Key Points
Transplant vs nontransplant approaches were compared in PMF patients grouped by DIPSS status.
The net benefit of transplant vs nontransplant is marked in higher-risk patients.
Abstract
Allogeneic hematopoietic stem cell transplantation (SCT) is the only curative option for patients with primary myelofibrosis (PMF), but information on its net advantage over conventional therapies is lacking. Using ad hoc statistical analysis, we determined outcomes in 438 patients <65 years old at diagnosis who received allogenic SCT (n = 190) or conventional therapies (n = 248). Among patients at low risk per the Dynamic International Prognostic Scoring System (DIPSS) model, the relative risk of death after allogenic SCT vs those treated with nontransplant modalities was 5.6 (95% CI, 1.7-19; P = .0051); for intermediate-1 risk it was 1.6 (95% CI, 0.79-3.2; P = .19), for intermediate-2 risk, 0.55 (95% CI, 0.36-0.83; P = .005), and for high risk, 0.37 (95% CI, 0.21-0.66; P = .0007). Thus, patients with intermediate-2 or high-risk PMF clearly benefit from allogenic SCT. Patients at low risk should receive nontransplant therapy, whereas individual counseling is indicated for patients at intermediate-1 risk.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3364.
Disclosures
Jorge Cortes, Associate Editor, has served as an advisor or consultant for Incyte, Novartis, and Gilead and received grants for clinical research from Incyte and Novartis. The authors and the CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the net benefit of allogeneic hematopoietic stem cell transplantation (SCT) vs conventional nontransplant therapies for primary myelofibrosis (PMF) among patients of low Dynamic International Prognostic Scoring System (DIPSS) risk status, based on findings of an ad hoc statistical analysis.
Discuss the net benefit of allogeneic SCT vs conventional nontransplant therapies for PMF among patients of intermediate-1 DIPSS risk status.
Determine the net benefit of alllogeneic SCT vs conventional nontransplant therapies for PMF among patients of intermediate-2 or high DIPSS risk status.
Release date: May 21, 2015; Expiration date: May 21, 2016
Introduction
Primary myelofibrosis (PMF) is a clonal myeloproliferative neoplasm with a high risk for clonal evolution and mortality. Powerful prognostic tools have been developed that assist clinicians in patient counseling and therapeutic decision making. The International Prognostic Scoring System (IPSS) is a clinic-based model to assess prognosis at the time of diagnosis1 ; the Dynamic IPSS (DIPSS) was designed to track changes in prognosis related to changes in scoring parameters over time.2 The DIPSS defines 4 risk categories—low, intermediate (int)-1, int-2, and high—which can be assigned on the basis of the instantaneous values of hemoglobin, white blood cell count (WBC), circulating blasts, constitutional symptoms, and patient age during follow-up.
Allogeneic hematopoietic stem cell transplantation (SCT) is currently the only curative treatment for myelofibrosis (MF)3,4 ; however, because of potential complications, careful patient counseling and accurate selection is mandatory. Conversely, conventional therapies including cytoreductive agents (hydroxyurea, busulfan, interferon-α); erythropoiesis-stimulating agents; androgens; immunomodulating drugs; and nonpharmacologic options such as blood transfusions, spleen irradiation, and splenectomy are mainly palliative.5 In the last decade, major advances have been achieved in the understanding of the molecular basis of MF, in particular the central role of the JAK-STAT pathway. These insights have thus led to the development of molecules with therapeutic anti-JAK2 properties.
Two critical questions in the management of MF are which patients may benefit from allogenic SCT and when the transplant should be carried out. Because no data from randomized prospective trials are available, we designed a retrospective study including patients stratified by DIPSS risk who received allogenic SCT (American and European multicenter collection) and patients who did not (independent European multicenter collection).
Patients and methods
The analysis used data from 2 independent international multicenter databases: one including only patients who received allogenic SCT at referral centers in Europe and in the United States (the transplant cohort) and the other including patients belonging to the DIPSS database, which is a European collection of PMF patients who did not receive any JAK inhibitor at the data cutoff and were censored at the time of allogenic SCT (the nontransplant cohort). In the transplant cohort, patients had the parameters composing the DIPSS score collected at the time of transplant, whereas in the nontransplant cohort, parameters were determined according to the DIPSS algorithm (ie, in a time-dependent way, at diagnosis, and longitudinally thereafter with at least 3 visits per year).2 By merging these 2 international databases, data on 673 patients with MF were available. Taking into account the commonly-used age threshold for indication of allogenic SCT, we restricted the analysis to patients aged 65 years or younger at diagnosis. We also excluded from the analysis patients with postpolycythemia vera MF and postessential thrombocythemia MF. Thus, 443 patients (188 and 255, respectively, in the 2 databases) diagnosed with PMF at an age <65 years were included in the analysis. Within the transplant cohort, there were no significant differences in outcome between reduced-intensity and myeloablative conditioning, or between HLA-matched unrelated and HLA-matched sibling donor transplants (data not shown); thus, data were combined for analysis. Age at diagnosis was higher in the nontransplant cohort (median, 55 vs 50 years) but was not significantly related to survival in any model and therefore was not included in the regressions.
The study was approved by the Institutional Review Board of the University of Hamburg and at all participating centers, and was conducted in accordance with the principles of the Declaration of Helsinki.
Survival was the focus of comparison; the main criterion of comparability of the 2 cohorts (transplant vs nontransplant) was the DIPSS risk status. To define the impact on the natural history of the disease, we considered the date of PMF diagnosis as the starting point of the time scale. Hence, patients of the transplant cohort entered the analysis when receiving allogenic SCT at a specific DIPSS status; they were compared with those of the nontransplant cohort who reached the same DIPSS status at the corresponding time. In the nontransplant cohort, the risk category is treated as a time-dependent attribute—that is, upon progression toward a higher-risk category, a patient would leave the set of subjects at risk in the former risk category and enter the “at-risk” set for the new one. By backdating allogenic SCT data from enrollment to the date of diagnosis, we generated left-truncated data, thereby potentially excluding candidate patients who may have died before they were able to undergo allogenic SCT. Regressions for left-truncated and right-censored survival data were computed according to the standard Andersen-Gill model.6 Proportional hazard models were built separately for the 4 DIPSS categories, considering the cohort as a discrete covariate. The relative risks (RR) between the 2 cohorts were estimated under the proportional-hazards approximation, and Wald tests were used to report significance. Statistical analyses were performed using R version 3.1.1 and the “survival” package version 2.37.7,8
Results and discussion
We studied 190 patients who received allogenic SCT and 248 who received conventional therapies. Available demographics are summarized in Table 1, reporting data at the time of transplant for allogenic SCT cohort and at the time of diagnosis for the nontransplant cohort.
Patient characteristics and results of patients with PMF receiving allogenic SCT (data at the time of transplant) or nonexperimental conventional therapy (data at the time of diagnosis)
| . | Allogenic SCT . | Conventional therapy . | ||||
|---|---|---|---|---|---|---|
| Patients (n) | 188 | 255 | ||||
| Median age at diagnosis, y (range) | 50 (20-65) | 55 (18-65) | ||||
| DIPSS, n | At transplant | At diagnosis* | ||||
| Low risk | 22 | 125 | ||||
| Int-1 | 38 | 75 | ||||
| Int-2 | 84 | 52 | ||||
| High risk | 44 | 3 | ||||
| Gender, n | ||||||
| Male | 108 | 154 | ||||
| Female | 80 | 101 | ||||
| Time from diagnosis to transplant, y (range) | 1.2 (0.0-22.2) | |||||
| Conditioning regimen, n | ||||||
| RIC | 91 | |||||
| MAC | 97 | |||||
| Donor, n | ||||||
| Matched unrelated or mismatch related | 102 | |||||
| Matched related | 86 | |||||
| Survival proportion† | Year | Year | ||||
| 1 | 5 | 10 | 1 | 5 | 10 | |
| Low risk | 100 | 69 (48-99) | 60 (38-95) | 98 (96-100) | 95 (90-99) | 92 (86-99) |
| Int-1 | 78 (55-100) | 52 (33-83) | 41 (24-70) | 97 (93-100) | 77 (67-89) | 63 (51-77) |
| Int-2 | 82 (68-98) | 50 (37-67) | 32 (21-48) | 77 (67-88) | 41 (32-54) | 11 (5-22) |
| High risk | 65 (46-92) | 32 (19-56) | 27 (15-49) | 67 (30-100) | 11 (3-44) | 1 (0-10) |
| . | Allogenic SCT . | Conventional therapy . | ||||
|---|---|---|---|---|---|---|
| Patients (n) | 188 | 255 | ||||
| Median age at diagnosis, y (range) | 50 (20-65) | 55 (18-65) | ||||
| DIPSS, n | At transplant | At diagnosis* | ||||
| Low risk | 22 | 125 | ||||
| Int-1 | 38 | 75 | ||||
| Int-2 | 84 | 52 | ||||
| High risk | 44 | 3 | ||||
| Gender, n | ||||||
| Male | 108 | 154 | ||||
| Female | 80 | 101 | ||||
| Time from diagnosis to transplant, y (range) | 1.2 (0.0-22.2) | |||||
| Conditioning regimen, n | ||||||
| RIC | 91 | |||||
| MAC | 97 | |||||
| Donor, n | ||||||
| Matched unrelated or mismatch related | 102 | |||||
| Matched related | 86 | |||||
| Survival proportion† | Year | Year | ||||
| 1 | 5 | 10 | 1 | 5 | 10 | |
| Low risk | 100 | 69 (48-99) | 60 (38-95) | 98 (96-100) | 95 (90-99) | 92 (86-99) |
| Int-1 | 78 (55-100) | 52 (33-83) | 41 (24-70) | 97 (93-100) | 77 (67-89) | 63 (51-77) |
| Int-2 | 82 (68-98) | 50 (37-67) | 32 (21-48) | 77 (67-88) | 41 (32-54) | 11 (5-22) |
| High risk | 65 (46-92) | 32 (19-56) | 27 (15-49) | 67 (30-100) | 11 (3-44) | 1 (0-10) |
These counts refer to PMF patients in each group at diagnosis. The number of patients in each group (risk set) increases or decreases at later times as a consequence of progressions, deaths, or loss of follow-up.
All data are given as 95% confidence interval (95% CI).
The RR of death among patients receiving allogenic SCT vs those receiving conventional therapies was 5.6 (95% CI, 1.7-19; P = .0051) for low-risk DIPSS, 1.6 (95% CI, 0.79-3.2; P = .19) for int-1 risk, 0.55 (95% CI, 0.36-0.83; P = .005) for int-2 risk, and 0.37 (95% CI, 0.21-0.66; P = .0007) for high-risk DIPSS patients. The 5-year proportions surviving in the transplant and nontransplant cohorts were 69% and 95% for low-risk, 52% and 77% for int-1, 50% and 41% for int-2, and 32% and 11% for high-risk patients, respectively. Analysis at alternative time points and 95% CIs are shown in Table 1.
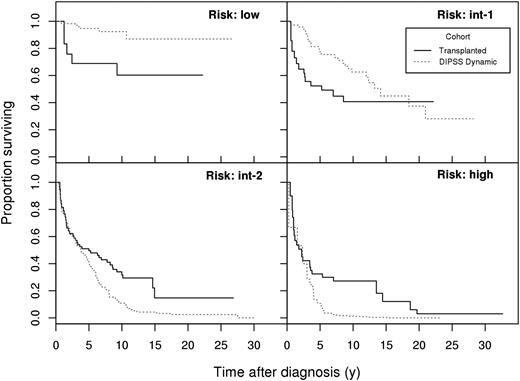
As illustrated in Figure 1, survival differences were pronounced beyond 5 years from diagnosis for int-2 and high-risk patients, and the survival curve for transplanted int-1 risk patients crossed the survival curve for the nontransplant cohort between 15 and 20 years. Hazard ratios were not constant over time, thus summarizing the risk in a single “average” RR figure ignores the specifics of the survival trends.
Survival probabilities for the 4 subgroups (DIPSS risk: low, int-1, int-2, high). DIPSS score is taken at stem cell transplant (solid, transplant cohort) or at the indicated time (dotted, nontransplant cohort). Time (horizontal axis) elapses from diagnosis.
Survival probabilities for the 4 subgroups (DIPSS risk: low, int-1, int-2, high). DIPSS score is taken at stem cell transplant (solid, transplant cohort) or at the indicated time (dotted, nontransplant cohort). Time (horizontal axis) elapses from diagnosis.
We could not address the potentially confounding factor of selection bias regarding allogenic SCT, nor the possible correlations between time of transplant and patient characteristics. Some of the concerns might be addressable in a Markov model, as reported for patients with myelodysplastic syndrome,9 although that model has other methodologic drawbacks.10
Despite the curative potential of allogenic SCT, inherent risks of therapy-related complications and mortality, dependent upon disease status and patient-specific risk factors, are of concern in patients with MF.11 Hence, there is a need to select patients carefully for allogenic SCT to obtain the maximum benefit with respect to survival and therapy-related complications. This study gives an indication of allogenic SCT per DIPSS status; however, conclusions are restricted to patients with primary and not with secondary myelofibrosis.
The recent implementation of the clinically-based risk scores with mutational profile (JAK2/MPL/CALR–triple-negative or ASXL-1–positive)12-14 will be of value in the future for risk stratification of MF patients and can help in the decision making of patients in the int-1 or low-risk DIPSS categories. The recognition of these mutations and the introduction of new treatment modalities such as JAK2 inhibition are changing the landscape for MF treatment. Ruxolitinib, the first JAK2 inhibitor approved for clinical practice, has led to early and sustained clinical benefits in patients with int-2 and high-risk MF, including spleen size reduction and improvement of symptom burden in 2 phase 3 trials.15,16 A survival benefit with ruxolitinib was shown in a long-term update of the COMFORT-I and COMFORT-II studies.12,17,18 Because the present analysis did not include ruxolitinib-treated patients, the impact of this drug compared with that of allogenic SCT could not be assessed, and results may be different if ruxolitinib patients can be included in subsequent studies.
In conclusion, this study indicates that non–ruxolitinib-treated PMF patients 65 years of age or younger at diagnosis with int-2 or high-risk disease are likely to benefit from allogenic SCT, whereas for patients with low-risk disease, nontransplant approaches may be appropriate. Individual counseling is indicated for int-1 risk patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the physicians, nurses, physician assistants, nurse practitioners, pharmacists, data manager of EBMT and at the Fred Hutchinson Cancer Research Center, the support staff caring for our patients, and the patients who participated in the studies that allowed for the present analysis.
This study was supported in part by the Hamburger Krebshilfe and the Roggenbuck Foundations (N.K.); National Institutes of Health, National Heart, Lung, and Blood Institute grants HL084054 and HL036444 and National Cancer Institute grants CA018029 and CA015704; grants from the Associazione Italiana per la Ricerca sul Cancro; the Special Program Molecular Clinical Oncology 5 × 1000 project (#1005, AIRC-Gruppo Italiano Malattie Mieloproliferative); Instituto de Salud Carlos III, Spanish Ministry of Health grant RD012/0036/0004 (F.C.); and the Associazione Italiana Leucemie–Onlus Varese.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors.
Authorship
Contribution: N.K. designed the study, analyzed and interpreted data, and wrote the manuscript; F.P. assisted in study design, analyzed and interpreted data, and edited the manuscript; T.G. assisted in study design, performed statistical analysis, and edited the manuscript; B.L.S., M.D., H.A., F.C., M.C., A.V., E.M., and D.B. assisted with data collection and edited the manuscript; T.Z. performed data collection and edited the manuscript; M.M. assisted with data interpretation and edited the manuscript; A.P. supported statistical interpretation and edited the manuscript; and H.J.D. assisted with study design and data interpretation and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nicolaus Kröger, Department of Stem Cell Transplantation, University Medical Center Hamburg-Eppendorf, Martinistraße 52, D-20246 Hamburg, Germany; e-mail: nkroeger@uke.uni-hamburg.de.
References
Author notes
H.J.D. and F.P. share senior authorship of this article.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal