Key Points
Itpkb produces the soluble messenger IP4, which limits cytokine-induced Akt/mTORC1 activation in HSC.
Itpkb loss in mice activates HSC and impairs their longevity and function, resulting in lethal hematopoietic failure and anemia.
Abstract
Tight regulation of hematopoietic stem cell (HSC) homeostasis ensures lifelong hematopoiesis and prevents blood cancers. The mechanisms balancing HSC quiescence with expansion and differentiation into hematopoietic progenitors are incompletely understood. Here, we identify Inositol-trisphosphate 3-kinase B (Itpkb) as an essential regulator of HSC homeostasis. Young Itpkb−/− mice accumulated phenotypic HSC, which were less quiescent and proliferated more than wild-type (WT) controls. Itpkb−/− HSC downregulated quiescence and stemness associated, but upregulated activation, oxidative metabolism, protein synthesis, and lineage associated messenger RNAs. Although they had normal-to-elevated viability and no significant homing defects, Itpkb−/− HSC had a severely reduced competitive long-term repopulating potential. Aging Itpkb−/− mice lost hematopoietic stem and progenitor cells and died with severe anemia. WT HSC normally repopulated Itpkb−/− hosts, indicating an HSC-intrinsic Itpkb requirement. Itpkb−/− HSC showed reduced colony-forming activity and increased stem-cell-factor activation of the phosphoinositide-3-kinase (PI3K) effectors Akt/mammalian/mechanistic target of rapamycin (mTOR). This was reversed by treatment with the Itpkb product and PI3K/Akt antagonist IP4. Transcriptome changes and biochemistry support mTOR hyperactivity in Itpkb−/− HSC. Treatment with the mTOR-inhibitor rapamycin reversed the excessive mTOR signaling and hyperproliferation of Itpkb−/− HSC without rescuing colony forming activity. Thus, we propose that Itpkb ensures HSC quiescence and function through limiting cytokine-induced PI3K/mTOR signaling and other mechanisms.
Introduction
All blood cells are derived from pluripotent long-term repopulating hematopoietic stem cells (HSC).1 Long-term repopulating HSC (LT-HSC) are kept quiescent in hypoxic bone marrow (BM) niches and self-renew by rare division. Infections or blood loss can induce temporary LT-HSC activation, proliferation, and differentiation via various short-term repopulating multipotent progenitor (MPP) and hematopoietic progenitor cell (HPC) intermediates into mature blood cells.2-4 Perturbed HSC homeostasis can cause BM failure, anemia, immunodeficiencies, or blood cancers. To avoid this, LT-HSC quiescence, proliferation, survival, and differentiation must be properly balanced.3,5,6 The underlying molecular mechanisms are incompletely understood, but highly important for regenerative medicine and blood cancer therapies. They include signaling from niche cytokines such as stem cell factor (SCF) through its receptor c-Kit on HSC, LT-HSC niche–cell interactions, and metabolic regulation.3,7,8 SCF signaling may need to be tuned into a window that ensures LT-HSC quiescence and self-renewal, but avoids activation and development of myeloproliferative disorders (MPD).9-14
Proto-oncogenic class I phosphoinositide-3-kinases (PI3K) and their effectors Akt, FoxO, and mammalian/mechanistic target of rapamycin complexes 1/2 (mTORC1/2) are important regulators of HSC homeostasis downstream of cytokine receptors.3,15-18 PI3K produce the membrane-lipid phosphatidylinositol(3,4,5)trisphosphate (PIP3), a recruiting and activating ligand for Akt and other effectors.19 Partial PI3K-loss in mice reduced fetal liver HSC competitive multilineage repopulating potential, HPC numbers, and SCF-induced proliferation.20 PI3K–Wnt/β-catenin cooperation promotes HSC self-renewal and expansion.21 PI3K-hyperactivity contributes to hematologic malignancies.19 Loss of PIP3-removing phosphatases severely dysregulates HSC homeostasis: induced phosphatase-and-tensin-homolog (PTEN) deletion caused HSC short-term proliferative expansion followed by depletion, but also MPD, acute myeloid (AML), and T-lymphoblastic leukemia due to leukemic stem cell expansion.22,23 The mechanism includes Akt/mTORC hyperactivation in HSC or other hematopoietic cells, although the relative importance of HSC intrinsic vs extrinsic PTEN function is controversial.18,24,25 SH2–domain-containing-inositol-5′-phosphatase-1 (SHIP-1)−/− mice showed increased HSC numbers, proliferation, and extramedullary hematopoiesis, reduced HSC BM homing and long-term reconstituting potential. They developed fatal MPD.26-29 SHIP controls HSC homeostasis primarily by acting in niche cells.30,31 HSC treatment with SCF or other cytokines activates Akt.10,11,32 Akt limits LT-HSC quiescence and promotes HSC function and differentiation by ensuring sufficient levels of reactive oxygen species.33 Constitutive Akt activation in HSC caused hyperproliferation, apoptosis, engraftment defects, HSC depletion, and MPD, T-cell lymphoma, or AML.34 Clearly, limiting PI3K/Akt signaling in HSC and niche cells is key for maintaining functional HSC and preventing blood cancers, but the mechanisms dampening PI3K/Akt-signaling within HSC remain ill understood.
Inositol(1,4,5)trisphosphate 3-kinases (Itpks) phosphorylate the Ca2+-mobilizing soluble second messenger inositol(1,4,5)trisphosphate into inositol(1,3,4,5)tetrakisphosphate (IP4). We along with others have identified receptor-induced IP4 production by Itpkb as an essential signaling component in thymocytes, B cells, natural killer (NK) cells, myeloid progenitors, and neutrophils.35-42 In its best understood in vivo function, IP4 dampens Akt-recruitment and activation as a soluble PIP3 competitor, but it is unclear whether this is broadly relevant.43 Among the 3 mammalian Itpks a/b/c, HSC only express Itpkb significantly.36 Whether Itpkb has any function in HSC is unknown. To elucidate such functions, we analyzed HSC homeostasis and function in Itpkb−/− mice. Our results unveil Itpkb as a novel, essential mediator of LT-HSC quiescence that dampens cytokine-induced PI3K signaling to Akt/mTORC1 within HSC and limits LT-HSC activation to prevent HSC-exhaustion and BM failure.
Methods
Mice
Our C57BL/6 Itpkb−/− mice were described in Sauer et al.42 All mice were housed in the The Scripps Research Institute (TSRI) specific pathogen free (SPF) vivarium. Animal care and handling were approved and supervised by the TSRI Institutional Animal Care and Use Committee and performed in compliance with all applicable regulatory standards. Most mice were analyzed at 6 to 12 weeks of age, long-term BM chimeras and aging mice at 30 to 80 weeks. Where indicated, mice were injected intraperitoneally with 10 mg/kg body weight rapamycin in 10% ethanol/4.5% polyethyleneglycol 400/4.5% Tween-80 or vehicle alone every other day for 10 days followed by analysis. In aging studies, Itpkb−/− mice were euthanized once moribund along with aged Itpkb+/+ controls.
Competitive BM chimeras
CD45.2+Itpkb+/+ or Itpkb−/− BM cells were mixed 1:1 with CD45.1+Itpkb+/+ competitor BM cells. Each 106 cells were injected intravenously into multiple lethally irradiated CD45.1+CD45.2+ recipients. These were fed trimethoprim/sulfamethoxazole broad-spectrum antibiotics in acidified drinking water44 for 4 weeks. Peripheral blood leukocyte reconstitution was analyzed after 6, 16, or 24 weeks. For serial transfers, BM from reconstituted 1° recipients was pooled after 26 to 29 weeks. 106 cells were injected into lethally irradiated CD45.1+CD45.2+ 2° recipients. Chimerism was assessed after 14 to 18 weeks (43 weeks after 1° transfer).
FACS analyses
BM cells were incubated with anti-CD16/32 antibodies or 5% rat serum, stained with a biotinylated lineage (Lin)-cocktail, fluorochrome-conjugated antibodies against the markers shown in the respective figure and streptavidin (SA), fixed and analyzed.
Viability was analyzed by staining with Annexin V/7-aminoactinomycin D (7-AAD). For cell-cycle analysis, permeabilized cells were stained with anti-Ki67 antibodies (BD Pharmingen) and/or Hoechst 33342. Alternatively, mice were injected intraperitoneally with 2 mg 5-bromodeoxyuridine (BrdU). Two hours later, HSC BrdU content was analyzed by fluorescence-activated cell sorter (FACS) (BD Pharmingen).
For biochemistry, cells were fixed with formaldehyde, permeabilized with BD perm buffer II, and stained with antibodies against Akt, pAktT308, or pS6S235/S236 (Cell Signaling Technology), the biotinylated Lin-cocktail, F(ab)2-fluorescein isothiocyanate, antibodies against HSC/MPP surface markers, and streptavidin-qdot 605 (Life Technologies).
Samples were run on a BD LSR-II flow-cytometer and analyzed with FlowJo. For details, see supplemental Methods on the Blood Web site.
Blood smears
Blood was collected into heparinized tubes, spread onto microscope slides, fixed, stained, and analyzed as described in Figure 5F by Antech Diagnostics (Irvine, CA).
Colony-forming unit assays34
Forty sorted Lin-Sca-1+c-Kit+ (LSK) CD150+CD48− cells were incubated in triplicates for up to 10 days in M3434 methylcellulose media (Stemcell Technologies) containing 0 (vehicle), 100 or 300 nM rapamycin in ethanol, or nothing. Colonies were counted on day 7 or 8.
RNA-Sequencing
In triplicates, we prepared RNA from FACS-sorted LSK CD34−CD150+CD48−Flk2− LT-HSC, and RNA sequencing libraries with NuGEN Ovation RNA-Seq System V2. RNA was sequenced using an Illumina HISeq Analyzer 2000, Casava v1.8.2 genome analyzer pipeline, TopHat v1.4.1/Bowtie2 genome-alignment, and Partek v6.6 messenger RNA (mRNA) annotation software. Statistical analyses were done with edgeR,45 excluding genes with false discovery rates >0.15, log2 (counts per million) ≤4, and fold-change magnitudes ≤1.4 to avoid undefined values and the poorly defined log (fold changes) for counts close to 0. Unsupervised clustering of 441 significantly changed genes was done with dChip46 using rank correlation and a centroid linkage method. Raw data have been deposited in NCBI’s gene expression omnibus,47 series accession no. GSE56613. Gene set enrichment analysis (GSEA) was performed as in Subramanian et al48 with gene set permutation, using gene sets from MSigDB or manually curated from Forsberg et al,49 Venesia et al,50 Ivanova et al,51 and Laurenti et al52 (supplemental Table 4). For details, see supplemental Methods.
In vitro stimulation
Lin− BM cells were rested for 60 minutes, incubated for 30 minutes with 10 µM Akt inhibitor VIII (Merck), 100 µM IP4/PM-ester (Sirius), or 1% DMSO, and stimulated for 5 minutes with 1 ng/mL SCF (PeproTech), fixed, stained for LT-HSC/MPP1-3 markers, and analyzed by FACS.
Statistics
We analyzed differences between two groups by 2-tailed Student t test, Welch’s t test or Wilcoxon signed-rank test, between multiple groups by 1-way analysis of variance/Bonferroni’s post test, and between survival groups by log-rank and Gehan-Breslow-Wilcoxon tests (Prism).
Results
Phenotypic HSC expansion in Itpkb−/− mice
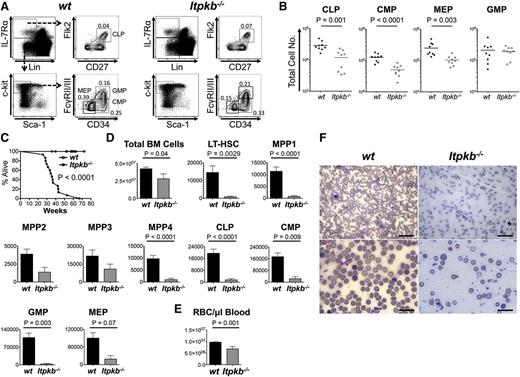
We first characterized phenotypic HSC in Itpkb−/− mice and WT littermates. Murine adult HSCs are enriched among lineage marker–deficient (Lin−) Sca-1+c-Kit+ (LSK) cells.53 They can be subclassified into LSK CD34-CD150+ LT-HSC and various LSK CD34+CD150+/− short-term reconstituting hematopoietic stem cell (ST-HSC)/MPP populations lacking LT-HSC function.1,4,54 LSK CD34+Flk-2− cells can generate LSK CD34+Flk-2+ MPP/lymphoid-primed multipotent progenitors,55 which lack self-renewal.56 LSK CD34+Flk-2−, but not Flk2+ cells support sustained myeloid and lymphoid reconstitution. We classified them as phenotypic ST-HSC/MPP and further distinguished LSK CD34+CD48−CD150+Flk-2− MPP1, LSK CD34+CD48+CD150+Flk-2− MPP2, and LSK CD34+CD48+CD150−Flk-2− MPP3.4 Nine- to 11-week-old Itpkb−/− mice had higher proportions of LT-HSC, MPP1-3, and LSK CD34+CD48+CD150−Flk-2+ MPP/MPP44 in the BM than WT mice, mildly, but significantly increased LT-HSC and MPP1, strongly increased MPP2/3, but comparable MPP4 numbers and total BM cellularity (Figure 1). In the spleens, Itpkb−/− mice had significantly increased MPP1-3, but WT–like LT-HSC and MPP4 numbers and total cellularity.
Phenotypic HSC expansion in Itpkb−/− mice. (A) FACS analysis of BM HSC and MPP subsets in Itpkb−/− and Itpkb+/+ (WT) mice. Lin−, CD3−CD4−CD8−CD11b−CD11c−GR-1−DX5−B220−CD19−Ter119−IL-7Rα−. LSK, Lin−Sca-1+c-Kit+. The cell types in each gate are indicated in the top panels and gated populations are above the panels. Numbers indicate percent of total BM cells in the respective gate. (B) Total phenotypic LT-HSC, MPP1-4, and cell numbers in the BM and spleens of WT or Itpkb−/− mice, pooled from 2 independent experiments. The P values for the indicated comparisons were obtained by unpaired t test (symbols, individual mice; horizontal bars, means). Age distributions of the mice were 9 to 11 weeks in the BM analysis (mean age = 9.8 weeks; standard deviation [SD] = 1 week; n = 6 per genotype) and in the spleen analysis (mean age = 9.6 weeks; SD = 0.9 weeks; n = 5 per genotype). Raw data is shown in supplemental Table 1.
Phenotypic HSC expansion in Itpkb−/− mice. (A) FACS analysis of BM HSC and MPP subsets in Itpkb−/− and Itpkb+/+ (WT) mice. Lin−, CD3−CD4−CD8−CD11b−CD11c−GR-1−DX5−B220−CD19−Ter119−IL-7Rα−. LSK, Lin−Sca-1+c-Kit+. The cell types in each gate are indicated in the top panels and gated populations are above the panels. Numbers indicate percent of total BM cells in the respective gate. (B) Total phenotypic LT-HSC, MPP1-4, and cell numbers in the BM and spleens of WT or Itpkb−/− mice, pooled from 2 independent experiments. The P values for the indicated comparisons were obtained by unpaired t test (symbols, individual mice; horizontal bars, means). Age distributions of the mice were 9 to 11 weeks in the BM analysis (mean age = 9.8 weeks; standard deviation [SD] = 1 week; n = 6 per genotype) and in the spleen analysis (mean age = 9.6 weeks; SD = 0.9 weeks; n = 5 per genotype). Raw data is shown in supplemental Table 1.
Itpkb−/− HSC have reduced quiescence
Compared with WT cells, Itpkb−/− LT-HSC, LSK CD34+CD150+ MPP1/2, and MPP3 each contained similar-to-mildly increased proportions of viable cells (Figure 2A). More ItpkB−/− than WT LSK Flk-2− LT-HSC/MPP1-3 were in the S/G2/M cell-cycle phases (Figure 2B). Itpkb−/− LSK CD34− LT-HSC had reduced proportions of quiescent cells in G0, WT–like proportions in G1 and each approximately threefold elevated proportions in S and G2/M (Figure 2B-C). Increased 5-bromo-2′-deoxyuridine incorporation confirmed LT-HSC hyperproliferation in Itpkb−/− mice (Figure 2D). Cell proliferation associates with an increased metabolism and cell size.8 Indeed, Itpkb−/− mice contained higher proportions of large, blastoid MPP than WT mice and tended to have more blastoid LT-HSC, although this was not statistically significant (Figure 2E-F).
Loss of HSC quiescence in Itpkb−/− mice. (A) Annexin V/7-AAD stain of the indicated phenotypic LT-HSC or MPP subsets in WT or Itpkb−/− BM. Numbers denote percent of cells per quadrant. Viable cells are double-negative. Representative of 2 independent experiments (nwt = 3; nKO =4). (B-C) Cell-cycle status of LSK Flk-2− LT-HSC/MPP1-3 and LSK CD34− LT-HSC in WT or Itpkb−/− mice, analyzed by Hoechst/Ki67 stain. (B) Representative data from 8 independent experiments. G0, Ki67−Hoechst 33342−. G1, Ki67+Hoechst 33342−. S, Ki67+Hoechst 33342int. G2/M, Ki67+Hoechst 33342hi. Numbers denote percent of cells per gate. (C) Percent of LT-HSC per cell-cycle phase for 11 mice/genotype (horizontal bars, means). KO, Itpkb−/−; WT, wild type. Statistical significance was determined by paired Student t test. (D) In vivo BrdU incorporation in LSK CD34− LT-HSC in WT or Itpkb−/− mice during a 2-hour pulse. Numbers denote percent of BrdU+ cells. BrdU−, uninjected WT control. Representative of 2 independent experiments. (E-F) Forward (FSC-A)/side-scatter (SSC-A) analysis of LSK CD34−CD150+ LT-HSC, LSK CD34+Flk-2− MPP1-3, and LSK CD34+Flk-2+ MPP4 in WT or Itpkb−/− BM. (E) Representative data from 5 independent experiments. Numbers denote percent of cells per gate. (F) Pooled data with means (horizontal lines) and P values for the indicated comparisons (unpaired t test; n = 11).
Loss of HSC quiescence in Itpkb−/− mice. (A) Annexin V/7-AAD stain of the indicated phenotypic LT-HSC or MPP subsets in WT or Itpkb−/− BM. Numbers denote percent of cells per quadrant. Viable cells are double-negative. Representative of 2 independent experiments (nwt = 3; nKO =4). (B-C) Cell-cycle status of LSK Flk-2− LT-HSC/MPP1-3 and LSK CD34− LT-HSC in WT or Itpkb−/− mice, analyzed by Hoechst/Ki67 stain. (B) Representative data from 8 independent experiments. G0, Ki67−Hoechst 33342−. G1, Ki67+Hoechst 33342−. S, Ki67+Hoechst 33342int. G2/M, Ki67+Hoechst 33342hi. Numbers denote percent of cells per gate. (C) Percent of LT-HSC per cell-cycle phase for 11 mice/genotype (horizontal bars, means). KO, Itpkb−/−; WT, wild type. Statistical significance was determined by paired Student t test. (D) In vivo BrdU incorporation in LSK CD34− LT-HSC in WT or Itpkb−/− mice during a 2-hour pulse. Numbers denote percent of BrdU+ cells. BrdU−, uninjected WT control. Representative of 2 independent experiments. (E-F) Forward (FSC-A)/side-scatter (SSC-A) analysis of LSK CD34−CD150+ LT-HSC, LSK CD34+Flk-2− MPP1-3, and LSK CD34+Flk-2+ MPP4 in WT or Itpkb−/− BM. (E) Representative data from 5 independent experiments. Numbers denote percent of cells per gate. (F) Pooled data with means (horizontal lines) and P values for the indicated comparisons (unpaired t test; n = 11).
To elucidate the molecular basis for the HSC expansion and how Itpkb loss affects LT-HSC properties, we analyzed the transcriptomes of sorted LSK CD34−CD48−CD150+Flk2− LT-HSC from Itpkb−/− vs WT mice by RNA sequencing. In all, 441 mRNAs were differentially expressed with false discovery rates <0.15, fold-changes >1.4, and average log2 (counts per million) >4 (Figure 3A-B; supplemental Table 2). Whole transcriptome GSEA48 unveiled downregulation of LT-HSC, quiescence and hypoxia-associated mRNAs, but upregulation of cell-cycle, mobilization, MPP, and pluripotency-associated, or hypoxia-suppressed genes in Itpkb−/− LT-HSC (Figure 3; supplemental Figure 1; supplemental Table 3). Interestingly, Itpkb−/− LT-HSC also enriched numerous genes normally associated with later HPC stages, developing and mature lymphoid and myeloid cells. Thus, Itpkb loss not only activates LT-HSC, but also induces LT-HSC differentiation.
Downregulation of quiescence and stemness-associated, upregulation of proliferation, mobilization, and differentiation-associated mRNAs in Itpkb−/− LT-HSC. The mRNA from 3 separate pools of Itpkb−/− or WT BM LSK CD34−CD150+CD48−Flk2− LT-HSC was analyzed by whole-transcriptome sequencing. (A) Scatter plot of mean expression for 23 998 transcripts (supplemental Table 3) in Itpkb−/− vs WT LT-HSC. Colors denote 441 significance-filtered mRNAs upregulated >1.4-fold in Itpkb−/− (red) or WT (blue) LT-HSC (supplemental Table 2). (B) Clustered heat map of log-transformed, normalized expression values in each sample for the 441 mRNAs. Red, upregulated, blue, downregulated over sample median (= 0). (C) GSEA results for the 23 998 transcripts. The results for significantly Itpkb−/− (NES >0) or WT LT-HSC (NES <0) enriched gene sets associated with the biological processes listed above each group are shown here and in supplemental Figure 1. Gene set descriptions are shown in supplemental Table 5. In the plots, all 23 998 transcripts are statistically rank-ordered left-to-right by decreasing relative expression level in Itpkb−/− vs WT LT-HSC. Gray histograms show phenotype correlation values for the ranked genes as signal-to-noise ratios. These are positive for mRNAs enriched in Itpkb−/− LT-HSC and negative for mRNAs enriched in WT LT-HSC. Vertical lines above the histograms denote positions of individual mRNAs within the considered gene set in the ranked list of all mRNAs. Red and blue horizontal bars mark mRNAs whose expression levels correlate positively (red, left) or negatively (blue, right) with the Itpkb−/− phenotype. Green curves show running enrichment scores for the gene set as the analysis walks down the ranked gene list. Peak asymmetry correlates with enrichment (left shift) or underrepresentation (right shift) of the respective gene set in Itpkb−/− LT-HSC. NES account for differences in gene set size and in correlations between gene sets and the expression dataset. They allow result comparison across gene sets.48 FDR, false discovery rate; NES, normalized enrichment score. P, nominal P value.48
Downregulation of quiescence and stemness-associated, upregulation of proliferation, mobilization, and differentiation-associated mRNAs in Itpkb−/− LT-HSC. The mRNA from 3 separate pools of Itpkb−/− or WT BM LSK CD34−CD150+CD48−Flk2− LT-HSC was analyzed by whole-transcriptome sequencing. (A) Scatter plot of mean expression for 23 998 transcripts (supplemental Table 3) in Itpkb−/− vs WT LT-HSC. Colors denote 441 significance-filtered mRNAs upregulated >1.4-fold in Itpkb−/− (red) or WT (blue) LT-HSC (supplemental Table 2). (B) Clustered heat map of log-transformed, normalized expression values in each sample for the 441 mRNAs. Red, upregulated, blue, downregulated over sample median (= 0). (C) GSEA results for the 23 998 transcripts. The results for significantly Itpkb−/− (NES >0) or WT LT-HSC (NES <0) enriched gene sets associated with the biological processes listed above each group are shown here and in supplemental Figure 1. Gene set descriptions are shown in supplemental Table 5. In the plots, all 23 998 transcripts are statistically rank-ordered left-to-right by decreasing relative expression level in Itpkb−/− vs WT LT-HSC. Gray histograms show phenotype correlation values for the ranked genes as signal-to-noise ratios. These are positive for mRNAs enriched in Itpkb−/− LT-HSC and negative for mRNAs enriched in WT LT-HSC. Vertical lines above the histograms denote positions of individual mRNAs within the considered gene set in the ranked list of all mRNAs. Red and blue horizontal bars mark mRNAs whose expression levels correlate positively (red, left) or negatively (blue, right) with the Itpkb−/− phenotype. Green curves show running enrichment scores for the gene set as the analysis walks down the ranked gene list. Peak asymmetry correlates with enrichment (left shift) or underrepresentation (right shift) of the respective gene set in Itpkb−/− LT-HSC. NES account for differences in gene set size and in correlations between gene sets and the expression dataset. They allow result comparison across gene sets.48 FDR, false discovery rate; NES, normalized enrichment score. P, nominal P value.48
Itpkb−/− HSC have intrinsically impaired function
To determine if the reduced “stemness” impairs Itpkb−/− LT-HSC function, we analyzed their competitive long-term repopulating potential. CD45.2+Itpkb−/− or WT BM was mixed 1:1 with CD45.1+ WT competitor BM and injected into lethally irradiated CD45.1+CD45.2+ recipients. Itpkb loss reduces peripheral T, B, and NK cell numbers by impairing their development after the HSC stage,35,38-42 but this does not affect peripheral blood macrophage/granulocyte content in young mice (supplemental Figure 2A-B). Thus, profoundly underrepresented Itpkb−/− donor-derived peripheral blood granulocytes/macrophages ≥24 weeks after engraftment indicated a strongly reduced competitive long-term repopulating ability of Itpkb−/− LT-HSC (Figure 4A). Although the injected BM mix had contained twofold more Itpkb−/− than WT LT-HSC (Figure 1), Itpkb−/− donor-derived LSK cells were strongly underrepresented in the host BM 26 to 29 weeks after engraftment (Figure 4B). Serial BM transfer revealed even further reduced Itpkb−/− vs WT BM LT-HSC, MPP1-3, LK CD34+ common myeloid progenitors/granulocyte monocyte progenitors, peripheral blood granulocytes, and macrophages 43 weeks after primary transfer (Figure 4C). Therefore, Itpkb−/− LT-HSCs have a severely reduced function in a competitive in vivo setting that is not rescued by a WT environment and the presence of WT hematopoietic cells, and is not caused by the lymphopenia of Itpkb−/− mice. This suggests a cell-intrinsic defect of Itpkb−/− HSC. Indeed, WT BM fully and similarly reconstituted WT and Itpkb−/− lethally irradiated hosts (Figure 4D and Wen et al 41 ).
Itpkb−/− HSC have a cell intrinsically reduced long-term repopulating potential. (A) Chimerism (percent of CD45.2+ cells) among donor-derived peripheral blood granulocytes and macrophages ≥24 weeks after injection of 1:1 mixed Itpkb−/− (gray bars) or WT (black bars) CD45.2+ donor and WT CD45.1+ competitor donor Lin− BM into lethally irradiated CD45.1+CD45.2+ recipients. Statistical significance for the indicated comparisons was determined by unpaired Student t test. Representative of 3 independent experiments. Representative FACS data in supplemental Figure 2C. (B) Donor-derived LSK cell chimerism ≥24 weeks after BM transfer. Pooled data from 2 independent experiments. Representative FACS data are shown in supplemental Figure 2D. (C) BM LT-HSC, MPP1-3, common myeloid progenitor/granulocyte monocyte progenitor, and blood granulocyte and macrophage chimerism in lethally irradiated 2° recipients 14 weeks after reconstitution with BM from 1° recipients 29 weeks after injection of 1:1 mixed Itpkb−/− or WT CD45.2+ and WT CD45.1+ competitor BM. Representative FACS data in supplemental Figure 2E. (D) BM total cell, LT-HSC, and MPP numbers in each 4 CD45.2+ lethally irradiated WT or Itpkb−/− hosts 12 weeks after injection with CD45.1+CD45.2+ WT BM. Bars denote means.
Itpkb−/− HSC have a cell intrinsically reduced long-term repopulating potential. (A) Chimerism (percent of CD45.2+ cells) among donor-derived peripheral blood granulocytes and macrophages ≥24 weeks after injection of 1:1 mixed Itpkb−/− (gray bars) or WT (black bars) CD45.2+ donor and WT CD45.1+ competitor donor Lin− BM into lethally irradiated CD45.1+CD45.2+ recipients. Statistical significance for the indicated comparisons was determined by unpaired Student t test. Representative of 3 independent experiments. Representative FACS data in supplemental Figure 2C. (B) Donor-derived LSK cell chimerism ≥24 weeks after BM transfer. Pooled data from 2 independent experiments. Representative FACS data are shown in supplemental Figure 2D. (C) BM LT-HSC, MPP1-3, common myeloid progenitor/granulocyte monocyte progenitor, and blood granulocyte and macrophage chimerism in lethally irradiated 2° recipients 14 weeks after reconstitution with BM from 1° recipients 29 weeks after injection of 1:1 mixed Itpkb−/− or WT CD45.2+ and WT CD45.1+ competitor BM. Representative FACS data in supplemental Figure 2E. (D) BM total cell, LT-HSC, and MPP numbers in each 4 CD45.2+ lethally irradiated WT or Itpkb−/− hosts 12 weeks after injection with CD45.1+CD45.2+ WT BM. Bars denote means.
To explore if homing defects contribute to the reduced repopulating ability of Itpkb−/− HSC, we coinjected differentially dye-labeled Itpkb−/− and WT HSC into lethally irradiated hosts. HSC downregulate surface c-Kit in a lymphopenic environment.50,57 Thus, we compared the proportions of Itpkb−/− cells among donor-derived Lin−Sca-1+ (LS) cells in input, recipient BM, and spleens 15 to 17 hours later (supplemental Figure 3). To ascertain that c-Kit loss did not confound our results, we also analyzed homing of Itpkb−/− vs WT LSK cells in unirradiated, nonlymphopenic recipients. In both cases, we did not find a statistically significant underrepresentation of Itpkb−/− HSC in host BM or spleens vs input (supplemental Figure 3B-C). Therefore, homing defects are unlikely to contribute to the severe repopulation defects of Itpkb−/− HSC.
To corroborate this, we analyzed the in vitro colony-forming unit (CFU)34 and cobblestone area-forming cell (CAFC)34 activities of equal numbers of sorted Itpkb−/− vs WT LT-HSC. Here, HSC tissue homing is not required. Consistent with their in vivo defects, Itpkb−/− vs WT LSK CD150+CD48− HSC had a significantly reduced CFU activity (Figure 7D). In CAFC assays, the presence of CAFC colonies after 4 weeks of culture associates with HSC activity.34 Compared with WT controls and consistent with their increased steady-state proliferation (Figure 2), Itpkb−/− LSK CD150+CD48− HSC tended to initially form more colonies, but then contracted significantly (P < .05) after ≥4 weeks (supplemental Figure 4A-C). Although the 2- and 3-week data did not reach statistical significance, this may suggest that Itpkb−/− HSC initially hyperexpand, but then exhaust, similar to PTEN−/− or myr-Akt expressing HSC.22,34
To confirm this in vivo, we conducted a kinetic competitive BM transfer study (supplemental Figure 4D-E). Consistent with the in vitro data and unimpaired homing, Itpkb−/− BM initially reconstituted phenotypic LT-HSC, MPP, HPC, and mature blood cells, except T and NKT cells. However, >16 weeks, all Itpkb−/− phenotypic LT-HSC, MPP, HPC, and mature hematopoietic cells disappeared. Thus, Itpkb−/− HSC have an intrinsically impaired function and exhaust over time in vitro and in vivo.
Itpkb−/− mice die prematurely with severe anemia
To determine if Itpkb−/− LT-HSC function is also impaired in a noncompetitive in vivo setting, we aged cohorts of Itpkb−/− and WT mice and analyzed their phenotypic LT-HSC, MPP, and HPC content over time. Consistent with beginning hematopoiesis defects, young Itpkb−/− mice had twofold to threefold lower mean BM common lymphoid progenitor, common myeloid progenitor and megakaryocyte-erythrocyte progenitor but not granulocyte-monocyte progenitor numbers than WT littermates (Figure 5A-B).
Itpkb−/− mice die prematurely with hematopoietic failure and lethal anemia. (A-B) BM HPC populations in 6- to 11-week-old WT or Itpkb−/− mice. Shown are representative FACS plots (A) and aggregated total numbers (B) from 3 independent experiments. Bars denote means. Statistical significance of genotype differences was determined by unpaired Student t test (n = 9). (C) Survival of WT (open circles) and Itpkb−/− (closed squares) mice was analyzed by Kaplan-Meier plot. Itpkb−/− mice were found dead or euthanized when moribund. WT littermates euthanized as controls for analysis or after exceeding the maximum lifespan of Itpkb−/− mice were censored. Median survival was 36 weeks. P < .0001 (nKO=14, nWT=23) (D-E) Bar graphs of mean ± standard deviation total numbers of BM cells, the indicated LT-HSC, MPP, or HPC populations (D), or (E) red blood cells/μL peripheral blood in age-matched old WT (n = 14, black bars) and Itpkb−/− (n = 4 for BM cell analysis; n = 3 for red blood cell analysis; gray bars) mice at time of euthanasia. Statistical significance of genotype differences was determined by unpaired Student t test. Representative raw data in supplemental Figure 5. (F) Blood smears from WT or Itpkb−/− mice were Wright stained and analyzed on a Nikon Eclipse E400 microscope equipped with ×10 (100 magnification, 0.25 numerical aperture), ×20 (200 magnification, 0.40 numerical aperture), ×50 oil (500 magnification, 0.90 numerical aperture), and ×100 oil (1000 magnification, 1.25 numerical aperture) lenses. Images were acquired on a DS-Fi1 Nikon Digital Sight system, cropped, resized, and tonal range and color balance adjusted using the “auto level” function in Photoshop CS6. All procedures were carried out at room temperature. Scale bars: 50 μm (top), 20 μm (bottom).
Itpkb−/− mice die prematurely with hematopoietic failure and lethal anemia. (A-B) BM HPC populations in 6- to 11-week-old WT or Itpkb−/− mice. Shown are representative FACS plots (A) and aggregated total numbers (B) from 3 independent experiments. Bars denote means. Statistical significance of genotype differences was determined by unpaired Student t test (n = 9). (C) Survival of WT (open circles) and Itpkb−/− (closed squares) mice was analyzed by Kaplan-Meier plot. Itpkb−/− mice were found dead or euthanized when moribund. WT littermates euthanized as controls for analysis or after exceeding the maximum lifespan of Itpkb−/− mice were censored. Median survival was 36 weeks. P < .0001 (nKO=14, nWT=23) (D-E) Bar graphs of mean ± standard deviation total numbers of BM cells, the indicated LT-HSC, MPP, or HPC populations (D), or (E) red blood cells/μL peripheral blood in age-matched old WT (n = 14, black bars) and Itpkb−/− (n = 4 for BM cell analysis; n = 3 for red blood cell analysis; gray bars) mice at time of euthanasia. Statistical significance of genotype differences was determined by unpaired Student t test. Representative raw data in supplemental Figure 5. (F) Blood smears from WT or Itpkb−/− mice were Wright stained and analyzed on a Nikon Eclipse E400 microscope equipped with ×10 (100 magnification, 0.25 numerical aperture), ×20 (200 magnification, 0.40 numerical aperture), ×50 oil (500 magnification, 0.90 numerical aperture), and ×100 oil (1000 magnification, 1.25 numerical aperture) lenses. Images were acquired on a DS-Fi1 Nikon Digital Sight system, cropped, resized, and tonal range and color balance adjusted using the “auto level” function in Photoshop CS6. All procedures were carried out at room temperature. Scale bars: 50 μm (top), 20 μm (bottom).
Giardia infections reduced mean survival of another line of Itpkb−/− mice to ∼11 weeks in a conventional vivarium.40 To reduce confounding effects of infections, we house our mice in a SPF vivarium. Here, Itpkb−/− mice had a longer but still reduced median lifespan of 36 weeks (Figure 5C). Aged Itpkb−/− mice at time of death had mildly reduced BM cellularity, but massively reduced BM phenotypic LT-HSC, MPP, and HPC numbers compared with age-matched WT mice (Figure 5D). As previously reported,36 Itpkb−/− mice had less erythrocytes (Figure 5E). Blood smears unveiled severe macrocytic, normo-to-hypochromic, regenerative anemia with moderate-to-marked polychromasia, and occasional erythrocyte basophilic stippling (Figure 5F). Thus, Itpkb−/− mice develop a progressive hematopoietic failure as they age and die with severe anemia. This ascribes critical physiological importance to their HSC defects.
Itpkb limits Akt/mTORC1 signaling in HSC
SCF signaling via c-Kit on HSC balances quiescence with activation and involves PI3K/Akt.32,58-61 The activities of PI3K, Akt, and downstream mTORC1 must be limited to ensure HSC quiescence and function.2,15,22,25,26,33,34 Recent data challenge the importance of PTEN/SHIP as HSC-intrinsic dampeners of PI3K signaling.24,30,31 The Itpkb product IP4 competitively limits PIP3-mediated Akt activation in neutrophils, myeloid progenitors, and NK cells.36,37,42 To explore if Itpkb may similarly limit PI3K signaling within HSC, we analyzed SCF-induced phosphorylation of Akt and the mTORC1 target, ribosomal protein S6 in LSK cells (Figure 6A-C), LT-HSC, and MPP subsets (Figure 6D) in vitro. Like others,62-64 we found double-peak phosphorylation patterns. This may suggest digital rather than analog responses65 or differential subpopulation responses. In vitro incubated Itpkb−/− vs WT LSK cells contained mildly elevated basal and significantly higher SCF-induced T308-phosphorylated Akt, despite reduced total Akt protein content (Figure 6A-C). Preincubation with Akt-inhibitor VIII or IP4/PM, a widely used nontoxic agent to increase cellular IP4 levels,42 reduced phospho-Akt in SCF-treated Itpkb−/− and WT LSK cells to basal levels. Therefore, the SCF response is Akt-specific and exogenous IP4 can inhibit SCF-induced Akt-activation in WT HSC and reverse the Akt hyperactivation in Itpkb−/− HSC. In vitro incubated Itpkb−/− and WT LT-HSC and MPP1-3 contained similar basal phospho-Akt and phospho-S6 amounts (Figure 6D). Interestingly, low SCF-doses, which barely activated Akt in WT LT-HSC/MPP strongly activated Akt in ItpkB−/− LT-HSC and MPP1-3. Pre-incubation with Akt-inhibitor VIII strongly reduced phospho-Akt content in SCF-treated WT, but not Itpkb−/− LT-HSC and MPP1-3. SCF-induction of phospho-S6 was strong in WT, but higher in Itpkb−/− LT-HSC and MPP1-3, particularly in MPP1/2. Thus, Itpkb−/− LT-HSC and MPP1-3 hyperactivate Akt and mTORC1 upon SCF treatment. Their reduced Akt-inhibitor sensitivity is consistent with a higher content of active Akt molecules.
Akt/mTORC1 hyperactivity in Itpkb−/− HSC. (A-B) WT (solid) or Itpkb−/− (hatched) LSK cells were treated in vitro with medium or SCF ± cell-permeable IP4/PM or Akt-inhibitor VIII (Akt-I) and analyzed by FACS for T308-phosphorylated Akt (pAktT308) content. (A) Representative FACS data from 4 independent experiments. (B) pAktT308 median fluorescence intensity (MFI) in WT (black squares) or Itpkb−/− (gray circles) LSK cells, normalized to the MFI of unstimulated WT cells (n = 4). Statistical significance of genotype differences was determined by unpaired Student t test. (C) Total Akt protein content in WT or Itpkb−/− LSK cells. Shaded histogram, isotype control. (D) WT (solid) or Itpkb−/− (hatched) LSK cells treated as in (A) were analyzed for pAktT308 and phospho-ribosomal protein S6 (pS6S235/S236) content in LSK CD34−CD48−CD150+ LT-HSC, and MPP1-3 subpopulations. (E) Itpkb−/− or WT LT-HSC enriched GSEA gene sets associated with Akt/mTOR signaling and metabolic activation were identified as in Figure 3C. Gene set descriptions are shown in supplemental Table 5.
Akt/mTORC1 hyperactivity in Itpkb−/− HSC. (A-B) WT (solid) or Itpkb−/− (hatched) LSK cells were treated in vitro with medium or SCF ± cell-permeable IP4/PM or Akt-inhibitor VIII (Akt-I) and analyzed by FACS for T308-phosphorylated Akt (pAktT308) content. (A) Representative FACS data from 4 independent experiments. (B) pAktT308 median fluorescence intensity (MFI) in WT (black squares) or Itpkb−/− (gray circles) LSK cells, normalized to the MFI of unstimulated WT cells (n = 4). Statistical significance of genotype differences was determined by unpaired Student t test. (C) Total Akt protein content in WT or Itpkb−/− LSK cells. Shaded histogram, isotype control. (D) WT (solid) or Itpkb−/− (hatched) LSK cells treated as in (A) were analyzed for pAktT308 and phospho-ribosomal protein S6 (pS6S235/S236) content in LSK CD34−CD48−CD150+ LT-HSC, and MPP1-3 subpopulations. (E) Itpkb−/− or WT LT-HSC enriched GSEA gene sets associated with Akt/mTOR signaling and metabolic activation were identified as in Figure 3C. Gene set descriptions are shown in supplemental Table 5.
Further supporting these findings, RNA-Sequencing/Gene Set Enrichment Analysis showed an underrepresentation in Itpkb−/− vs WT LT-HSC of mRNAs downregulated by transgenic Akt1, and enrichment of mRNAs downregulated by activated FoxO3 or cell treatment with the mTORC1 inhibitor rapamycin (Figure 6E; supplemental Tables 3 and 5). Akt inhibits FoxO3. Enrichment of these mRNAs thus supports Akt and mTORC1 hyperactivity in Itpkb−/− HSC.
BM hypoxic niches promote LT-HSC quiescence by switching their glucose metabolism from oxidative phosphorylation (OP) to anaerobic glycolysis, and by limiting protein synthesis through dampened PI3K/mTOR signaling.25,66,67 Further supporting their activation and PI3K-hyperactivity, Itpkb−/− LT-HSC underexpressed mRNAs enriched in hypoxia-adapted cells, but overexpressed genes downregulated by hypoxia (supplemental Figure 1), or involved in glucose, ion, and amino acid transport, TCA cycle, respiratory electron transport, OP, nucleotide metabolism, transcription, and translation (Figure 6E).
The defects of Itpkb−/− HSC result from mTORC1 hyperactivity and other mechanisms
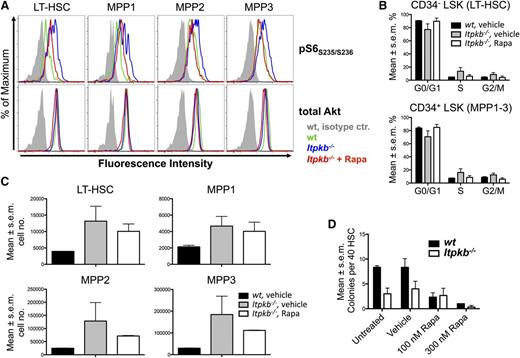
To determine whether the Akt/mTORC1 hyperactivity in Itpkb−/− LT-HSC/MPP1-3 contributes to their functional defects, we compared how rapamycin injection affects LT-HSC/MPP1-3 phospho-S6 content, cell-cycle status, and numbers in Itpkb−/− vs WT mice (Figure 7A-C). Further supporting Akt/mTORC hyperactivity, all subsets contained more phospho-S6 in vehicle-injected Itpkb−/− than WT mice, but similar total Akt protein (Figure 7A). Rapamycin injection reduced the phospho-S6 content in Itpkb−/− LT-HSC and MPP1-3 close to WT levels. Unexpectedly, vehicle injection mildly increased LT-HSC and MPP1-3 proliferation unrelated to genotype, and impaired Ki-67 staining of Itpkb−/−, but not WT cells (supplemental Figure 6). This precludes flow cytometric separation of G0 and G1 phase. Thus, we used Hoechst staining alone for cell-cycle analysis (Figure 7B; supplemental Figure 7). Vehicle-injected Itpkb−/− vs WT mice reproduced the LT-HSC and MPP1-3 accumulation and significantly increased proportions of LT-HSC in S and G2/M phase seen in uninjected Itpkb−/− mice (Figures 1, 2B-D, and 7B-C; supplemental Figure 7). Rapamycin injection reversed the LT-HSC and MPP1-3 hyperproliferation in Itpkb−/− mice to that in vehicle-injected WT mice (Figure 7B; supplemental Figure 7). Rapamycin also tended to reverse the LT-HSC/MPP1-3 accumulation in Itpkb−/− mice, but this was variable and statistically insignificant (Figure 7C).
The mTOR hyperactivity contributes to the hyperproliferation of Itpkb−/− HSC. (A-C) Rapamycin (Rapa) injection can reverse the mTOR hyperactivation in, and hyperproliferation of LT-HSC and MPP in Itpkb−/− mice with little effect on LT-HSC and MPP numbers. WT or Itpkb−/− mice were intraperitoneally injected with rapamycin or vehicle every other day for 10 days, followed by FACS analysis of pS6S235/S236 content (A) (n = 2), cell-cycle phase distribution (B) (Hoechst stain, n = 2), and total numbers (C) (n = 2) of LT-HSC and MPP subsets. Total Akt protein content was determined as a loading control. Bar graphs show mean percent of cells per indicated cell-cycle phase (B) or mean cell numbers for the indicated population (C). Error bars denote standard error of the mean. Representative of 2 (A) or 3 (B-C) independent experiments. Representative FACS data for (B) with gates are shown in supplemental Figure 7. In (A), vehicle injected WT and Itpkb−/− mice, and rapamycin-injected Itpkb−/− mice are represented by green, blue, and red open histograms, respectively. Gray solid histograms, isotype controls. In (B-C) these mice are represented by black solid bars, gray solid bars, and open bars, respectively. (D) Itpkb−/− LT-HSC have reduced in vitro CFU activity and reduced sensitivity to rapamycin. Each 40 sorted WT (black solid bars) or Itpkb−/− (open bars) LT-HSC were incubated in separate wells in M3434 methylcellulose media containing the indicated amounts of rapamycin (Rapa), vehicle, or nothing. Shown are mean numbers of colonies per well on day 7 (n = 3). Error bars denote standard error of the mean. Representative of 3 independent experiments.
The mTOR hyperactivity contributes to the hyperproliferation of Itpkb−/− HSC. (A-C) Rapamycin (Rapa) injection can reverse the mTOR hyperactivation in, and hyperproliferation of LT-HSC and MPP in Itpkb−/− mice with little effect on LT-HSC and MPP numbers. WT or Itpkb−/− mice were intraperitoneally injected with rapamycin or vehicle every other day for 10 days, followed by FACS analysis of pS6S235/S236 content (A) (n = 2), cell-cycle phase distribution (B) (Hoechst stain, n = 2), and total numbers (C) (n = 2) of LT-HSC and MPP subsets. Total Akt protein content was determined as a loading control. Bar graphs show mean percent of cells per indicated cell-cycle phase (B) or mean cell numbers for the indicated population (C). Error bars denote standard error of the mean. Representative of 2 (A) or 3 (B-C) independent experiments. Representative FACS data for (B) with gates are shown in supplemental Figure 7. In (A), vehicle injected WT and Itpkb−/− mice, and rapamycin-injected Itpkb−/− mice are represented by green, blue, and red open histograms, respectively. Gray solid histograms, isotype controls. In (B-C) these mice are represented by black solid bars, gray solid bars, and open bars, respectively. (D) Itpkb−/− LT-HSC have reduced in vitro CFU activity and reduced sensitivity to rapamycin. Each 40 sorted WT (black solid bars) or Itpkb−/− (open bars) LT-HSC were incubated in separate wells in M3434 methylcellulose media containing the indicated amounts of rapamycin (Rapa), vehicle, or nothing. Shown are mean numbers of colonies per well on day 7 (n = 3). Error bars denote standard error of the mean. Representative of 3 independent experiments.
To study if mTORC1 inhibition can restore other functions of Itpkb−/− LT-HSC, we analyzed rapamycin effects on their CFU activity in vitro (Figure 7D). Rapamycin (100 nM) strongly reduced WT LT-HSC CFU activity, but did not further impair the CFU activity of Itpkb−/− LT-HSC, resulting in similar CFU activities between genotypes. High-dose rapamycin severely impaired the CFU activities of WT and Itpkb−/− LT-HSC. Therefore, Itpkb−/− LT-HSCs have reduced rapamycin sensitivity consistent with mTORC1 hyperactivity, but their reduced CFU-activity likely reflects contributions of additional defects.
Altogether, the data suggest that Itpkb−/− LT-HSC and MPP1-3 hyperproliferate due to mTORC1 hyperactivity, but also have functional defects unrelated to mTORC1.
Discussion
Here, we identify Itpkb as a novel critical regulator of HSC homeostasis that limits PI3K/Akt/mTORC1 signaling in HSC and ensures their quiescence. Itpkb−/− mice accumulated phenotypic LT-HSC mildly and MPP1-3 more strongly with reduced LT-HSC quiescence, but increased viability, proliferation, size, and expression of activation-, mobilization-, proliferation- and differentiation-associated genes. Itpkb−/− LT-HSC had a strongly reduced in vivo competitive long-term repopulating potential and reduced in vitro CAFC and CFU activities. They initially reconstituted mixed chimeras, but then exhausted. Itpkb−/− mice died prematurely with BM failure and severe anemia. In vitro, Itpkb−/− HSC showed increased SCF activation of Akt and downstream mTORC1, reversed by exogenous IP4/PM. RNA-Sequencing unveiled transcriptome changes consistent with Akt/mTORC1 hyperactivity, FoxO-inhibition, and a switch from anaerobic glycolysis to oxidative phosphorylation and protein synthesis. This does not reflect the lymphopenia of Itpkb−/− mice, as lymphopenic Rag−/− mice have intact HSC68 and Itpkb−/− HSC exhausted even in nonlymphopenic mixed BM chimeras. Rapamycin treatment reversed the mTORC1-hyperactivity in, and hyperproliferation of LT-HSC and MPP1-3 in Itpkb−/− mice, but did not rescue the CFU-activity of Itpkb−/− LT-HSC.
Others previously reported WT-like LSK cell proportions in another strain of Itpkb−/− mice, but did not study HSC further.36 The difference to our data likely reflects different gating, genetic backgrounds, and housing conditions, or potential expression of a truncated Itpkb protein in those,40 but not our39 Itpkb−/− mice.
The reduced quiescence, but increased proliferation and activity of Itpkb−/− HSC, their reduced CAFC and CFU activities, but intact homing, and the initial reconstitution of mixed BM chimeras followed only later by Itpkb−/− HSC loss all suggest that the reduced function of Itpkb−/− HSC primarily reflects reduced self-renewal, consistent with the HSC-loss in old Itpkb−/− mice. Hematopoietic exhaustion and anemia can explain their reduced lifespan, although we cannot exclude contributions of mild undetected infections even in SPF housing.
Limiting PI3K/Akt/mTOR signaling is critical for HSC homeostasis,2,15 but the HSC intrinsic dampening mechanisms remain unclear. Several reports have proposed HSC intrinsic dampening by PTEN.17,18,22,23,25 However, in one study, conditional PTEN deletion in HSC did not affect HSC homeostasis and function. Instead, HSC were mobilized indirectly via production of mobilizing cytokines by PTEN−/− myeloid cells.24 Similarly, SHIP-1 may primarily control HSC homeostasis by acting in niche cells.30,31 Our data present Itpkb as a possible alternative that may dampen HSC intrinsic PI3K signaling via IP4/PIP3 antagonism, a mechanism also found in NK cells, neutrophils, and myeloid progenitors.36,37,42
Conclusively discerning HSC-intrinsic from HSC-extrinsic Itpkb functions will require conditional Itpkb deletion. However, Itpkb expression in HSC,36 similar WT and Itpkb−/− host repopulation by WT HSC, reversal of the Akt hyperactivation in SCF-stimulated sorted Itpkb−/− HSC by exogenous IP4, the reduced long-term repopulating potential of Itpkb−/− HSC in mixed BM chimeras, and their reduced in vitro CAFC and CFU activities all suggest a HSC-intrinsic Itpkb requirement.
SCF signaling via c-Kit activates PI3K/Akt in HSC and is required for LT-HSC quiescence and self-renewal. However, excessive c-Kit signaling may activate HSC, reduce LT-HSC function, promote differentiation, and may cause MPD.2,9,10,12-14,60,61 Based on the increased SCF sensitivity, viability, proliferation, and differentiation of Itpkb−/− HSC, and on the rapamycin ability to reverse their mTORC1 hyperactivity and hyperproliferation in vivo, we propose that Itpkb may act in HSC to limit SCF-signaling into a window that promotes quiescence, but avoids activation and exhaustion.
HSC have reduced mitochondrial content and produce adenosine triphosphate by anaerobic glycolysis instead of oxidative phosphorylation.8 Redirected glycolytic flux into mitochondria reduced HSC quiescence and function.67 Conversely, increased glycolysis and reduced mitochondrial aerobic metabolism impaired proliferation, differentiation, and repopulating activity of HSC lacking the PTEN-like mitochondrial phosphatase PTPM1.69 So, glycolysis and mitochondrial metabolism are both important for HSC homeostasis and function, but need to be properly balanced. The underlying mechanisms include Akt-assurance, but FoxO-dampening of reactive oxygen species, cytokine-activation, but AMPK-inhibition of mTOR, and probably promotion of mitochondrial pyruvate oxidation through PTPM1.8,17,33,63,69-72 Akt activates mTORC1 but inactivates FoxO.19 In Itpkb−/− LT-HSC, SCF mediated hyperphosphorylation of Akt and S6 protein, downregulation of mRNAs suppressed by transgenic Akt1, and enrichment of mRNAs downregulated by mTOR-inhibition or FoxO-activation suggest Akt/mTORC1 hyperactivation and FoxO-inhibition. We speculate that this contributes to the upregulation of oxidative phosphorylation, TCA cycle/respiratory chain, and mitochondrial mRNAs in Itpkb−/− LT-HSC, and that the metabolic changes contribute to their functional defects. A recent study suggests that loss of PTEN cell intrinsically depletes HSC by increasing protein synthesis in an mTORC-dependent manner.25 Thus, in the future, it will be important to determine to what extent the altered balance of glycolysis and mitochondrial oxidative phosphorylation, and the elevated protein biosynthesis and anabolic metabolism suggested by our RNA-Sequencing data contribute to the exhaustion of Itpkb−/− HSC.
SHIP-1 loss expands HSC and impairs homing and long-term engraftment HSC extrinsically.30,31 HSC lacking Itpkb or PTEN,22 or expressing myrAkt34 did not show significant homing defects. Thus, SHIP-1 controls homing through a unique mechanism. SHIP-1−/− mice develop MPD.28,29 PTEN-loss and myr-Akt expression both cause transient HSC expansion followed by depletion and reduced long-term engraftment associated with variable MPD, T-cell lymphoma, or AML.22,23,34 Itpkb−/− mice have not yet shown overt MPD, T cell lymphoma, or AML. Rapamycin treatment rescued the exhaustion and CAFC/CFU-activities of PTEN−/−, TSC1−/−, or myr-Akt expressing HSC.22,23,34,64,73,74 Rapamycin reversal of the HSC hyperproliferation and mTORC1 hyperactivity in Itpkb−/− mice supports contributions of mTORC1-hyperactivation to their HSC defects. However, the inability of rapamycin to increase the CFU-activity of Itpkb−/− LT-HSC points toward additional, mTORC1-unrelated defects that remain to be elucidated, reminiscent of the mTORC1-independent HSC mobilization in TSC1−/− mice.74 Alternatively, recently discovered mTORC1 requirements for HSC regeneration and function63,72 can explain both how rapamycin impairs WT LT-HSC CFU-activity and the difficulty of rescue experiments.
The differences between SHIP-1−/−, PTEN−/−, myr-Akt transgenic, and Itpkb−/− mice may reflect different experimental systems, varying HSC-extrinsic contributions to the SHIP-1−/−, and PTEN−/− phenotypes24,30,31 and other factors. SHIP-1 loss increases PIP3-levels, but may also reduce PI(3,4)P2 production or perturb noncatalytic SHIP-1 functions.75 PTEN loss causes PIP3 accumulation, but may also reduce levels of its product PI(4,5)P2, a phospholipase C substrate and protein ligand.76 Moreover, PIP3 controls multiple effectors beyond Akt that can be differentially impacted by IP4.35,43 Finally, IP4 can have ill understood PIP3-unrelated functions, Itpks can have IP4-unrelated functions, and Itpkb can control different effectors depending on cell type and context.42,43,77 Clearly, more detailed mechanistic studies are needed to discern the respective contributions of Akt/mTORC1-dampening by IP4 and other mechanisms to HSC control by Itpkb.
Altogether, our results identify Itpkb as an important novel regulator of HSC homeostasis that limits cytokine-induced PI3K-signaling to Akt/mTORC1 in HSC and promotes LT-HSC quiescence to prevent exhaustion and BM failure. Itpkb loss activates and expands HSC without causing obvious T-cell lymphoma, or AML. Thus, transient Itpkb inhibition may be useful to therapeutically mobilize or expand HSC without impairing their function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Juan-Carlos Zúñiga-Pflücker for OP9 cells; Tannishtha Reya, Argyrios Theofilopoulos, and other colleagues for valuable discussions and critical reading of the manuscript; Lyn'Al Nosaka and Boreth Eam for technical help; Michelle Courtney for pathology analyses; and the TSRI vivarium for expert mouse care.
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases grant AI070845, National Institute of General Medical Sciences grant GM100785, NIH National Center for Advancing Translational Sciences grant UL1 TR000109, and The Leukemia and Lymphoma Society Scholar Award 1440-11 to K.S., Deutsche Forschungsgemeinschaft fellowship SI 1547/1-1 to S.S., and National Institutes of Health pre-doctoral training grant AI007606 to L.W. This is The Scripps Research Institute manuscript no. 27025.
Authorship
Contribution: S.S. and S.R. designed and conducted research; L.S. and S.R.H. conducted RNA sequence experiments and raw data analysis; C.C. and B.B. performed experiments; L.W. assisted with mouse work; K.S. conceived, designed, and supervised this research; S.S. and K.S. analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.S. is the Institute of Immunology, Center of Infectious Diseases, College of Veterinary Medicine, University of Leipzig, Leipzig, Germany.
Correspondence: Karsten Sauer, The Scripps Research Institute, Mail Stop IMM-24, 10550 N Torrey Pines Rd., La Jolla, CA 92037; e-mail: ksauer@scripps.edu.
References
Author notes
S.S. and S.R. contributed equally to this study.

![Figure 1. Phenotypic HSC expansion in Itpkb−/− mice. (A) FACS analysis of BM HSC and MPP subsets in Itpkb−/− and Itpkb+/+ (WT) mice. Lin−, CD3−CD4−CD8−CD11b−CD11c−GR-1−DX5−B220−CD19−Ter119−IL-7Rα−. LSK, Lin−Sca-1+c-Kit+. The cell types in each gate are indicated in the top panels and gated populations are above the panels. Numbers indicate percent of total BM cells in the respective gate. (B) Total phenotypic LT-HSC, MPP1-4, and cell numbers in the BM and spleens of WT or Itpkb−/− mice, pooled from 2 independent experiments. The P values for the indicated comparisons were obtained by unpaired t test (symbols, individual mice; horizontal bars, means). Age distributions of the mice were 9 to 11 weeks in the BM analysis (mean age = 9.8 weeks; standard deviation [SD] = 1 week; n = 6 per genotype) and in the spleen analysis (mean age = 9.6 weeks; SD = 0.9 weeks; n = 5 per genotype). Raw data is shown in supplemental Table 1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/18/10.1182_blood-2014-06-583187/4/m_2786f1.jpeg?Expires=1767703520&Signature=4LNLdAjumsu5TSe7q40q0QwN3INxoPjgFiwT6AQjPEdMPq7x-QRdS2g6v3duf9XQ4K~VFqT8ioSR1YZlCZ81ESfZfA5MhUAll81ZHfhC~o53VA1jRn8EjykxE0wMREIJxY23oaMeUqh83gTYGdBeM3jlDwQHV5bJF6qb-47qL047B3umOCXFiOVOOdXUbN5aE637Xa~C-md04eSdhE5oC-qoB4B~tgh6pKrTb0VJA9X2jZ0AAS1BbC-by6rDPPhIyX5TBVpA8uIUN~NYylfu0oa498zbooQmWz~sk61YyxSun2LDUZitSrGZip0LJ4qu1~-rQECiX0xL9Qf558yKnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal