Key Points
IL-13 is an autocrine factor for CTCL.
IL-13 and its receptors represent novel markers of CTCL malignancy and potential therapeutic targets for intervention.
Abstract
Cutaneous T-cell lymphomas (CTCLs) primarily affect skin and are characterized by proliferation of mature CD4+ T-helper cells. The pattern of cytokine production in the skin and blood is considered to be of major importance for the pathogenesis of CTCLs. Abnormal cytokine expression in CTCLs may be responsible for enhanced proliferation of the malignant cells and/or depression of the antitumor immune response. Here we show that interleukin-13 (IL-13) and its receptors IL-13Rα1 and IL-13Rα2 are highly expressed in the clinically involved skin of CTCL patients. We also show that malignant lymphoma cells, identified by the coexpression of CD4 and TOX (thymus high-mobility group box), in the skin and blood of CTCL patients produce IL-13 and express both receptors. IL-13 induces CTCL cell growth in vitro and signaling through the IL-13Rα1. Furthermore, antibody-mediated neutralization of IL-13 or soluble IL-13Rα2 molecules can lead to inhibition of tumor-cell proliferation, implicating IL-13 as an autocrine factor in CTCL. Importantly, we established that IL-13 synergizes with IL-4 in inhibiting CTCL cell growth and that blocking the IL-4/IL-13 signaling pathway completely reverses tumor-cell proliferation. We conclude that IL-13 and its signaling mediators are novel markers of CTCL malignancy and potential therapeutic targets for intervention.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are a heterogeneous group of lymphomas that primarily affect the skin. The most common forms of CTCL,1,2 mycosis fungoides (MF) and Sézary syndrome (SS), are characterized by proliferation of mature CD4+ T-helper cells.3 Patients with MF usually develop cutaneous patches and plaques and have an indolent course with a 5-year survival rate of ∼87%.4-6 In the early stages, T cells reside in the skin and only a few circulate in peripheral blood.7 However, as the disease progresses, the outcome is often fatal8,9 and the 5-year survival rate for patients with widespread manifestation of CTCL beyond the skin is reduced to 25%.10 In SS, skin-homing malignant T cells are found in peripheral blood and they infiltrate skin profusely, causing scaling erythroderma and severe pruritus. CTCL is difficult to diagnose, especially in the early stages, because of the absence of specific markers for malignant lymphocytes, delaying timely treatment and resulting in poor clinical outcomes.2,8
A striking feature of CTCL is the restriction of lymphocyte proliferation to the skin, which implies that the affected cells are dependent on the specific cutaneous microenvironment, including cytokines and adhesion molecules. Malignant skin-infiltrating cells are accompanied by dermal infiltrates of nonmalignant T cells and other mononuclear cells. These infiltrating cells, as well as resident cells such as keratinocytes and fibroblasts, produce a variety of cytokines that modulate cutaneous inflammation11 and are important constituents of the local environment of tumors, fostering proliferation, survival, and migration.12 In the inflammatory context, cytokines that are derived from inflammatory cells play a key role in restricting immune functions and act concomitantly with suppressive inflammatory cytokines that are secreted by the tumor cells themselves.13
Attempts to associate a unique cytokine profile with the disease based on skin or blood samples have generally indicated that a shift from Th1 to Th2 cytokine production14-18 accompanies disease progression. Furthermore, Th2 cell–specific transcription factors, such as GATA-3 and JunB, were highly overexpressed in SS, as detected by cDNA microarray analysis.19 Consequently, a hypothesis emerged in which immune-suppressive Th2 cytokines may promote local growth of the malignant lymphocyte clone.
IL-13 plays a critical role in pathologic processes such as asthma,20 fibrosis,21,22 and cancer.21,23 Several studies implicate IL-13 as an autocrine factor for several tumors that express IL-13Rα1, the signaling receptor for IL-13,24 including Hodgkin lymphoma,25,26 B-CLL,27 and breast carcinoma.28 A variety of other human cancer cells such as those derived from glioma,29,30 squamous cell carcinoma of head and neck,31 pancreatic cancer,32 and breast cancer33 overexpress IL-13Rα2, the decoy receptor for IL-13,34 and this expression represents an important tumor biomarker. In addition, recent studies of IL-13 reveal its central role in a novel immunoregulatory pathway in which natural killer T cells suppress tumor immunosurveillance.23 Thus by several different mechanisms, IL-13 can promote growth or survival of certain tumors through direct action on the tumor and/or by acting through suppression of immunosurveillance. However, previous studies have shown that IL-13 can also inhibit tumor cell growth.33,35-38 These apparently contradictory results may be explained by the fact that IL-13 activity may vary on different tumors and that the earlier studies used tumor cell lines rather than primary tumor cells.
In the present study we provide new insight into the pathogenesis of CTCL by showing that IL-13 and its receptors are expressed by tumor cells in the clinically involved skin of CTCL patients. Furthermore, we demonstrate activation of the IL-13–signaling pathway in the skin and blood of CTCL patients and that the addition of IL-13 neutralizing antibodies or soluble IL-13Rα2 to Sézary cell cultures inhibit tumor-cell proliferation, implicating IL-13 as an autocrine factor for the tumor. We conclude that IL-13 and its receptors are novel tumor markers for CTCL as well as potential therapeutic targets.
