Key Points
Absence of B7-H3 expression in allogeneic recipients or on allogeneic donor T cells leads to accelerated GVHD lethality.
Increased GVHD lethality is a result of increased T-cell proliferation, colon inflammatory cytokines, and intestinal permeability.
Abstract
Members of the B7 family have been shown to be important for regulating immune responses by providing either positive or negative costimulatory signals. The function of B7-H3 has been controversial. We show that B7-H3 is upregulated in graft-versus-host disease (GVHD) target organs, including the colon, liver, and lung. Infusion of allogeneic donor T cells into B7-H3−/− vs wild-type (WT) recipients resulted in increased GVHD lethality associated with increased T-cell proliferation, colonic inflammatory cytokines, and destruction of epithelial barriers. Allogeneic B7-H3−/− vs WT donor T cells also had increased T-cell proliferation and GVHD lethality associated with increased proliferation and cytokine secretion in the spleen, intraepithelial lymphocyte inflammatory cytokines, and intestinal permeability. Both resting and activated regulatory T cells (Tregs) lack B7-H3 messenger RNA. Consistent with these data, GVHD was augmented in recipients of B7-H3−/− Treg-depleted grafts. In two delayed lymphocyte infusion (DLI) models, T cells lacking B7-H3 are capable of providing graft-versus-leukemia (GVL) effects. We conclude that B7-H3 is responsible for providing a negative costimulatory signal. Our studies provide support for developing and testing new therapies directed toward the B7-H3 pathway, including approaches to augment host B7-H3 early after bone marrow transplantation to prevent GVHD and to develop potent antagonistic antibodies later after transplant to facilitate DLI-mediated GVL without GVHD complications.
Introduction
Graft-versus-host disease (GVHD) remains the leading cause of morbidity and mortality after bone marrow transplantation (BMT). Novel GVHD strategies remain a high priority. B7-H3 is a B7 family member whose function in immune regulation has yet to be clearly defined. B7-H3 is a type I transmembrane protein and the most highly conserved B7 family member between mice and humans.1 A wide range of cells express B7-H3, including activated T cells, natural killer cells, dendritic cells (DCs), and macrophages1-3 along with nonhematopoietic cells, including fibroblasts, synoviocytes, osteoblasts, and epithelial cells.4-6 Although TLT-2 was identified as a receptor for B7-H3,7 others have shown no evidence for this in mice or humans,8 thus confounding elucidation of the biologic response of the B7-H3 pathway.
Initial studies identified B7-H3 as a positive costimulatory molecule because of its capability of promoting T-cell proliferation and interferon gamma (IFN-γ) secretion.1 Tumor B7-H3 overexpression promoted an antitumor response leading to tumor regression and cytotoxic T lymphocyte amplification.9 When a B7-H3−/− mouse was used in an allograft rejection model, there was no difference in graft prolongation unless treatment included cyclosporine A or rapamycin, which led to increased allograft survival.10 These studies indicate that B7-H3 can act as a positive costimulatory molecule. However, both stimulatory1,7,9,10 and inhibitory2,8,11,12 properties have been described. With respect to the latter, B7-H3−/− mice have augmented T-cell proliferation to anti-CD3ε monoclonal antibodies (mAbs) or allogeneic stimulators.2 Conversely, mouse B7-H3 can inhibit T-cell activation and effector cytokine production and lead to exacerbated experimental autoimmune encephalomyelitis.11 In a cardiac allograft model, B7-H3−/− recipients of major histocompatibility complex mismatched grafts had accelerated graft rejection under the cover of cytolytic T lymphocyte-associated antigen 4 (CTLA4) immunoglobulin (CTLA4-Ig), which prolongs graft acceptance.12
Because of these controversies and the unknown function of B7-H3 in BMT recipients, we sought to define the role B7-H3 plays during acute GVHD. We show that B7-H3 is upregulated in GVHD target organs in mice and in the intestine of GVHD patients. B7-H3−/− recipients had accelerated GVHD lethality, more damage to the epithelial layer of the colon, and an increased percentage of inflammatory cytokine secretions from intraepithelial lymphocytes, consistent with B7-H3 as a negative costimulatory pathway member. Recipients of B7-H3−/− donor T cells had accelerated GVHD lethality and increased damage to the epithelial layer of the colon. Lamina propria and intraepithelial lymphocytes showed increased inflammatory cytokine secretion. These results suggest that B7-H3 signaling negatively regulates T cells directly and indirectly during GVHD and that inhibiting B7-H3 increases T-cell effectors and GVHD lethality.
Methods
Details on mice, BMT, quantitative polymerase chain reaction (qPCR), carboxyfluorescein diacetate succinimidyl ester labeling, flow cytometry, and fluorescein isothiocyanate (FITC)–dextran permeability assays are provided in supplemental Data, available on the Blood Web site. Research was conducted in accordance with the Declaration of Helsinki.
Results
B7-H3 expression is upregulated in target organs during acute GVHD in mice and humans
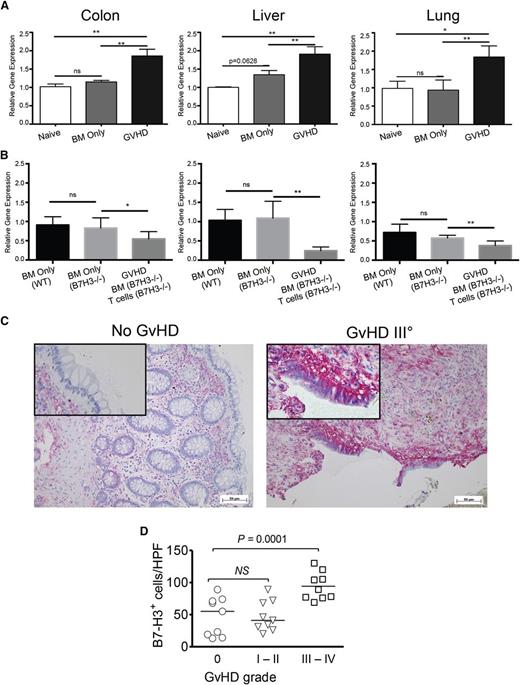
B7-H3 is expressed on activated T cells, epithelial cells, and Ag-presenting cells, including DCs, B cells, and macrophages.13 Inflammatory cytokines increase B7-H3 expression on DCs and monocytes.1 To determine whether B7-H3 is induced during acute GVHD, C57BL/6 (B6; H2b) irradiated recipients were given BALB/c (H2d) BM with or without purified donor T cells. GVHD target organs (colon, ileum, liver, lung, and spleen) were harvested on days 7, 14, and 21 posttransplant, and B7-H3 expression assessed. Because none of the six commercially available anti-murine B7-H3 antibodies provided a sufficient signal-to-noise ratio (not shown), quantitative reverse-transcription PCR (qRT-PCR) was performed. Whereas non-BMT and BM only recipients had comparable B7-H3 expression, mice receiving allogeneic wild-type (WT) T cells had highly upregulated B7-H3 messenger RNA (mRNA) by day 21 posttransplant in the colon, liver, and lung (Figure 1A), consistent with recipients rejecting cardiac allografts.10 Host B7-H3 expression was a major contributor in each organ because there was no expression difference in recipients of B6-WT vs B7-H3−/− BM (Figure 1B). Adding B7-H3−/− donor T cells paradoxically reduced recipient B7-H3 expression, suggesting that host B7-H3+ cells were destroyed by B7-H3−/− T cells, which cause significantly more GVHD than WT T cells or B7-H3+ T cells contribute to B7-H3 expression in GVHD tissues.
B7-H3 expression is upregulated in target organs during acute GVHD. (A) B6-WT mice were lethally irradiated and infused with 107 BALB/c non-T-cell–depleted (NTCD) BM with or without 2 × 106 BALB/c-purified T cells (3 experiments were performed). (B) BALB/c mice were lethally irradiated and infused with 107 B6-WT BM only, B7-H3−/− BM only, or B7-H3−/− BM plus 1 × 106 purified B7-H3−/− T cells. Mice were euthanized on day 21 after transplantation, and the colon, liver, and lung were examined for B7-H3 mRNA by qPCR. Two experiments were performed with 5 mice per group per experiment; 1 representative experiment is depicted. (C) Representative colon sections of a patient without GVHD (left) and a patient with intestinal GVHD grade 4 (right) are shown. Red signal: detection of B7-H3+ cells. (D) Quantification of B7-H3 staining in intestinal sections from multiple patients is shown. *P < .05; **P < .01. ns, not significant. HPF, high power field.
B7-H3 expression is upregulated in target organs during acute GVHD. (A) B6-WT mice were lethally irradiated and infused with 107 BALB/c non-T-cell–depleted (NTCD) BM with or without 2 × 106 BALB/c-purified T cells (3 experiments were performed). (B) BALB/c mice were lethally irradiated and infused with 107 B6-WT BM only, B7-H3−/− BM only, or B7-H3−/− BM plus 1 × 106 purified B7-H3−/− T cells. Mice were euthanized on day 21 after transplantation, and the colon, liver, and lung were examined for B7-H3 mRNA by qPCR. Two experiments were performed with 5 mice per group per experiment; 1 representative experiment is depicted. (C) Representative colon sections of a patient without GVHD (left) and a patient with intestinal GVHD grade 4 (right) are shown. Red signal: detection of B7-H3+ cells. (D) Quantification of B7-H3 staining in intestinal sections from multiple patients is shown. *P < .05; **P < .01. ns, not significant. HPF, high power field.
To determine whether GVHD patients had increased B7-H3 expression, intestinal biopsies from patients who had undergone allogeneic hematopoietic cell transplantation (supplemental Table 1) were analyzed by immunohistochemistry for B7-H3 expression. We observed increased levels of B7-H3 in patients with grade 3 or 4 intestinal GVHD, whereas no significant difference was detected in patients with no GVHD vs grade 1 to 2 GVHD (Figure 1C-D).
Absence of B7-H3 expression on host cells increases allogeneic proliferation in vitro and GVHD lethality in vivo
We asked whether allogeneic T cells would increase their proliferation when B7-H3 was blocked. In a mixed leukocyte reaction of BALB/c-purified T cells and irradiated B6-WT or B7-H3−/− BM–derived DC stimulators (10:1), both CD4+ and CD8+ T cells stimulated with B7-H3−/− DC stimulators had significantly increased proliferation compared with B6-WT DC stimulators (Figure 2A). No inhibition of WT T-cell responses was seen in cocultures of WT and B7-H3−/− CD4+ or CD8+ T cells in an anti-CD3/CD28 mAb bead-driven carboxyfluorescein diacetate succinimidyl ester assay, indicating that B7-H3 does not function in trans to directly affect other T cells that may be capable of binding to each other (supplemental Figure 1).
B7-H3−/− recipients have accelerated GVHD lethality. (A) A mixed leukocyte reaction (MLR) was performed by coculturing BALB/c-purified T cells that were carboxyfluorescein diacetate succinimidyl ester (CFSE) –labeled with irradiated B6 or B7-H3−/− DC stimulators (10:1). Cells were analyzed by flow cytometry on day 9. Cells were gated on H2Kb-positive, viability dye–negative CD4+ or CD8+ events and were analyzed for dilution of CFSE (n = 5). One of 2 representative experiments is shown. (B) B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 2 × 106 BALB/c-purified T cells. Survival plot of B6-WT (green solid circle) vs B7-H3−/− (red open circle) is shown (n = 16 per group; P < .0001). B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 CD25-depleted BALB/c-purified T cells. Survival plot of WT (solid circle) vs B7-H3−/− (open circle) is shown (n = 16 per group pooled from 2 experiments with comparable results; P < .0001). (C) B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 2 × 106 BALB/c-purified T cells. Mice were analyzed for clinical (left panel) and weight (right panel) scores (n = 8). One experiment was performed. (D) Mice were transplanted as in (C). Twenty-one days after transplant, colons were harvested, sectioned, and stained by hematoxylin and eosin (H&E). Sections were scored for pathology (n = 4). One of 2 representative experiments is shown. (E) B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 BALB/c-purified T cells. Mice were sacrificed on day 7, and splenocytes were analyzed for percentage of α4β7, IL-2, and Ki-67 expression (n = 5). One experiment was performed. *P < .05; **P < .01; ***P < .001.
B7-H3−/− recipients have accelerated GVHD lethality. (A) A mixed leukocyte reaction (MLR) was performed by coculturing BALB/c-purified T cells that were carboxyfluorescein diacetate succinimidyl ester (CFSE) –labeled with irradiated B6 or B7-H3−/− DC stimulators (10:1). Cells were analyzed by flow cytometry on day 9. Cells were gated on H2Kb-positive, viability dye–negative CD4+ or CD8+ events and were analyzed for dilution of CFSE (n = 5). One of 2 representative experiments is shown. (B) B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 2 × 106 BALB/c-purified T cells. Survival plot of B6-WT (green solid circle) vs B7-H3−/− (red open circle) is shown (n = 16 per group; P < .0001). B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 CD25-depleted BALB/c-purified T cells. Survival plot of WT (solid circle) vs B7-H3−/− (open circle) is shown (n = 16 per group pooled from 2 experiments with comparable results; P < .0001). (C) B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 2 × 106 BALB/c-purified T cells. Mice were analyzed for clinical (left panel) and weight (right panel) scores (n = 8). One experiment was performed. (D) Mice were transplanted as in (C). Twenty-one days after transplant, colons were harvested, sectioned, and stained by hematoxylin and eosin (H&E). Sections were scored for pathology (n = 4). One of 2 representative experiments is shown. (E) B6 or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 1 × 106 BALB/c-purified T cells. Mice were sacrificed on day 7, and splenocytes were analyzed for percentage of α4β7, IL-2, and Ki-67 expression (n = 5). One experiment was performed. *P < .05; **P < .01; ***P < .001.
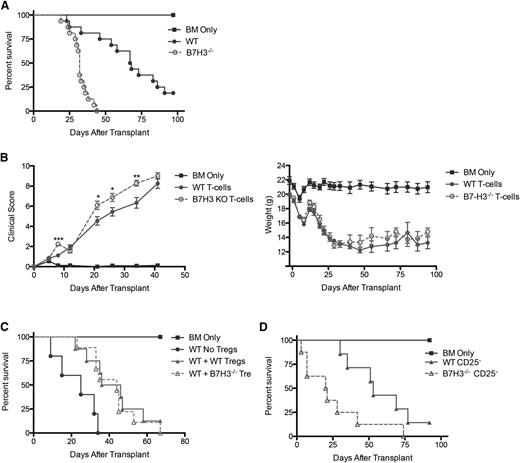
To determine whether host B7-H3 would regulate acute GVHD-induced mortality, B6-WT or B7-H3−/− mice were given BALB/c BM with or without purified T cells. Figure 2B shows survival data, with B7-H3−/− recipients having significantly accelerated lethality compared with B6-WT (median survival time [MST], 11 vs 57 days; P < .0001). Supplemental Figure 2 shows a similar trend using a different strain combination of B10.BR donor T cells (MST, 35 vs 52 days; P = .0737). Regulatory T cells (Tregs) present in the donor graft can suppress GVHD-induced lethality.14 GVHD lethality acceleration in B7-H3−/− recipients was not dependent on donor Tregs, evidenced by marked GVHD acceleration (P < .0001) following purified CD25-depleted T cell administration (Figure 2B). There was no difference in the percentage of Tregs present in WT or B7-H3−/− recipients at day 7 posttransplant (data not shown). B7-H3−/− vs B6-WT mice that were lethally irradiated and given BALB/c BM and 1 × 106 BALB/c-purified T cells had significantly increased clinical scores (days 7-30; P < .05; Figure 2C) and accelerated weight loss curves (Figure 2C). Histologic analysis depicted in Figure 2D shows that the colon of B7-H3−/− recipients on day 7 and day 21 post-BMT had significantly increased pathology, consistent with the upregulation of B7-H3 in the colon. Histologic scores of the ileum, liver, lung, and spleen were not significant (data not shown). Experiments were performed by using a known in vivo blocking anti-B7-H3 mAb (AA1.5,11,15 rat IgG2a) from days –1 to +5 and then 2 (supplemental Figure 3A) or 3 times (supplemental Figure 3B-C) per week. In recipients of Treg-depleted or replete grafts, GVHD was not accelerated (supplemental Figure 3A-C). Because anti-B7-H3 mAb did not phenocopy B7-H3−/− recipients or donor T cells, studies were also performed with another known in vivo blocking anti-B7-H3 mAb, clone MJ1816 rat IgG1, given every other day (supplemental Figure 3D). Whereas B7-H3−/− recipients had accelerated GVHD compared to B6-WT recipients, WT recipients treated with anti-B7-H3 mAb did not. Thus, additional studies focused exclusively on B7-H3–deficient recipients and donors.
To investigate the mechanism of reduced survival, we evaluated donor T cells localized to the spleen early post-BMT. α4β7 is an important homing integrin on alloreactive T cells in the development of intestinal GVHD.17 The percentage of α4β7 integrin-expressing T cells was significantly increased in B7-H3−/− recipients compared with WT recipients (Figure 2E: CD4+, 21.7% vs 28.5%; CD8+, 62.9% vs 70.2%), which correlates with the increased colon histopathology scores. Splenic T cells were analyzed for interleukin-2 (IL-2) expression by intracellular cytokine staining. There was a significant increase in the number of IL-2–expressing T cells obtained from B7-H3−/− recipients compared with B6-WT recipients for donor CD4+ (87.2% vs 78.6%) and CD8+ (91.3% vs 86.7%) T cells. Ki-67 staining was used to determine that the number of proliferating T cells obtained from B7-H3−/− recipients was also significantly greater than B6-WT T cells (CD4+, 12.7% vs 9.7%; CD8+, 5.5% vs 4.0%). The total number of T cells expressing α4β7, IL-2, and Ki-67 was also significantly increased (supplemental Figure 4) along with the mean fluorescent intensity (data not shown). Together, these data suggest that B7-H3 expression in the host can restrain donor T-cell proliferation, T-effector generation, and gut homing.
Absence of B7-H3 expression in recipients results in increased gut injury
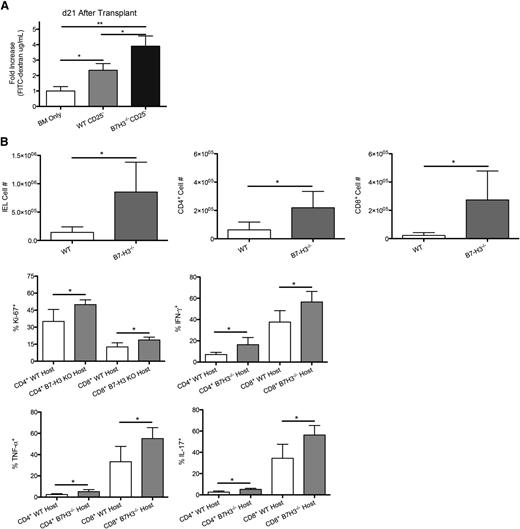
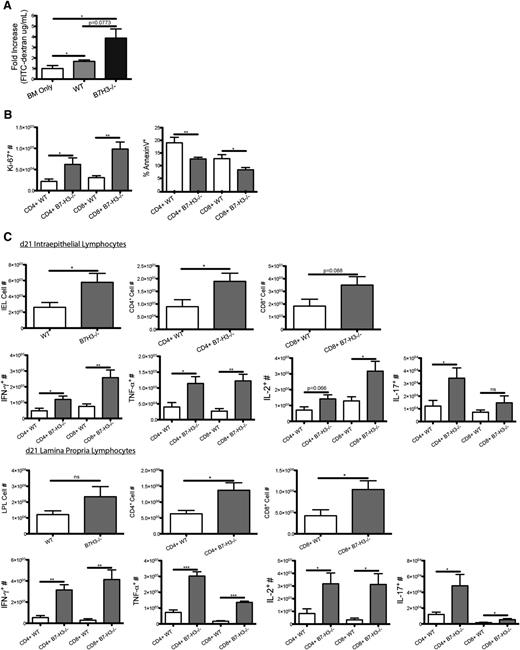
To further investigate the mechanism of decreased survival, we evaluated a gastrointestinal tract that had an increased histopathology score. B6-WT or B7-H3−/− recipients were lethally irradiated and given BALB/c BM and purified CD25-depleted T cells from BALB/c mice. To measure gut epithelial integrity, we used an FITC-dextran assay in which the loss of integrity results in leakage of FITC-dextran from the intestine into peripheral blood. FITC-dextran was administered orally to mice on day 21, and serum levels were measured 4 hours later. B7-H3−/− recipients had a significantly increased level of serum FITC-dextran (1.47 μg/mL) compared with recipients of WT CD25-depleted T cells (0.65 μg/mL), indicating decreased epithelial integrity (P < .05; Figure 3A).
Increased gut injury in B7-H3−/− vs WT recipients. (A) B6-WT or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c-purified CD25-depleted T cells. On day 21, 16 mg of FITC-dextran was administered orally to mice, and serum levels were measured 4 hours later (n = 4). One experiment was performed. (B) B6-WT or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 2 × 106 BALB/c-purified T cells. Mice were euthanized on day 21, and intraepithelial lymphocytes were analyzed for total cell numbers (n = 4; P < .05). Intraepithelial lymphocytes were analyzed for Ki-67 as well as effector cytokines IFN-γ, IL-17, and TNF-α (n = 4; P < .05). One experiment was performed. *P < .05; **P < .01.
Increased gut injury in B7-H3−/− vs WT recipients. (A) B6-WT or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 3 × 106 BALB/c-purified CD25-depleted T cells. On day 21, 16 mg of FITC-dextran was administered orally to mice, and serum levels were measured 4 hours later (n = 4). One experiment was performed. (B) B6-WT or B7-H3−/− mice were lethally irradiated and infused with 107 BALB/c NTCD BM and 2 × 106 BALB/c-purified T cells. Mice were euthanized on day 21, and intraepithelial lymphocytes were analyzed for total cell numbers (n = 4; P < .05). Intraepithelial lymphocytes were analyzed for Ki-67 as well as effector cytokines IFN-γ, IL-17, and TNF-α (n = 4; P < .05). One experiment was performed. *P < .05; **P < .01.
To determine how B7-H3 deficiency affected T cells within the intestine itself, intraepithelial lymphocytes (IELs) were isolated from the colon on day 21 posttransplant. Significantly more infiltrating donor IELs were found in B6 B7-H3−/− vs WT recipients (Figure 3B). When analyzing the number of proliferating IELs, both CD4+ and CD8+ donor T cells had an increased percentage of Ki-67+ cells in B7-H3−/− vs WT recipients (Figure 3B). On day 21 after transplant, IELs were isolated, restimulated, and stained for IFN-γ, IL-17, and tumor necrosis factor α (TNF-α). The percentage of expression of IFN-γ, IL-17, and TNF-α in CD4+ and CD8+ T cells was significantly increased in B7-H3−/− vs WT recipients (Figure 3B) as were the total cell numbers (supplemental Figure 5). Although we observed significant differences in the IELs, there was no significant difference found in the lamina propria lymphocytes (LPLs) (data not shown). These data suggest that during acute GVHD in which B7-H3 is absent in recipient tissues, the GI tract is populated with increased numbers of T effector cells producing proinflammatory cytokines.
Absence of B7-H3 expression on donor T cells leads to accelerated GVHD lethality
T cells that were activated with phorbol 12-myristate 13-acetate/ionomycin show an increased expression of B7-H3.1 To determine whether B7-H3 expression on donor T cells would have an impact on GVHD lethality by inhibiting T-cell responses (analogous to B7-H3's role on host tissues), lethally irradiated WT BALB/c mice were given B6-WT BM with or without purified T cells from B6-WT or B7-H3−/− mice to induce GVHD. Figure 4A shows survival data, with B7-H3−/− donor T cells having a significantly accelerated lethality compared with WT donor T cells (MST, 32 vs 67.5 days; P < .0001). Consistent with survival data, Figure 4B shows that mice that received B7-H3−/− donor T cells had significantly increased clinical scores (day 8 and days 21-34; P < .05). Whereas the histopathology scores showed that the mice had severe GVHD (supplemental Figure 6), no significant difference between the WT and B7-H3−/− groups was seen during clinical scoring.
B7-H3−/− donor T cells augment GVHD. (A) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6-WT or B7-H3−/−–purified T cells. Survival plot of WT (solid circle) vs B7-H3−/− (open circle) is shown (n = 16 per group; P < .0001). Two experiments with comparable results were pooled. (B) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6 or B7-H3−/−-purified T cells. Mice were analyzed for weights and clinical scores, the latter of which were not scored beyond day 41 because they had reached mean values of >8 in both groups (n = 8 per group). One experiment was performed. (C) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM, 1 × 106 B6-purified T cells, and 0.5 × 106 B6-WT or B7-H3−/−-purified Tregs. Survival plot of B6-WT Tregs (solid triangle) vs B7-H3−/− Tregs (open triangle) is shown (n = 8-9 per group; P < .0001). One experiment was performed. (D) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6-WT or B7-H3−/− CD25-depleted–purified T cells. Survival plot of WT (solid circle) vs B7-H3−/− (open circle) is shown (n = 8 per group; P < .05). One experiment was performed. *P < .05; **P < .01. KO, knockout.
B7-H3−/− donor T cells augment GVHD. (A) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6-WT or B7-H3−/−–purified T cells. Survival plot of WT (solid circle) vs B7-H3−/− (open circle) is shown (n = 16 per group; P < .0001). Two experiments with comparable results were pooled. (B) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6 or B7-H3−/−-purified T cells. Mice were analyzed for weights and clinical scores, the latter of which were not scored beyond day 41 because they had reached mean values of >8 in both groups (n = 8 per group). One experiment was performed. (C) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM, 1 × 106 B6-purified T cells, and 0.5 × 106 B6-WT or B7-H3−/−-purified Tregs. Survival plot of B6-WT Tregs (solid triangle) vs B7-H3−/− Tregs (open triangle) is shown (n = 8-9 per group; P < .0001). One experiment was performed. (D) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6-WT or B7-H3−/− CD25-depleted–purified T cells. Survival plot of WT (solid circle) vs B7-H3−/− (open circle) is shown (n = 8 per group; P < .05). One experiment was performed. *P < .05; **P < .01. KO, knockout.
B7-H3−/− recipients were hypersusceptible to GVHD caused by Treg-replete or Treg-depleted grafts. However, such data do not provide insight regarding whether B7-H3 is expressed on Tregs or whether its absence on donor Tregs, if Tregs do indeed express B7-H3, would alter GVHD lethality. qRT-PCR studies of resting and day 3 anti-CD3/CD28 mAb plus IL-2–activated Tregs were performed. There was no difference between resting and activated Tregs, neither of which expressed B7-H3 mRNA in contrast to concurrently analyzed mRNA from the lung tissue of GVHD mice from Figure 1 (data not shown). Consistent with these data indicating an absence of B7-H3 mRNA in resting or activated Tregs at a suboptimally effective but biologically relevant ratio of 1:2 donor Tregs to T cells, we did not see any reduced in vivo suppression of GVHD when adding back freshly isolated Tregs from B7-H3−/− vs WT donors (Figure 4C). Additionally, Figure 4D shows that acceleration of GVHD lethality in recipients of B7-H3−/− donor T cells was not dependent on donor Tregs because Treg depletion of B7-H3−/− vs WT donor T cells resulted in significant GVHD acceleration. Furthermore, there was no difference in the percentage of Tregs present in recipients of WT or B7H3−/− donor T cells at day 7 after transplant (data not shown). These data confirm that the absence of B7-H3 expression on donor T cells results in augmented GVHD lethality.
Absence of B7-H3 expression on donor T cells results in increased donor T-cell proliferation and activation
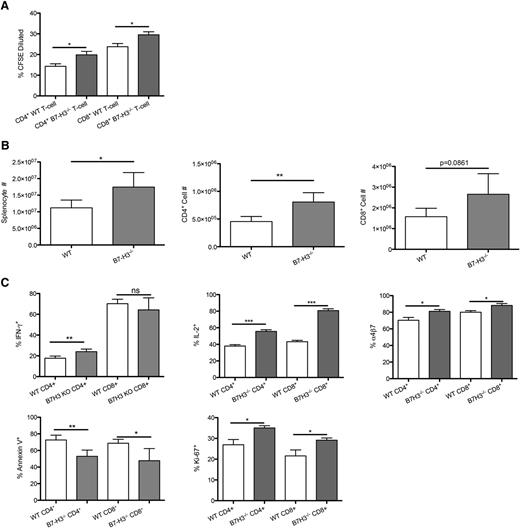
To determine whether allogeneic T cells would increase their in vitro and in vivo proliferation when B7-H3 was absent, we performed an in vitro mixed leukocyte reaction of B6-WT or B7-H3−/−- purified T cells with irradiated BALB/c BM-derived DC stimulators (10:1). Figure 5A shows that B7-H3−/− vs WT CD4+ and CD8+ T cells stimulated with BALB/c DC stimulators had significantly increased proliferation on day 5, indicating an inhibitory regulatory function of B7-H3 on T-cell alloresponses. Next, splenocytes were isolated on day 7 from recipients of B7-H3−/− vs WT donor T cells. In contrast to WT recipients, mice receiving B7-H3−/− donor T cells had significantly more total splenic donor T cells, donor CD4+ T cells, and a trend (P = .086) toward more donor CD8+ T cells (Figure 5B). There were significantly more B7-H3−/− donor T cells that were proliferating, measured by Ki-67 expression, compared with WT T cells (CD4+, 35% vs 26%; CD8+, 29% vs 22%). Splenic T cells were restimulated and analyzed for IFN-γ and IL-2 by intracellular cytokine staining. Figure 5C shows that recipients of B7-H3−/− compared with WT donor T cells had a significantly higher percentage of IFN-γ–expressing CD4+ T cells (24.02% vs 17.7%). Although the percentage of IFN-γ–expressing CD8+ T cells was not significantly different (64.22% vs 70.23%), the total number of IFN-γ–expressing CD8+ T cells was significantly higher (supplemental Figure 7; 1.6 × 106 vs 1.1 × 106). The percentage of IL-2–expressing T cells (CD4+, 55.62% vs 37.98%; CD8+, 80.74% vs 42.18%) was also a significantly increased as were the total cell numbers. In addition to having a higher number of cells proliferating, the proportion of cells undergoing apoptosis was significantly reduced in B7-H3−/− vs WT donor T cells (CD4+, 52.9% vs 72.6%; CD8+, 47.7% vs 68.7%). The number of α4β7 integrin–expressing cells was significantly increased in B7-H3−/− recipients compared with WT donor T cells (CD4+, 81.08% vs 70.40%; CD8+, 88.22% vs 80.0%). Compared with WT, B7-H3−/− donor T cells had increased proliferation, effector cytokine secretion, and α4β7 integrin expression; they were also less susceptible to apoptosis.
B7-H3−/− T cells have increased activation and proliferation and decreased apoptosis. (A) MLR was performed by coculturing B6-WT or B7-H3−/−-purified T cells that were CFSE labeled with irradiated BALB/c DC stimulators (10:1). Cells were analyzed by flow cytometry on day 5. Cells were gated on H2Kb-positive, viability dye–negative CD4 or CD8 positive events and were analyzed for dilution of CFSE (n = 5). One of 2 representative experiments is shown. (B) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6-WT or B7-H3−/−-purified T cells. Mice were euthanized on day 7, and splenocytes were analyzed for total cell numbers (n = 4). One experiment was performed. (C) Splenocytes were analyzed for Ki-67, IFN-γ, IL-2, and annexin V α4β7 expression (n = 4). One experiment was performed. *P < .05; **P < .01; ***P < .001.
B7-H3−/− T cells have increased activation and proliferation and decreased apoptosis. (A) MLR was performed by coculturing B6-WT or B7-H3−/−-purified T cells that were CFSE labeled with irradiated BALB/c DC stimulators (10:1). Cells were analyzed by flow cytometry on day 5. Cells were gated on H2Kb-positive, viability dye–negative CD4 or CD8 positive events and were analyzed for dilution of CFSE (n = 5). One of 2 representative experiments is shown. (B) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM and 1 × 106 B6-WT or B7-H3−/−-purified T cells. Mice were euthanized on day 7, and splenocytes were analyzed for total cell numbers (n = 4). One experiment was performed. (C) Splenocytes were analyzed for Ki-67, IFN-γ, IL-2, and annexin V α4β7 expression (n = 4). One experiment was performed. *P < .05; **P < .01; ***P < .001.
Absence of B7-H3 expression on donor T cells results in increased gut injury in recipients
To further investigate the mechanism of decreased survival, we used the FITC-dextran assay. FITC-dextran was administered orally to mice on day 21, and serum levels were measured 4 hours later. On day 21, mice that received B7-H3−/− donor T cells had a modestly increased level of FITC-dextran in the serum (1.477 μg/mL) compared with recipients of WT donor T cells (0.6497 μg/mL) indicating decreased epithelial integrity (P = .077; Figure 6A). IELs were isolated on day 21 posttransplant. When we analyzed the number of proliferating IELs, Ki-67+ cell numbers in recipients of B7-H3−/− vs WT T cells was increased approximately threefold for both CD4+ and CD8+ T cells (Figure 6B). Additionally, the proportion of T cells undergoing apoptosis (annexin V-positive) was significantly reduced. This suggests that B7-H3−/− donor T cells are proliferating more while dying less and leading to increased gut injury. On day 21 after transplant, IELs and LPLs were isolated, re-stimulated, and stained for expression of IFN-γ, IL-2, IL-17, and TNF-α. Significantly more total donor IELs, more CD4+ cells, and a trend toward more CD8+ T cells were found in recipients of B7-H3−/− vs WT T cells in addition to significantly increased CD4+ and CD8+ LPLs (Figure 6C). IEL expression of IFN-γ and TNF-α in CD4+ and CD8+ T cells, IL-2 in CD8+ T cells, and IL-17 in CD4+ T cells was significantly increased in recipients of B7-H3−/− vs WT donor T cells. LPL expression of IFN-γ, TNF-α, IL-2, and IL-17 was significantly increased in recipients of B7-H3−/− vs WT donor T cells (Figure 6C). These data suggest that in acute GVHD in which B7-H3 is absent on donor T cells, lymphocytes accumulate in the GI tract by increased proliferation and survival with increased cytokine production leading to increased damage to the GI tract, which is associated with decreased overall survival.
B7-H3−/− donor T cells have increased GVHD-induced gut injury. (A) BALB/c mice were lethally irradiated and infused with 107 B6-WT BM and 1 × 106 B6-WT or B7-H3−/−-purified T cells. On day 21, 16 mg of FITC-dextran was administered orally to mice, and serum levels were measured 4 hours later (n = 4). One experiment was performed. (B) BALB/c mice were lethally irradiated and infused with 107 B6 BM and 1 × 106 B6-WT or B7-H3−/−-purified T cells. Mice were euthanized on day 21 after transplant, and intraepithelial lymphocytes were analyzed for Ki-67 and annexin V expression (n = 4). One experiment was performed. (C) BALB/c mice were lethally irradiated and infused with 107 B6-WT BM and 1 × 106 B6-WT or B7-H3−/−- purified T cells. Mice were euthanized on day 21 after transplant, and intraepithelial lymphocytes and lamina propria lymphocytes were analyzed for CD4 or CD8 and IFN-γ, TNF-α, IL-2, or IL-17 coexpression (n = 4). One experiment was performed. *P < .05; **P < .01; ***P < .001.
B7-H3−/− donor T cells have increased GVHD-induced gut injury. (A) BALB/c mice were lethally irradiated and infused with 107 B6-WT BM and 1 × 106 B6-WT or B7-H3−/−-purified T cells. On day 21, 16 mg of FITC-dextran was administered orally to mice, and serum levels were measured 4 hours later (n = 4). One experiment was performed. (B) BALB/c mice were lethally irradiated and infused with 107 B6 BM and 1 × 106 B6-WT or B7-H3−/−-purified T cells. Mice were euthanized on day 21 after transplant, and intraepithelial lymphocytes were analyzed for Ki-67 and annexin V expression (n = 4). One experiment was performed. (C) BALB/c mice were lethally irradiated and infused with 107 B6-WT BM and 1 × 106 B6-WT or B7-H3−/−- purified T cells. Mice were euthanized on day 21 after transplant, and intraepithelial lymphocytes and lamina propria lymphocytes were analyzed for CD4 or CD8 and IFN-γ, TNF-α, IL-2, or IL-17 coexpression (n = 4). One experiment was performed. *P < .05; **P < .01; ***P < .001.
B7-H3−/− T cells can mediate graft-versus-leukemia without inducing GVHD when given later after BMT
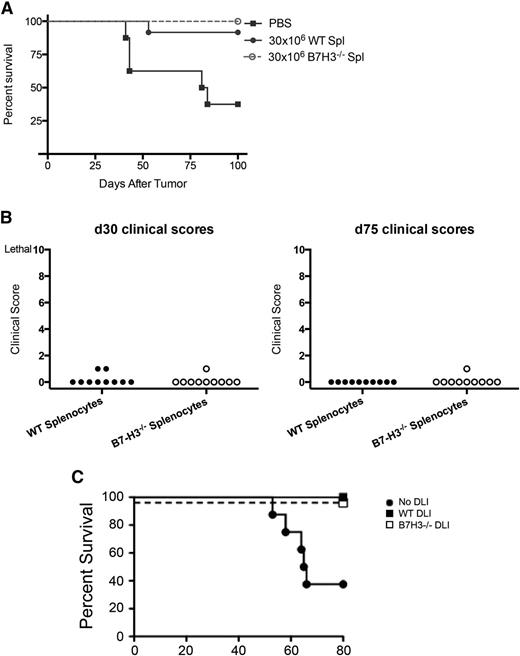
Because B7-H3 appears to negatively regulate T cells and cause higher lethality in our acute GVHD model, we sought to determine whether they could be used to facilitate graft-versus-leukemia (GVL) without inducing lethal GVHD. Because of the marked acceleration of GVHD lethality early post-BMT, we used a model of delayed lymphocyte infusion (DLI). In this setting, blocking a negative regulatory pathway (programmed cell death protein 1 [PD1]/programmed death-ligand 1 [PD-L1] pathway) can provide a potent means of achieving GVL effects without GVHD effects, an approach that uses negative regulatory molecules such as anti-PD-1 or anti-CTLA4 to achieve GVL without GVHD late post-BMT.18 Lethally irradiated BALB/c mice were given 107 BALB/c BM. Cohorts were infused with B6-WT or B7-H3−/− splenocytes at a dose of 30 × 106 (day 50) in saline without supplemental cells as a control. Three days later, mice were challenged with A20luc lymphoma cells (106). Mice were monitored for survival as well as GVHD clinical scores. Mice that received no DLI had more than 50% tumor-related lethality (Figure 7A). Mice that received WT or B7-H3−/− splenocytes had only 8% and 0% lethality, respectively, and did not have clinical GVHD (Figure 7B), despite infusing 30 × 106 splenocytes. In other studies, B6 non-T-cell–depleted BM was used for allogeneic BMT (Figure 7C). On day 28, an infusion of B6-WT or B7-H3−/− splenocytes at a dose of 25 × 106 was followed 3 days later by 4 × 106 A20luc lymphoma cells. Whereas 70% of mice receiving BM and A20luc alone succumbed to tumor, no deaths (Figure 7C) or weight loss (not shown) were seen in either DLI group. These data indicate that B7-H3−/− T cells are well-tolerated later post-BMT without increasing GVHD while mediating a GVL effect in a manner similar to that of WT T cells.
B7-H3−/− splenocytes retain a GVL effect. (A) BALB/c mice were lethally irradiated and infused with 107 BALB/c T-cell-depleted BM. Mice were infused with phosphate-buffered saline (PBS) (no DLI) or either 30 × 106 B6-WT or B7-H3−/− splenocytes (Spl) (DLI; day 50). Three days later, the mice were infused with 106 A20luc tumor cells (n = 10 per group). One experiment was performed. No DLI vs B6-WT DLI, P = .0092; no DLI vs B7H3−/− DLI, P = .0014. (B) Mice were monitored for clinical scores that were not significant on day 30 or day 75 (n = 10 per group). One experiment was performed. No DLI vs WT or B7H3 −/− DLI, P ≤ .01. (C) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM. Mice were then infused with 25 × 106 splenocytes (DLI; day 28) from B6-WT or B6-B7-H3−/− donors (n = 8 per group; 1 experiment was performed) or PBS (no DLI) as a control. Three days later, the mice were infused with 4 × 106 A20luc tumor cells (day 31). Survival after tumor infusion is shown (n = 7-8 mice per group; 1 experiment was performed). For PBS vs B6-WT DLI or B6-B7-H3−/− DLI, P < .01.
B7-H3−/− splenocytes retain a GVL effect. (A) BALB/c mice were lethally irradiated and infused with 107 BALB/c T-cell-depleted BM. Mice were infused with phosphate-buffered saline (PBS) (no DLI) or either 30 × 106 B6-WT or B7-H3−/− splenocytes (Spl) (DLI; day 50). Three days later, the mice were infused with 106 A20luc tumor cells (n = 10 per group). One experiment was performed. No DLI vs B6-WT DLI, P = .0092; no DLI vs B7H3−/− DLI, P = .0014. (B) Mice were monitored for clinical scores that were not significant on day 30 or day 75 (n = 10 per group). One experiment was performed. No DLI vs WT or B7H3 −/− DLI, P ≤ .01. (C) BALB/c mice were lethally irradiated and infused with 107 B6 NTCD BM. Mice were then infused with 25 × 106 splenocytes (DLI; day 28) from B6-WT or B6-B7-H3−/− donors (n = 8 per group; 1 experiment was performed) or PBS (no DLI) as a control. Three days later, the mice were infused with 4 × 106 A20luc tumor cells (day 31). Survival after tumor infusion is shown (n = 7-8 mice per group; 1 experiment was performed). For PBS vs B6-WT DLI or B6-B7-H3−/− DLI, P < .01.
Discussion
We demonstrate the important role for the B7-H3 pathway in acute GVHD in major histocompatibility complex–disparate recipients. B7-H3 was found to be upregulated in GVHD organs, including colon, liver, and lung on day 21 posttransplant in mice and in intestinal biopsies of patients with grade 3 to 4 acute GVHD. B7-H3−/− recipients showed accelerated GVHD lethality. B7-H3−/− donor T cells also accelerated GVHD lethality. Both recipients of B7-H3−/− donor T cells and recipients of B7-H3−/− cells had more damage to the epithelial layer of the colon and an increased percentage of inflammatory cytokine secretion. Our results indicate that B7-H3 acts as a negative costimulatory molecule in the context of GVHD.
GVHD leads to an upregulation of B7-H3 mRNA in target organs, likely because of IFN-γ, which is increased during acute GVHD.1,2 Other negative regulatory pathways have been shown to play an important role in regulating acute GVHD.19-22 Because B7-H3 is a B7 homolog, similar to the PD-1/PD-L1 pathway, it is not surprising that during acute GVHD, B7-H3 would be upregulated in GVHD target organs and that the absence of this inhibitory receptor would accelerate GVHD.23 Because B7-H3−/− donor T cells accelerated GVHD lethality, B7-H3 expression plays a cell-intrinsic role in suppressing T-cell responses. Intriguingly, Tregs do not express B7-H3, as assessed by qRT-PCR, and the accelerated GVHD lethality of B7-H3−/− vs WT T cells is accounted for entirely by the non-Treg population, pointing to a T-cell intrinsic (donor) and extrinsic (host) role of B7-H3 in acute GVHD.
Our studies using B7-H3−/− recipient mice showed significantly increased GVHD compared with that in B6-WT recipient mice, supporting previous studies in which inhibiting B7-H3 in a Th1-mediated model led to earlier onset of experimental autoimmune encephalitis2 and an increased cardiac graft rejection.12 Although Tregs have a known inhibitory role in GVHD,14 depletion of CD25+ cells from the donor graft still resulted in increased GVHD lethality in B7-H3−/− recipients, indicating that B7-H3 expression on donor T cells downregulates GVHD through a thymus-derived Treg-independent mechanism.
Studies have shown that an increased level of cytokine production in the gut, mainly by infiltrating donor T cells, can mediate acute GVHD.24,25 Increased histopathology scores were found in the colon of B7-H3−/− recipients at days 7 and 21 after transplant. Our data show that a higher percentage of both CD4+ and CD8+ donor T cells were secreting inflammatory cytokines (IFN-γ, TNF-α, and IL-17) and had increased T effector proliferation in the intraepithelial layer of the colon in B7-H3−/− recipients at day 21 posttransplant. These data are consistent with other studies, which showed that inhibiting B7-H3 led to increased IFN-γ secretion as well as increased proliferation.2,11,12 Increased T effector proliferation and inflammatory cytokine secretion could mean increased gut epithelial damage, as is supported by our finding of increased serum concentration of orally administered FITC-dextran. We conclude that B7-H3−/− recipients have an increased GVHD lethality rate as a result of an increase of inflammatory cytokine secretion in the gut and decreased integrity of the epithelial barrier.
With regard to the T-cell intrinsic properties of B7-H3, activated T cells have been shown to express B7-H3, and expression can be upregulated by IFN-γ.1,2 By using B7-H3−/− donor T cells, we showed that depletion of CD25+ cells from the donor graft still resulted in increased GVHD lethality. Analysis of the spleen at day 7 posttransplant showed that there was an increased number of proliferating donor T cells (Ki-67+) as well as a reduced percentage of donor cells undergoing apoptosis (annexin V-positive). We observed more IFN-γ production in recipients of B7-H3−/− donor T cells, consistent with other studies, which showed that inhibiting B7-H3 led to increased IFN-γ secretion as well as increased proliferation.2,11,12 Increased gut epithelial damage could result from increased T effector proliferation, inflammatory cytokine secretion, and increased epithelial damage demonstrated by an increased serum concentration of orally administered FITC-dextran, likely contributing to accelerated GVHD-induced lethality.
After observing that B7-H3 negatively regulated T cells, we hypothesized that B7-H3−/− donor T cells could be used to facilitate GVL without inducing GVHD by using a delayed DLI model. Our results show that when mice were challenged with tumor and given DLI 3 days later, the mice that received B7-H3−/− donor T cells had no clinical GVHD while achieving a GVL response comparable to that of WT donor T cells. Despite the high level of acute GVHD seen upon transfer of B7-H3−/− donor T cells at the time of transplant, performing a DLI at day 53 or day 28 after BM transfer circumvents GVHD. We conclude that B7-H3−/− T cells can provide a potent GVL effect without GVHD in this model of delayed DLI.
The role of B7-H3 remains controversial, with reports of both positive1,7,9,10 and negative2,8,11,12 costimulatory roles in models of autoimmunity and tumor immunobiology. A possible explanation for such conflicting data may be that B7-H3 is capable of binding to more than one receptor. Members of the B7/CD28 family are known to be capable of being both activators and negative regulators of the T-cell response. It is therefore possible that B7-H3 may have different ligands that can provide positive or negative signals. Currently, only one potential ligand has been suggested, the triggering receptor expressed on myeloid cell-like transcript (TLT-2), which has been known to interact with B7-H3 and enhance CD8 T-cell activation and conversely inhibit contact hypersensitivity responses by using an mAb to block this interaction.7 Although these data suggest that TLT-2 may function as a ligand for B7-H3, others have not confirmed this interaction in mice or humans.8 To better understand the mechanisms of the role B7-H3 has in the immune system, all potential ligands will need to be identified.
Another possible explanation for the contrasting data are the physiological differences between the models used to study B7-H3. Suh et al2 demonstrated that B7-H3 is a negative regulator that preferentially affects Th1 responses. B7-H3 expression on DCs was upregulated in the presence of IFN-γ but was suppressed in the presence of IL-4. B7-H3−/− mice developed more severe airway inflammation than did WT mice under conditions in which T helper cells were differentiated toward Th1 rather than Th2. Under Th1 conditions, B7-H3−/− mice developed more severe airway inflammation than WT mice with more activated T cells in the bronchoalveolar lavage, and they had increased IFN-γ production. These results were not seen under a Th2-mediated airway hypersensitivity that showed no apparent differences between the B7-H3−/− and WT groups. In a model of cardiac transplantation in which Th1 responses mediate rejection, using a B7-H3 blocking antibody or B7-H3−/− recipients led to accelerated rejection in one study that used mice with deletion of the second exon of the Ig domain,12 whereas B7-H3−/− mice that had a deletion of the second and third exon10 provided a contrasting conclusion in terms of B7-H3 function for unknown reasons. In our acute GVHD studies, which were dominated by Th1 responses, we showed that inhibiting B7-H3 by using B7-H3−/− recipients as well as B7-H3−/− donor T cells led to accelerated GVHD lethality, along with increased IFN-γ production and T effector proliferation. This is in agreement with the above cardiac allograft rejection studies that used the same B7-H3−/− strain, which led to the conclusion that B7-H3 functions as a negative regulatory molecule.12
In summary, the B7-H3 pathway acts as a suppressor of acute GVHD. Our data point to the development and testing of approaches to increase B7-H3 expression early post-BMT to reduce GVHD and to the development of potent antagonistic B7-H3 mAbs later post-BMT to enhance DLI-mediated GVL.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Wayne Hancock for valuable discussions of this work.
This work was supported in part by National Institutes of Health grants R01 HL56067 from the National Heart, Lung, and Blood Institute, and R01 AI 34495, P01 AI 056299, and T32 AI 007313 from the National Institute of Allergy and Infectious Diseases.
Authorship
Contribution: R.G.V. designed and performed research, provided and analyzed the data, and wrote the paper; R.F. performed experiments, provided data, and edited the paper; K.K. performed experiments, provided data, and edited the paper; C.M.-H. and A.S. performed experiments and edited the paper; P.A.T. performed experiments, provided advice, and edited the paper; M.J.O., A.P.-M., A.S.-G., E. L., and R.Z. provided data and edited the paper; W.J.M., J.S.S., D.H.M., and G.J.F. designed research and edited the paper; T.W.M. provided mice and edited the paper; J.P.A. and M.v.d.B. provided advice and edited the paper; and B.R.B. designed, organized, and supervised research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, Department of Pediatrics, MMC 109, University of Minnesota, Minneapolis, MN 55455; e-mail address: blaza001@umn.edu.
References
Author notes
R.F., K.K., M.v.d.B., R.Z., and B.R.B. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal