. Nilotinib also induced MMR more rapidly than did imatinib; among patients who achieved MMR, those in the nilotinib arm achieved the response in approximately half the time of those in the imatinib arm. In ENESTnd, analysis of the kinetics of molecular responses achieved with nilotinib and imatinib revealed a similar pattern,4 and the improvement in MMR rates with nilotinib vs imatinib was maintained throughout 5 years of follow-up.11 Furthermore, although deep molecular responses, which have been shown to predict improved OS and can lead to successful maintenance of treatment-free remission,12-14 were not evaluated in ENESTchina, nilotinib resulted in consistently higher rates of deep molecular response than imatinib in ENESTnd.11
In contrast to the pattern observed for MMR rates, cytogenetic response rates were similar between arms. This was particularly true at later time points, when most evaluated patients (in both arms) were in CCyR. Six-month rates of CCyR were numerically higher with nilotinib vs imatinib (no statistical difference); by 24 months, CCyR rates were comparable between arms. Early CCyR is a well-established surrogate marker for survival in patients with CML,15,16 and as such is designated as an optimal response at 6 months in the 2013 ELN recommendations.17 At later time points, however, MMR becomes a key predictor of outcome,12,18 and MMR is the designated optimal response at 12 months and beyond.17 The apparent discrepancy between CCyR and MMR rates suggests that responses were deeper with nilotinib vs imatinib.
Baseline patient characteristics suggest that the population enrolled in ENESTchina may have had more favorable prognosis vs those in ENESTnd. Compared with patients in ENESTnd, patients in ENESTchina tended to be younger (median ages of patients in ENESTnd were 47 years [nilotinib arms] and 46 years [imatinib arm] compared with 41 and 39 years, respectively, in ENESTchina) and had lower Sokal risk scores (in ENESTnd, 37%, 36%, and 28% of patients in each arm were in the low, intermediate, and high Sokal risk groups, respectively, compared with 51%-52%, 32%-33%, and 16%, respectively, in ENESTchina).3 The reason for these differences is unknown, although epidemiological data suggest that CML tends to occur at a younger age in Asian patients than in other populations,19 and because Sokal risk score calculation takes patient age into account,20 the younger age of patients in this study can partially explain the Sokal risk score distribution.
Unlike ENESTnd, in which nilotinib resulted in higher rates of freedom from progression to AP/BC than imatinib,3-7 rates of freedom from progression to AP/BC were high and identical in both arms of ENESTchina. The rates of OS and CCyR were also high and similar in both arms. Although data cannot be compared between studies, it is notable that the rates of freedom from progression to AP/BC, OS, and CCyR achieved with imatinib were all numerically higher in ENESTchina vs ENESTnd, whereas the rates achieved with nilotinib were similar between studies3-7 ; the reason for this difference is unknown. It may be partially explained by the relatively favorable prognosis of the patients in ENESTchina (in terms of Sokal risk score and age) or potential for improved management of imatinib-treated patients in ENESTchina vs earlier studies. The low frequency of BCR-ABL1 mutations in both arms of ENESTchina may similarly be related to the low Sokal risk scores and high response rates among patients in this study.
Safety data were consistent with those from other studies.3-5,21 No new safety concerns were identified for either drug. With 2 years of follow-up, rash, myalgia, nasopharyngitis, eyelid edema, and diarrhea were the only nonlaboratory AEs that occurred in >10% of patients in either arm. A single cerebrovascular AE and no cardiac or peripheral arterial events were reported. The young age and low frequency of preexisting cardiovascular risk factors among patients in this study, as well as the limited follow-up duration for this analysis, may have contributed to the lack of observed cardiovascular safety issues; in other studies with longer follow-up, higher frequencies of cardiovascular events (ie, ischemic heart disease, ischemic cerebrovascular events, and peripheral artery disease) were reported during nilotinib treatment, particularly in older patients and those with preexisting risk factors.7,22,23 Moreover, although new onset of diabetes during treatment with nilotinib has been reported in other studies, including ENESTnd,24,25 no patient in ENESTchina without a prior history of diabetes mellitus developed grade 3/4 glucose elevation or an AE of diabetes mellitus by the cutoff date for this analysis.
Grade 1/2 cholesterol elevations (corresponding to total cholesterol levels between the upper limit of normal and 10.34 mmol/L26 ) were more common in the nilotinib arm (43.6%) than in the imatinib arm (8.3%). Grade 1/2 serum glucose elevations (corresponding to serum glucose levels between the upper limit of normal and 13.9 mmol/L26 ) were also somewhat more common with nilotinib. Active monitoring and management of laboratory abnormalities and other potential complications are important for patients receiving nilotinib or any other TKI therapy,1,17 and, if test results warrant intervention, these complications should be managed according to local treatment guidelines and standards of practice. In particular, therapeutic and/or lifestyle interventions may be recommended, even for some patients with cholesterol levels in the grade 0 or 1 range.27-30 For patients who require lipid-lowering medication, the possibility of drug-drug interactions should be considered. Some statins are metabolized via the CYP3A4 pathway, which also metabolizes, and is inhibited by, TKIs such as imatinib and nilotinib.31
In conclusion, results from ENESTchina demonstrated superior rates of MMR at 12 months with nilotinib 300 mg twice daily vs imatinib 400 mg once daily in Chinese patients with newly diagnosed Ph+ CML-CP.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Priscille Bourquelot and Svetlana Jevtic (Novartis Pharma AG) and Prof Qian Jiang (Peking University People’s Hospital, Beijing, China) for ongoing medical and scientific support, as well as Staci Heise and Karen Kaluza (ArticulateScience, LLC) for medical editorial assistance with this manuscript. The authors also thank all other ENESTchina clinical investigators: Drs Zhen Xia, Yang Yu, Huiying Qiu, Sai Lou, Xiong Ni, Jian Huang, Yejiang Lou, Zhen Cai, Weiyan Zheng, Jimin Shi, Jingsong He, De Zhou, Jinsong Jia, Hao Jiang, Bingcheng Liu, Ming Hong, Lei Fan, Kourong Miao, Li Wang, Run Zhang, Yadan Wang, Yong You, Wenjuan He, Jing Li, Liangfang Zhu, Langhui Zhang, Ting Yang, Tingbo Liu, Yanjuan Lin, Danhui Fu, Rong Zhan, Nainong Li, Zhihong Zheng, Jing Zheng, Jianyu Weng, Zesheng Lu, Chengwei Luo, Min Dai, Jiazhuo Liu, Li Zhang, and Xuemei Qin.
The ENESTchina study and work presented here were sponsored and funded by Novartis Pharmaceuticals Corporation. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
Authorship
Contribution: S.H., H.D.M., M.B., and X.H. designed research; Jianxiang Wang, J.J., Y.H., X.D., J.L., H.Z., J.H., Jianmin Wang, M.H., X.H., and F.M. performed research; X.H. contributed vital new reagents or analytical tools; Jianxiang Wang, H.H., H.Z., J.H., Jianmin Wang, H.D.M., and F.M. collected data; Jianxiang Wang, Z.-X.S., G.S., H.Z., S.H., H.D.M., C.-E.O., Y.Y., M.B., X.H., and C.T. analyzed and interpreted data; C.-E.O. performed statistical analysis; and all authors have drafted and approved the manuscript.
Conflict-of-interest disclosure: Jianxiang Wang acted as a consultant for Novartis and Bristol-Myers Squibb. G.S. acted as a consultant and attended a speakers’ bureau for Novartis, Bristol-Myers Squibb, Pfizer, and Ariad. S.H. and H.D.M. are Novartis Pharma AG employees and stockholders. C.E.-O. is an employee of Novartis Pharma AG. C.T. is an employee of Novartis Pharmaceuticals Corporation. Y.Y. is an employee of Beijing Novartis Pharma Company, Limited. M.B. acted as a consultant to, received honoraria from, and attended a speaker bureau for Novartis, Bristol-Myers Squibb, Pfizer, and Ariad, and attended an advisory board for Novartis and Ariad. The remaining authors declare no competing financial interests.
Correspondence: Xiaojun Huang, Peking University People’s Hospital, Peking University Institute of Hematology, 11 Xizhimen South Street, Beijing 100044, China; e-mail: xjhrm@medmail.com.cn.