Material and methods
Patient samples
Skin and blood samples were obtained from 32 patients with a confirmed diagnosis of nontransformed CTCL, including 24 MF and 8 SS patients at the Cutaneous Oncology Center, University of Pittsburgh Medical Center. Patients were well characterized in terms of disease type, clinical features, and therapy. All enrolled SS patients had elevated numbers of CD4+ cells in their blood and loss of CD26 expression on CD4+ cells.6 A Giemsa-stained blood smear confirmed Sézary cell morphology. Patient age ranged from 22 to 93 years, with a median of 69 ± 17.6. The male-to-female ratio was 1:2.2. African Americans made up 3 of 32 patients; the rest were of mixed European descent. Patients were staged according to the most recent consensus,39 including those with leukemic blood involvement.40 The maximum clinical stage refers to the highest clinical stage at any point in the patient’s clinical course and is not necessarily at the time of last follow-up. Patient therapies were divided into 3 categories: skin-directed only, 1 to 3 systemic therapies, and >3 systemic therapies. Controls included atopic dermatitis (AD, n = 5), psoriasis (n = 5), and human normal skin (NS, n = 3) obtained from The Health Sciences Tissue Bank, University of Pittsburgh. Blood from normal donors (NDs) was obtained from the Central Blood Bank of Pittsburgh. All participants signed a written consent document. Clinical information and biological specimens were de-identified and coded. Research protocols involving human subjects were approved by the Institutional Review Board of the University of Pittsburgh.
Immunohistochemistry and confocal microscopy
Sequential paraffin-embedded skin biopsies (5 μm) from 24 patients with a confirmed MF diagnosis were stained with the following antihuman primary mAbs: rat anti-IL-13 (Abcam), rabbit anti-IL-13Rα1 (Sigma), mouse anti-IL-13Rα2 (Abcam), or rabbit anti-pSTAT-6 (Abcam), as previously described.41 Skin samples were observed using an Olympus BX40 microscope. Images were acquired using a Leica DFC420 camera and Leica Application Suite version 2.7.1 R1. Results are expressed as a percentage of positive cells of the entire infiltrate after quantification of 20 high-power fields (HPF; magnification ×400).
Double-color immunofluorescence staining was performed on frozen samples cryo-sectioned at 7 µm.42 Skin samples from 5 patients with a confirmed diagnosis of CTCL were analyzed; NS and AD skin were used as controls. The following combinations of primary antibodies were used: (1) anti-CD4 (Abcam), -TOX (Sigma); (2) anti-CD4, -IL-13 (Abcam); (3) anti-TOX (Sigma), -IL-13 (Abcam); (4) anti-CD4 (Abcam), -IL-13Rα1 (Sigma); (5) anti-TOX (Sigma), -IL-13Rα1 (R&D Systems); (6) anti-CD4 (R&D Systems), -IL-13Rα2 (Abcam); and (7) anti-TOX (Sigma), -IL-13Rα2 (Abcam). Confocal images were obtained on an Olympus FluoView 1000 confocal microscope using an oil immersion 100× objective.
Flow cytometry
CD4+ T cells were isolated from peripheral blood mononuclear cell samples by negative selection using the EasySep enrichment kit (StemCell Technologies). The CD4+ T-cell–enriched fraction contained >98% CD4/CD3+ cells as determined by flow cytometry (data not shown). Surface IL-13Rα1 and IL-13Rα2 expression by circulating CD4+ T cells from 5 SS patients and 4 NDs was determined by flow cytometry using the following mAbs: IL-13Rα1 and IL-13-Rα2 (R&D Systems). Intracellular IL-13Rα2 expression was determined by intracellular cytokine staining.43
For pSTAT-6 determination, freshly isolated CD4+ T cells from SS patients or NDs were incubated for 15 minutes with IL-4 (10 ng/mL), IL-13 (30 ng/mL), or medium alone at 37°C. Cells were permeabilized in ice-cold 90% methanol and then stained with an anti-pSTAT-6 mAb (Cell Signaling). A fluorochrome-conjugated irrelevant isotype (mouse IgG) provided a negative control.
Labeled cells were analyzed on a 3-laser, 9-detector LSR II instrument (Becton Dickinson) using FlowJo software (Tree Star).
Analysis of cell proliferation after neutralization of IL-13
CD4+ T cells purified from the peripheral blood of SS patients (1 × 106/mL) were cultured in 96-well microplates for 5 days in the presence of anti-IL-13 antibody (6.5 μg/mL, R&D Systems), soluble IL-13Rα2 (2.5 μg/mL, R&D Systems), or medium alone. Incubation time and concentration of IL-13 inhibitors used were determined in B-cell lines such as Raji and SKW 6.4 (data not shown). IL-4 was inhibited with an anti-IL-4 antibody (10 μg/mL, R&D Systems), whereas pSTAT-6 inhibition was obtained with the specific pSTAT-6 inhibitor AS151749944 (100 nM,45 Axon, Medchem). All cultures were performed in complete RPMI 1640 medium (GIBCO, Invitrogen) in the presence of rhIL-2 (10 ng/mL, PeproTech) and IL-7 (5 ng/mL, PeproTech).46 Cells were activated by beads coated with anti-CD3 and anti-CD28 mAbs (Dynabeads CD3/CD28 T cell Expander, Dynal Biotech-Invitrogen, bead-to-cell ratio: 1:1) or by Phytohemagglutinin (10 U/mL, Sigma). In some experiments cells were treated with 10, 50, 100, or 500 ng/mL IL-13 (Peprotech). Cell proliferation was evaluated by MTT assay (Vybrant MTT Cell Proliferation Assay Kit, Life Technologies). Optical density was measured at 570 nm on a SpectraMax 340PC384 microplate reader (Molecular Devices). Results are expressed as percent change of absorbance from untreated control cells, which is set at 100% and represents mean values of triplicate measurements from 3 to 4 independent experiments.
Statistical analysis
Statistical analyses were performed using Prism (GraphPad Software). We used the unpaired 2-tailed Student t test to compare 2 groups. Multiple-group comparisons were made by 1-way analysis of variance (ANOVA) followed by a post hoc Dunnett test or a Tukey test. We considered P < .05 (indicated with * in the figures) as significant, P < .01 (**) as very significant, and P < .001 (***) as highly significant.
Results
IL-13 expression by tumor cells in the skin lesions of CTCL patients
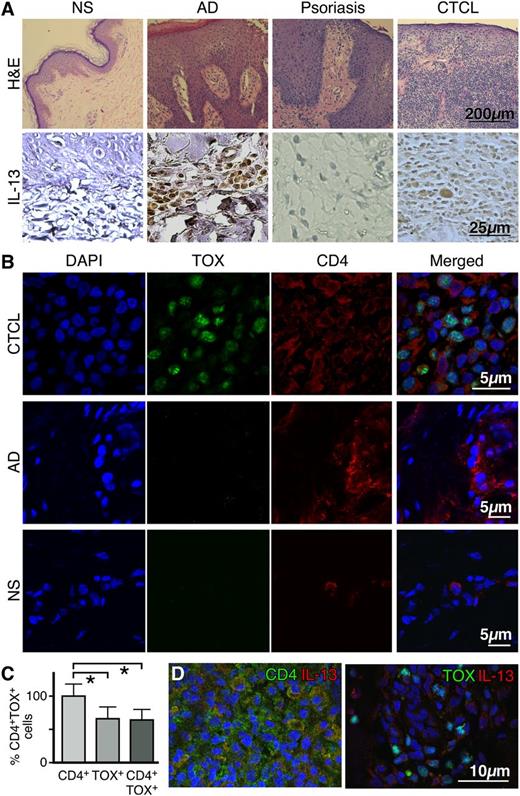
By conventional immunohistochemistry, we compared IL-13 expression in skin lesions of CTCL patients with that of NS, AD, and psoriasis skin. We analyzed samples from CTCL patients ranging from the IA- IVA stages of disease. All patients had an established CD4+ malignant phenotype. As expected, NS was negative for IL-13 expression,41 whereas high numbers of IL-13+ cells were found in the epidermis and dermis of AD skin47 and none were found in psoriasis.48 In CTCL skin biopsies, IL-13 protein was detected in mononuclear cells associated with malignant cell aggregates (Figure 1A). The amount of malignant infiltrate ranged from paucicellular in early-stage disease and in SS to florid malignant infiltration of the skin by the tumor-forming aggregates in stage IIB. Interestingly, we found that IL-13 immunopositivity correlates with disease progression, because the percentage of IL-13+ cells increased from 11.25 ± 6.3% and 13.3 ± 5.8% in stages IA (n = 5) and IB (n = 3), respectively, to 42.5 ± 15% in stage IIB disease (n = 4), to 55 ± 7.1% in stage IVA disease (n = 5) (P < .01, Figure 2B and supplemental Table 1).
IL-13 is expressed by tumor cells in the skin lesions of CTCL patients. (A, upper panel) Representative hematoxylin and eosin stains of normal skin (NS), atopic dermatitis (AD), psoriasis, and CTCL skin (original magnification ×100). (A, lower panel) Immunohistochemical staining for IL-13 expression in skin biopsies from NS (n = 3), AD (n = 5), psoriasis (n = 5), or CTCL (n = 17) (original magnification ×400). (B) Double-color immunofluorescence labeling of frozen skin samples from NS (n = 3), AD (n = 3), and CTCL (n = 7) biopsies stained with antibodies to CD4 (surface staining) and TOX (nuclear staining). A representative example is shown (original magnification ×1000). (C) Proportion of CD4+ and TOX+ cells in the skin of CTCL patients. Error bars are mean ± standard deviation (SD) (n = 7). Statistics were derived by Student t test. (D) Representative example of double-color immunofluorescence staining for CD4 and IL-13 (upper panel) or TOX and IL-13 (lower panel) (original magnification ×400). Skin samples from 7 CTCL patients were analyzed giving similar results. 4,6 diamidino-2-phenylindole stains nuclei.
IL-13 is expressed by tumor cells in the skin lesions of CTCL patients. (A, upper panel) Representative hematoxylin and eosin stains of normal skin (NS), atopic dermatitis (AD), psoriasis, and CTCL skin (original magnification ×100). (A, lower panel) Immunohistochemical staining for IL-13 expression in skin biopsies from NS (n = 3), AD (n = 5), psoriasis (n = 5), or CTCL (n = 17) (original magnification ×400). (B) Double-color immunofluorescence labeling of frozen skin samples from NS (n = 3), AD (n = 3), and CTCL (n = 7) biopsies stained with antibodies to CD4 (surface staining) and TOX (nuclear staining). A representative example is shown (original magnification ×1000). (C) Proportion of CD4+ and TOX+ cells in the skin of CTCL patients. Error bars are mean ± standard deviation (SD) (n = 7). Statistics were derived by Student t test. (D) Representative example of double-color immunofluorescence staining for CD4 and IL-13 (upper panel) or TOX and IL-13 (lower panel) (original magnification ×400). Skin samples from 7 CTCL patients were analyzed giving similar results. 4,6 diamidino-2-phenylindole stains nuclei.
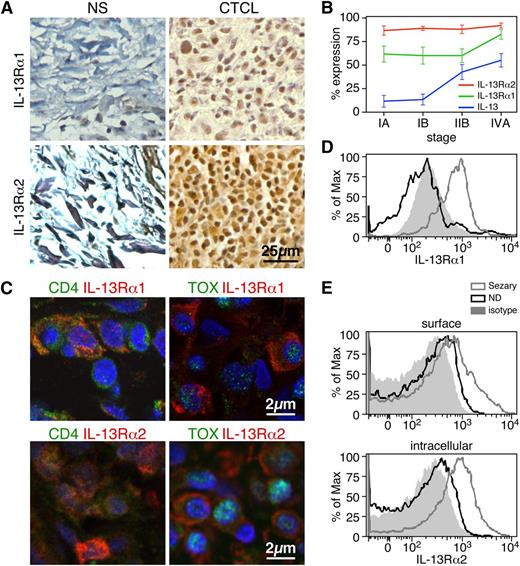
IL-13 receptors are expressed by tumor cells in the skin lesions of CTCL patients and in the peripheral blood CD4+ T cells of Sézary patients. (A) Expression by immunohistochemistry of IL-13Rα1 (upper panel) and IL-13Rα2 (lower panel) in biopsies from CTCL and normal skin (original magnification ×400). Representative examples are shown (n = 17 CTCL, n = 3 NS). (B) The percentages of IL-13+, IL-13Rα1+, and IL-13Rα2+ cells in CTCL skin biopsies at different disease stages are shown. Error bars are mean ± SD. (C) Representative examples of IL-13Rα1 or IL-13Rα2 costaining with CD4 or TOX by immunofluorescence of frozen CTCL skin biopsies (original magnification ×1000, n = 7). Flow cytometry expression of IL-13Rα1 (D) or IL-13Rα2 (E) in CD4+ T cells from the peripheral blood of SS patients. Representative examples out of 5 SS patients tested.
IL-13 receptors are expressed by tumor cells in the skin lesions of CTCL patients and in the peripheral blood CD4+ T cells of Sézary patients. (A) Expression by immunohistochemistry of IL-13Rα1 (upper panel) and IL-13Rα2 (lower panel) in biopsies from CTCL and normal skin (original magnification ×400). Representative examples are shown (n = 17 CTCL, n = 3 NS). (B) The percentages of IL-13+, IL-13Rα1+, and IL-13Rα2+ cells in CTCL skin biopsies at different disease stages are shown. Error bars are mean ± SD. (C) Representative examples of IL-13Rα1 or IL-13Rα2 costaining with CD4 or TOX by immunofluorescence of frozen CTCL skin biopsies (original magnification ×1000, n = 7). Flow cytometry expression of IL-13Rα1 (D) or IL-13Rα2 (E) in CD4+ T cells from the peripheral blood of SS patients. Representative examples out of 5 SS patients tested.
To establish whether IL-13 expression was specific to tumor cells or was associated with the inflammatory cells infiltrating the tumor, we performed multicolor immunofluorescence analysis of well-characterized CTCL skin biopsies staining for IL-13 and tumor markers. Malignant CTCL cells were identified both by CD4 positivity and TOX expression.49-51 Indeed, TOX is only expressed by ∼60% of CD4+ T cells in skin biopsies from patients with confirmed CTCL diagnosis, and it is not expressed by NS or skin from AD (Figure 1B-C), a benign inflammatory skin condition that presents some clinical and histologic features common to CTCL50,52 and harbors a large number of infiltrating CD4+ T lymphocytes. Representative images of IL-13/CD4 or IL-13/TOX costaining are shown in Figure 1D and supplemental Figure 1A. The abnormal phenotype of TOX+ cells was confirmed by light microscopy. We found that IL-13 is expressed by the increasing number of CD4+ cells and TOX+ cells in CTCL skin lesions as the disease progresses. Similar results were obtained by all samples tested. Furthermore, consistent with previous findings,18 we found that IL-13 is also produced by peripheral blood CD4+ T cells from patients with SS, the leukemic variant of CTCL (data not shown). Altogether, these data indicate that IL-13 is not only expressed by inflammatory cells in the tumor microenvironment, but also by tumor cells and likely has an important role in the pathogenesis of CTCL.
IL-13 receptor expression in CTCL skin lesions
IL-13 receptors are expressed in a broad range of cell types, including hematopoietic and nonhematopoietic cells, but not T cells.53 As expected, both receptors are expressed by fibroblasts, endothelial cells, epithelial cells, eosinophils, and mononuclear cells from CTCL-affected tissues as well as from NS (Figure 2A) and skin with other inflammatory conditions (data not shown). However, in CTCL skin biopsies, a large number of mononuclear cells in the tumor-cell infiltrate show immunopositivity for both receptors (Figure 2A). Figure 2B shows that both receptors are expressed very highly, with IL13Rα2 being expressed on almost 100% of mononuclear cells through all disease stages. To establish expression of the receptors by tumor cells, we determined their coexpression with CD4 and TOX by double-color immunofluorescence (Figure 2C). We found that IL-13 receptors are indeed expressed by CD4+ and TOX+ cells in CTCL skin biopsies, whereas few cells coexpressed CD4 and IL-13 receptors in NS and AD skin, and none coexpressed TOX and the IL-13 receptors (supplemental Figure 1B). With multicolor flow cytometry, we then determined IL-13 receptor expression by CD4+ T cells from the peripheral blood of SS patients. The hallmark of SS is expansion of clonotypic CD4+ malignant T cells in the blood. Likewise, we found an increased proportion of CD4+ T cells in the blood of 5 patients tested compared with that of 4 NDs used as controls (78 ± 13% vs 35 ± 8%, respectively). In Figure 2D, we report a representative example for the IL-13Rα1 surface expression of circulating CD4+ T cells in SS patients and NDs (75.3 ± 7% vs 6.5 ± 4%, respectively, n = 5). Similarly, SS CD4+ T cells also express high levels of IL-13Rα2, both intracellularly and on the surface. Representative examples are depicted in Figure 2E, whereas the combined results from multiple patients and controls (n = 5) for surface IL-13Rα2 expression are 26.1 ± 4% vs 1.7 ± 1.3%, respectively, and for intracellular expression are 55.4 ± 3.8% vs 2.3 ± 1.1%, respectively. Moreover, peripheral blood CD4+ T cells from patients with atopic dermatitis lacked IL-13 receptors (supplemental Figure 1C). Thus, these results demonstrate that IL-13 receptors are expressed in the blood and skin of CTCL patients and represent novel markers of malignancy in CTCL.
IL-13 neutralization in Sézary cells
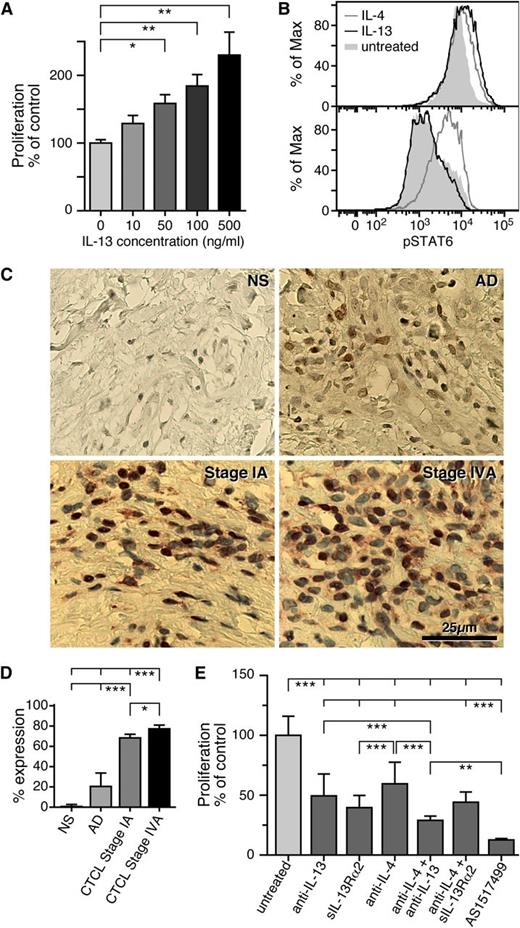
To determine whether IL-13 affects proliferation of Sézary cells in vitro, we purified CD4+ T cells from the peripheral blood of SS patients and cultured them for 5 days with various doses of IL-13. Proliferation of SS CD4+ T cells was assessed by MTT proliferation assay (Figure 3A). We found that IL-13 induces proliferation in a dose-dependent manner, implicating IL-13 as a potential growth factor for SS cells. In comparing the growth-promoting effect of IL-13 to IL-2, another important growth factor for Sézary cells,54 we found that similar concentrations of IL-13 and IL-2 (ie, 100 ng/mL) have comparable effects on Sézary CD4+ T-cell proliferation (supplemental Figure 2B).
Blocking the IL-13–signaling pathway inhibits SS CD4+ T-cell proliferation. (A) Freshly isolated CD4+ T cells from SS patients were cultured in vitro for 5 days and treated with 10, 50, 100, or 500 ng/mL of IL-13. Proliferation was determined by MTT assay and data are depicted as means ± SD compared with untreated cells. Statistics were gathered by ANOVA followed by post hoc Dunnett test. (B) CD4+ T cells from SS patients (upper panel) or NDs (lower panel) were treated for 15 minutes with IL-4 or IL-13 and pSTAT-6 was determined by intracellular staining as described in Material and methods. Shown is a representative example of 5 independent experiments giving similar results. (C) Immunohistochemical analysis of pSTAT-6 from NS (n = 3), AD skin (n = 3), and stage I (n = 4) and stage IV (n = 4) CTCL skin biopsies. Representative examples are shown (original magnification ×400). (D) The percentages of pSTAT-6+ cells after quantification of 20 HPF for each sample are shown. Error bars are mean ± SD. Statistics were gathered by ANOVA followed by post hoc Tukey test. (E) Effect of a soluble IL-13Rα2 (2.5 μg/mL), an anti-IL-13 antibody (6.5 μg/mL), an anti-IL-4 antibody (10 μg/mL), or a pSTAT-6 inhibitor (AS1517499, 100 nM) on SS CD4+ T-cell proliferation. The proliferation inhibition rate was detected by MTT assay after 5 days in culture; the data are shown as means ± SD; ***P < .001 compared with the untreated cells. Statistics were gathered by ANOVA followed by post hoc Tukey test.
Blocking the IL-13–signaling pathway inhibits SS CD4+ T-cell proliferation. (A) Freshly isolated CD4+ T cells from SS patients were cultured in vitro for 5 days and treated with 10, 50, 100, or 500 ng/mL of IL-13. Proliferation was determined by MTT assay and data are depicted as means ± SD compared with untreated cells. Statistics were gathered by ANOVA followed by post hoc Dunnett test. (B) CD4+ T cells from SS patients (upper panel) or NDs (lower panel) were treated for 15 minutes with IL-4 or IL-13 and pSTAT-6 was determined by intracellular staining as described in Material and methods. Shown is a representative example of 5 independent experiments giving similar results. (C) Immunohistochemical analysis of pSTAT-6 from NS (n = 3), AD skin (n = 3), and stage I (n = 4) and stage IV (n = 4) CTCL skin biopsies. Representative examples are shown (original magnification ×400). (D) The percentages of pSTAT-6+ cells after quantification of 20 HPF for each sample are shown. Error bars are mean ± SD. Statistics were gathered by ANOVA followed by post hoc Tukey test. (E) Effect of a soluble IL-13Rα2 (2.5 μg/mL), an anti-IL-13 antibody (6.5 μg/mL), an anti-IL-4 antibody (10 μg/mL), or a pSTAT-6 inhibitor (AS1517499, 100 nM) on SS CD4+ T-cell proliferation. The proliferation inhibition rate was detected by MTT assay after 5 days in culture; the data are shown as means ± SD; ***P < .001 compared with the untreated cells. Statistics were gathered by ANOVA followed by post hoc Tukey test.
Next, we investigated signaling via the IL-13 pathway in SS. We determined the phosphorylation of the transcription factor signal transducer and activator of transcription-6 (pSTAT-6), one of the most significant steps during IL-13 activation.55 CD4+ T cells were purified from the peripheral blood of SS patients and NDs and treated with IL-4, IL-13, or medium alone. The levels of pSTAT-6 were determined by flow cytometry (Figure 3B). Interestingly, all 5 SS samples demonstrated constitutive STAT-6 phosphorylation compared with ND CD4+ T cells (87.23 ± 8.53% vs 14.39 ± 7.75%, respectively; P < .01). As expected, we found that IL-4 is able to activate STAT-6 in CD4+ T cells from patients and NDs, because of activation of the IL-4Rα expressed by T cells. Conversely, IL-13 can only signal through IL-13Rα1, which is normally not expressed by T cells.53 Although IL-13 did not induce STAT-6 activation in ND CD4+ T cells, we found it to be a potent activator in freshly isolated CD4+ T cells from patients, indicating signaling through IL-13Rα1 (Figure 3B). STAT-6 activation was then studied in CTCL skin biopsies at different disease stages. We found high numbers of pSTAT-6+ mononuclear cells associated with malignant cell aggregates in CTCL skin biopsies of all samples tested (Figure 3C). However, patients with stage IV disease had more pSTAT-6+ cells compared with stage I patients (Figure 3D, P < .05). pSTAT-6 staining was mainly localized in the nucleus; however, some cells exhibited cytoplasmic staining. As expected, there was no expression of pSTAT-6 in NS and only few pSTAT-6+ cells were found in AD skin (Figure 3D, P < .001).
Because SS cells produce IL-13,18 we analyzed whether blocking IL-13 by an anti-IL-13 antibody or a soluble form of the decoy IL-13Rα2 could affect proliferation of SS tumor cells in vitro. Cells were cultured for 5 days and activated in vitro by anti-CD3/CD28 or phytohemagglutinin. With MTT assay, we detected a highly significant antiproliferative effect after IL-13 blocking (Figure 3E) that was confirmed by carboxyfluorescein diacetate succinimidyl ester staining (supplemental Figure 2A) and by Ki67 staining (data not shown). The viability of SS CD4+ T cells after IL-13 blocking was examined by Annexin V staining and Trypan blue. No significant differences between treated and untreated cells were detected (data not shown). We also found that an anti-IL-4–neutralizing antibody is able to reduce SS CD4+ T-cell proliferation (Figure 3E and supplemental Figure 2B), although IL-13 blocking via sIL-13Rα2 is more powerful (Figure 3E, P < .01). Moreover, we demonstrated that anti-IL-13 and anti-IL-4 antibodies synergize in reducing SS CD4+-cell proliferation (Figure 3E, P < .01), whereas sIL-13Rα2 has no synergistic effect with the anti-IL-4 antibody. Strikingly, we found that by targeting pSTAT-6, a common mediator of IL-4 and IL-13 signaling, proliferation of CD4+ Sézary cells was completely reversed (Figure 3E and supplemental Figure 2C). Altogether, our results indicate that IL-13 represents a growth factor for CTCL and that inhibition of IL-13 or its signaling pathway are potential therapeutic targets for intervention.
Discussion
Cytokines orchestrate the inflammatory microenvironment of tumors. As such, they represent a prime target for taming tumor-promoting cancer-related inflammation and developing innovative diagnostic tools. Strategies to modulate the host microenvironment and/or unmask immunosurveillance may offer additional interesting approaches when used in combination with direct antitumor therapies. We provide here the first evidence that tumor cells in the skin lesions of CTCL patients produce IL-13 and express IL-13Rα1 and IL-13Rα2. We also show that IL-13 induces SS CD4+ T-cell proliferation in vitro and signaling through IL-13Rα1. Finally, we demonstrate that neutralization of IL-13 or blocking the IL-13 signaling pathway in SS cells inhibits proliferation. These important results identify novel tumor markers for CTCL as well as therapeutic targets for intervention.
Previous work implicates IL-13 as an important autocrine factor for several tumors,25-28 and the expression of IL-13Rα2 represents an important biomarker for several solid malignancies.29-33 However, little is known about the role of IL-13 in CTCL, apart from the finding that both CTCL tumor cell lines56 and SS malignant cells produce IL-13 in vitro.18 Although it is well established that CTCL disease progression is associated with Th2 skewing of the cytokine profile and increasing immunosuppression,18 the role of IL-13 as a regulator of tumor-cell proliferation has never been addressed. Using the expression of CD4 and TOX to identify malignant lymphoma cells, we found that tumor cells in CTCL skin lesions express IL-13 and both IL-13 receptors highly and constitutively. Identification of malignant cells is difficult in CTCL because of a lack of established markers. Our group and others have shown that TOX is ectopically and habitually expressed in malignant cells and can therefore serve as a specific marker of the disease.57 Furthermore, we demonstrated that it is expressed only by tumor cells in CTCL skin biopsies and not by NS or skin with other benign inflammatory dermatoses. Remarkably, we found that IL-13 is used by SS tumor cells as a growth factor in vitro, because it induces SS CD4+ T-cell proliferation in a dose-dependent manner, and its neutralization inhibits tumor-cell proliferation. Because a significant overlap exists between phenotypic presentations and immunologic pathogenesis of both MF and SS, it is not surprising that the same cytokines may play a role in the pathogenesis of both diseases. Based on our data, we conclude that IL-13 plays an increasingly important role in progression from early to advanced stages.
Activation of STAT-6 is an important step in the IL-13/IL-4–signaling pathway. Indeed, activation of IL-13 signaling was detected after IL-13 treatment in CD4+ T cells from patients but not in CD4+ T cells from controls. Interestingly, we found that the levels of pSTAT6 were high in freshly-isolated SS CD4+ T cells, whereas STAT6 is rarely activated in normal CD4+ lymphocytes. Constitutive activation of STAT6 was observed in other malignancies, such as classical Hodgkin lymphoma58 and primary mediastinal large B-cell lymphoma,59 which was shown to result from autocrine secretion of IL-13 by tumor cells. Significantly, we found that pSTAT-6 is also highly expressed in CTCL skin biopsies, implying activation of the IL-13/IL-4–signaling pathway in both the skin and the blood of CTCL patients. However, although antibody-mediated neutralization of IL-13 or soluble IL-13Rα2 in Sézary CD4+ T cells resulted in a significant reduction of SS-cell proliferation, complete abrogation was not observed, suggesting that IL-4 produced by tumor cells and/or inflammatory cells may contribute, in addition to IL-13, to malignant cell proliferation. Indeed, it is well established that CTCL patients exhibit elevated levels of IL-4 in their skin or blood,14,16,18 and we found that an anti-IL-4 antibody decreases SS CD4+ T-cell proliferation in vitro, although less efficiently than IL-13 neutralization. Remarkably, we found that anti-IL-13 and anti-IL-4 antibodies synergize in inhibiting SS CD4+ T-cell growth. Blocking of IL-4 and IL-13 may represent a novel therapeutic strategy for these patients. Importantly, blocking IL-4/IL-13 by the fully human monoclonal antibody dupilumab was shown to be effective in clinical trials in treating asthma and atopic dermatitis, 2 highly Th2-skewed diseases.60,61 However, we reached the highest inhibition of SS CD4+ T-cell proliferation by targeting pSTAT-6, the common mediator of IL-4 and IL-13 signaling. IL-13–induced activation of STAT-6 occurs through IL-13Rα1 signaling.24 Indeed, we found high numbers of IL-13Rα1+ cells among freshly isolated peripheral blood CD4+ T cells from SS patients, as well as in skin biopsies of MF patients, where large numbers of CD4+IL-13Rα1+ and TOX+IL-13Rα1+ cells were found, confirming the expression by malignant cells. This finding is intriguing because CTCL is a T cell–origin tumor, yet T cells do not express IL-13 receptors. Thus, our results demonstrate that CTCL tumors produce IL-13 and use it as a growth factor in an autocrine manner.
IL-13Rα2, the decoy receptor of IL-13,62 binds IL-13 with high affinity and is highly expressed in some tumors.21 Although IL-13Rα2 expression is induced by IL-13,43 likely modulating IL-13 bioavailability, its precise role in cancer is unknown. We found high numbers of IL-13Rα2+ cells in the tumor infiltrate of CTCL patients, independent of disease stage. Furthermore, a large number of CD4+IL-13Rα2+ and TOX+IL-13Rα2+ cells were found in CTCL skin lesions, as well as among CD4+ T cells in SS patient blood, indicating that IL-13Rα2 is expressed by malignant cells. Such overexpression of IL-13Rα2 across all stages of CTCL is highly abnormal, suggesting that this molecule plays a role in disease pathogenesis, possibly through a regulatory feedback mechanism reactive to IL-13 production. IL-13Rα2 has also been exploited as a possible target for cancer therapy. A cytotoxin, IL-13-PE38 composed of human IL-13 and a mutated form of a Pseudomonas exotoxin,63 induced specific killing of IL-13Rα2+ tumor cells in vitro and in vivo64,65 and showed survival benefit in phase 1/2 clinical trials of patients with glioblastoma multiforme at doses that do not produce significant toxicity.66 Therefore, IL-13Rα2 expression in CTCL might represent a marker of malignancy that can be exploited for targeted therapy of the disease.
In addition to its role as a mediator of tumor cell growth, IL-13 may also affect the immune response to CTCL. It was recently shown that IL-13 and other type-2 cytokines produced by Sézary cells contribute to inhibition of the antitumor type 1 immune response.18 IL-13 may act directly on infiltrating cells, including B cells, eosinophils, and macrophages,21 or it may indirectly stimulate the release of chemokines, which regulate reactive infiltrate recruitment. Several chemokines are expressed in CTCL-involved tissues, including CCL17,67,68 CCL22,67 and eotaxin.67 CCL17 and CCL22 attract Th2 cells that specifically express the chemokine receptors CCR469 and CCR10,69 whereas eotaxin attracts cells such as eosinophils and activated Th2 cells that express the chemokine receptor CCR3.70 Other aspects of the CTCL phenotype such as eosinophilia, increased IgE production, and bias toward Th2 cells may also be attributable to the effects of IL-13. In addition, although the profibrotic activity of IL-13 is well known,21 the secretion of IL-13 by CTCL cells may induce a profibrotic phenotype on dermal fibroblasts and underlie the pathogenesis of fibrosis observed in CTCL skin tumors.
We conclude that IL-13 and its receptors are novel biomarkers of CTCL malignancy and critical factors in the disease’s etiology. Further, the role of IL-13 in autocrine growth of CTCL cells and paracrine stimulation of the reactive infiltrate suggests that IL-13 and its molecular pathway are highly relevant targets for therapeutic intervention.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood Commentary on this article in this issue.
Acknowledgments
The authors thank Brittany L. O’Neill for providing frozen CTCL skin biopsies and Lisa Grandinetti and Sue McCann for providing blood samples from Sezary patients. We also thank Jason Devlin for immunofluorescence confocal microscopy.
The study was supported by the National Institutes of Health, National Cancer Institute (Skin SPORE P50 CA121973), Career Development Award (P.F.), and by the Clinical and Translational Science Institute BaCCoR Award, National Institutes of Health (8UL 1 TR000005-07) (P.F., L.J.G.).
Authorship
Contribution: L.J.G., and P.F. designed the study; P.F., S.V., and D.B.S. contributed data; P.F., L.J.G., and S.V. performed statistical analysis and made figures; P.F. and L.J.G. contributed to manuscript writing; and all authors approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for L.J.G. is Columbia University Medical Center, Herbert Irving Pavilion, New York, NY.
Correspondence: Patrizia Fuschiotti, Department of Immunology, University of Pittsburgh School of Medicine, 200 Lothrop St, BST E1046, Pittsburgh PA 15261; e-mail: paf23@pitt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal