Key Points
Platelet-delivered ADAMTS13 inhibits arterial thrombosis after vascular injury.
Platelet-delivered ADAMTS13 also prevents thrombotic thrombocytopenic purpura.
Abstract
ADAMTS13 metalloprotease cleaves von Willebrand factor (VWF), thereby inhibiting platelet aggregation and arterial thrombosis. An inability to cleave ultralarge VWF resulting from hereditary or acquired deficiency of plasma ADAMTS13 activity leads to a potentially fatal syndrome, thrombotic thrombocytopenic purpura (TTP). Plasma exchange is the most effective initial therapy for TTP to date. Here, we report characterization of transgenic mice expressing recombinant human ADAMTS13 (rADAMTS13) in platelets and its efficacy in inhibiting arterial thrombosis and preventing hereditary and acquired antibody-mediated TTP in murine models. Western blotting and fluorescent resonance energy transfer assay detect full-length rADAMTS13 protein and its proteolytic activity, respectively, in transgenic (Adamts13−/−PltA13), but not in wild-type and Adamts13−/−, platelets. The expressed rADAMTS13 is released on stimulation with thrombin and collagen, but less with 2MesADP. Platelet-delivered rADAMTS13 is able to inhibit arterial thrombosis after vascular injury and prevent the onset and progression of Shigatoxin-2 or recombinant murine VWF-induced TTP syndrome in mice despite a lack of plasma ADAMTS13 activity resulting from the ADAMTS13 gene deletion or the antibody-mediated inhibition of plasma ADAMTS13 activity. These findings provide a proof of concept that platelet-delivered ADAMTS13 may be explored as a novel treatment of arterial thrombotic disorders, including hereditary and acquired TTP, in the presence of anti-ADAMTS13 autoantibodies.
Introduction
ADAMTS13, a plasma metalloprotease that cleaves von Willebrand factor (VWF), is produced primarily in hepatic stellate cells1 as well as in other cells, including endothelial cells,2,3 megakaryocytes, and platelets.4,5 Plasma ADAMTS13 concentration ranges from 0.5 to 1.5 mg/L. Deficiency of plasma ADAMTS13 activity resulting from hereditary6 or acquired etiologies7,8 results in an accumulation of ultralarge (UL) VWF on endothelial cell surface and in plasma, leading to the formation of disseminated thrombi in small arterioles and capillaries. This is the characteristic feature of TTP. Patients with TTP often present with severe thrombocytopenia and microangiopathic hemolytic anemia with various degrees of end organ damage.9 TTP is seen in neonates, young children, and adults. The annual incidence rate is 3 to 10 per million populations. Hereditary TTP is caused by mutations in the ADAMTS13 gene,6,10 but acquired TTP is caused by autoantibodies against ADAMTS13.11 Without treatment, the mortality rate is ∼85% to 90%.9,12
Plasma infusion suffices in treating hereditary TTP,13 but plasma exchange is often required to achieve remission for acquired TTP with inhibitors.11,14 Plasma exchange removes immunoglobulin G (IgG) autoantibodies against ADAMTS13, ULVWF multimers, and perhaps inflammatory cytokines while replenishing ADAMTS13 enzyme. In patients with high-titer inhibitors, the infused ADAMTS13 from plasma is often not sufficient to override autoantibodies and correct the underlying ADAMTS13 deficiency.8 In this case, a prolonged course of plasma exchange plus adjunctive immunosuppressive therapies, including cyclophosphamide, cyclosporine, or rituximab, may be required.8,15-18 Severe ADAMTS13 deficiency and persistence of autoantibodies correlate with increased mortality rate and/or relapses.8,19 Our groups20 and others21 have shown that anti-ADAMTS13 IgGs bind to multiple domains on ADAMTS13, but their inhibition appears to be mediated most commonly through the binding to the spacer domain.22-26 Conservative modifications in the antibody-binding epitopes have generated 2 ADAMTS13 variants that are more resistant to inhibition by autoantibodies, which may be developed as a novel therapeutic for acquired TTP resulting from inhibitors.22
Here, we characterize transgenic mice that express human ADAMTS13 in platelets and report its efficacy in murine models of arterial thrombosis and TTP. A similar strategy has been used to express factor VIII to treat hemophilia A with inhibitors, with limited success.27 Our results demonstrate that platelet-delivered rADAMTS13 is efficacious for inhibiting arterial thrombosis after vascular injury and prevents the onset of Shigatoxin-2 (Stx-2)-or recombinant murine VWF (rmVWF)-induced TTP in the absence or the presence of inhibitors. The findings provide a proof of concept that platelet-derived ADAMTS13 may be explored as a novel therapeutic strategy for arterial thrombosis and TTP, even in the face of autoantibodies.
Materials and methods
Preparation of murine VWF
Preparation of scFv4-20 antibody
Generation of transgenic mice
A human ADAMTS13 cDNA containing 2 flanking NotI sites was amplified by a polymerase chain reaction as described.31,32 After digestion with NotI, a human ADAMTS13 gene was inserted into a pBSK vector33 which contains a megakaryocyte-specific murine glycoprotein Ib (GPIbα) promoter (∼2.6 kb), a 5′ untranslated region, the first exon, and part of the first intron, followed by human ADAMTS13 (4.3 kb) flanked by 5′ and 3′ untranslated regions. After digestion with XhoI/SalI, the released insert was used to generate transgenic mice by pronuclear injections, following a standard method. An institutional animal care and use committee at the Children’s Hospital of Philadelphia approved the study protocol.
Genotyping and reverse transcription-polymerase chain reaction
Genomic DNA was isolated from a tail of the founder animals and their offspring, using a DNA-Mini Kit (Qiagen, Valencia, CA). The transgene was amplified by polymerase chain reaction (PCR), using primers that correspond to the GPIbα promoter region (forward: 5′-aggggtggaaaaggagagaa-3′) and 5′-SV40 untranslated region (reverse: 5′-aaaggcaacatccactgagg-3′). Knockout (KO) genotype was confirmed by another set of PCR reactions, as described previously.34
Total RNAs were isolated from washed platelets of wild-type (WT), KO, and transgenic (TG) mice with a TRIzol reagent (Life Technologies, Grand Island, NY) according to the manufacturer’s recommendation. Reverse transcription PCR (RT-PCR) was performed using a forward primer (5′-tctgttcctccaaaggactg-3′) and a reverse primer (5′-cacagaggggagggcatctt-3′). As a positive control, murine platelet factor 4 (PF4)33 was amplified with primers (5′-gtaga-actttatctttgggt-3′ and 5′-aattttcctcccattcttca-3′).
Isolation of murine platelets
Platelet-rich plasma was prepared by centrifugation at 100g for 10 minutes from anticoagulated whole blood in the presence of prostaglandin E1(PGE1) (1 µM) and apyrase (0.1 U/mL). Platelets were obtained after an additional spin at 400g for 10 minutes and suspended in a Tyrode’s buffer containing glucose (0.1%, wt/vol), human serum albumin (0.35%, wt/vol), calcium (2 mM), and magnesium (1 mM) or lysed in a lysis buffer or a sodium dodecyl sulfate sample buffer, depending on the purpose of the experiment.
Assay of ADAMTS13 activity
ADAMTS13 activity in plasma and platelet releasate or lysate was determined by the cleavage of a fluorogenic VWF73 (rF-VWF73), as described.35 Normal human plasma was used for calibration (1 U/mL activity). Murine plasma ADAMTS13 activity was determined by the cleavage of a commercial fluorescent resonance energy transfer VWF73 peptide (Peptide International, Louisville, KY), as described.36
Western blotting
All proteins in platelet releasate or lysate with or without agonist stimulation were precipitated by 0.6N perchloric acid and used for western blotting with mouse anti-ADAMTS13 IgG (5F6) (kindly provided by David Motto at Puget Sound Blood Center, University of Washington, Seattle, WA) or mouse anti-β actin IgG, or rabbit anti-VWF IgG followed by IRDy800-labeled goat anti-mouse IgG or IRDy700 goat anti-rabbit IgG, as described.22,37
Immunofluorescence microscopy
Platelets were either air-dried on a glass slide or cultured on a fibrinogen-coated surface. Megakaryocytes were cultured on a chamber slide with Dulbecco’s modified Eagle medium with 10% fetal bovine serum and thrombopoietin (100 ng/mL), as described.38 Platelets and megakaryocytes were fixed with 4% paraformaldehyde in phosphate-buffered saline for 10 minutes, permeabilized with 1% Triton X-100, and stained with mouse anti-ADAMTS13 IgG (clone 360) (1:50) and/or rabbit anti-CD41 (1:100), rabbit anti-murine PF4 IgG (1:500) or rabbit anti-VWF IgG (1:100), or rabbit anti-Lamp1 IgG (1:100) (Abcam), followed by Alexa488-conjugated anti-mouse IgG (1:200) or Alexa594-conjugated anti-rabbit IgG (1:100) (Life Technologies), as described.3,39 F-actin was stained with Alexa350-labeled phalloidin (Life Technologies). Nuclei were counterstained with 4′,6 diamidino-2-phenylindole (DAPI) in mounting medium (Vector Laboratories, Burlingame, CA). Images were obtained either under a Nikon fluorescence microscope or an Olympus confocal microscope.
Megakaryocyte ploidy
Megakaryocyte ploidy was measured by double staining and flow cytometry (LSR Fortessa; BD Biosciences), as described.40
Platelet aggregation assay
Platelets (0.5 mL, 2∼4 × 108 cells/mL) were incubated at 37°C with a buffer or various agonists, including type 1 fibrillar collagen (2-10 μg/mL), α-thrombin (0.1-1.0 U/mL), or 2MesADP (30-60 μM). Light transmission was monitored with a PAP4 light scattering platelet aggregometer (BioData Corporation, Horsham, PA) for 3 minutes. The numeric data were expressed as the percentage of light transmission as a function of time.
Intravital microscopic imaging
Mice (∼4 weeks) were anesthetized with intraperitoneal injection of pentobarbital. One hundred microliters rhodamine-6G (200 µg/mL) in phosphate-buffered saline was infused via retro orbital sinus perplex. Mesenteric vessels were exposed to 10% FeCl3 for 3 minutes.28,41 A Nikon fluorescent microscope equipped with a high-resolution digital camera determined the accumulation of fluorescent platelets and leukocytes in injured vessels. The time lapse was set for 30 minutes, with acquisition of an image every 10 seconds. The relative fluorescent intensity was determined off-line, using the NIS-Elements software.
Murine models of TTP
Mice (CAST/Ei) (4-8 weeks old) were challenged by an intravenous bolus of Stx-2 (20∼250 pg/g body weight; Toxin Technology, Sarasota, FL)42 or rmVWF (5∼10 µg/g body weight). Complete blood counts were determined before and daily for 3-7 days after Stx-2 or rmVWF challenge, using a Hemavet M2950HV analyzer (Drew Scientific, Waterbury, CT).
To generate acquired ADAMTS13 deficiency, WT and TG mice (CAST/Ei) were injected with scFv4-20 (10 μg/g body weight). Eight hours after antibody administration, mice were challenged with an intravenous bolus of rmVWF. Whole blood was collected 1 day before and 8 and 18 hours after rmVWF challenge and anticoagulated with sodium citrate for complete blood count and plasma ADAMTS13 activity.
Histology
Tissues were collected from mice that died or were euthanized at the end of experiments, fixed in 4% paraformaldehyde in phosphate-buffered saline, and paraffin-embedded before sectioning. Tissue sections were stained with hematoxylin-eosin. Digital images were obtained using a Nikon light microscope at the magnification of ×200.
Statistical analysis
Student t test and analysis of variance (ANOVA) were used to determine the significance of the differences between the groups.
Results
Characterization of transgenic mice
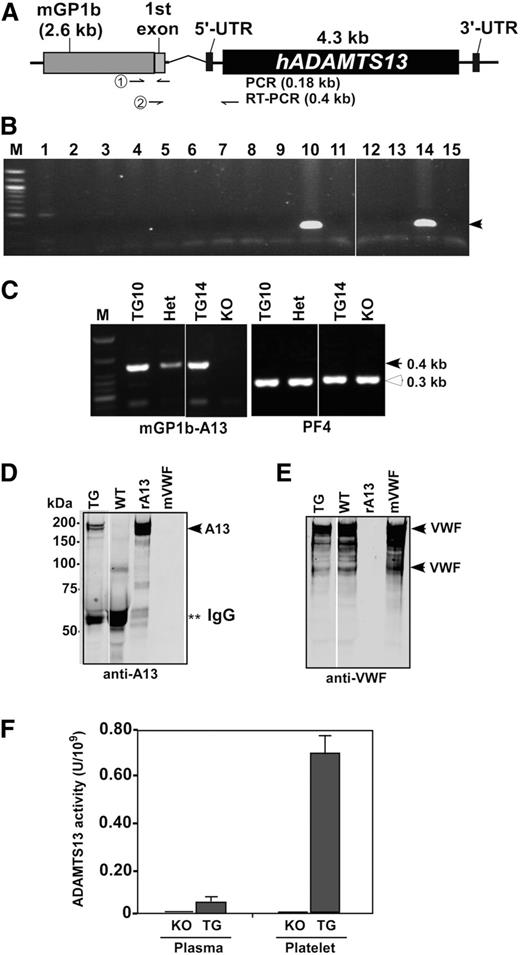
A partial (XhoI/SalI) fragment containing a murine GP1b promoter and human ADAMTS13 gene (Figure 1A) was used to generate transgenic mice. Two founders were obtained, demonstrated by the presence of transgene (∼0.18 kb) in genomic DNA (Figure 1B), and crossed for at least 6 generations with KO (CAST/Ei) mice to generate mice with platelet-specific expression of rADAMTS13 in an Adamts13-deficient background (ie, Adamts13−/−PltA13 or TG).
Schematic transgenic construct and characterization of transgenic mice. (A) An expression cassette consists of a murine platelet glycoprotein 1b (mGP1b) proximal promoter sequence (∼2.6 kb), a part of its first exon, an intron, and a full-length human ADAMTS13 cDNA (∼4.3 kb) that is flanked by 5′ and 3′ untranslated regions (UTRs). Primer pairs used for genotyping and RT-PCR, respectively, are indicated in the arrows. (B) Genotyping shows 2 positive (∼0.18 kb) transgenic founders (#10 and #14). (C) RT-PCR demonstrates the platelet-specific expression of transgene mRNA (arrowhead, ∼0.4 kb) in the homozygous transgenic (TG1 and TG2) and heterozygous transgenic (Het) mice, but not in KO, mice. As a positive control, murine PF4 mRNA (open arrowhead, ∼0.3 kb) was amplified in all mice. (D) Western blotting with anti-ADAMTS13-Dis IgG demonstrates a full-length rADAMTS13 protein in duplet (∼195 kDa) (left panel, arrowhead) in transgenic platelets (lane 1), but not in nontransgenic WT platelets (lane 2). Lanes 3 and 4 contain only rADAMTS13 (rA13, arrowhead) and murine VWF (mVWF, arrowheads) for the control. **Fragments of IgG. (E) Western blotting of the same membrane in D with polyclonal anti-VWF IgG; therefore, murine VWF (arrowheads) are detected in lanes 1, 2, and 4, but not in lane 3. (F) ADAMTS13 activity in platelet lysate of KO and TG mice is determined by the cleavage of rF-VWF73 substrate. Normal human plasma is used for calibration, defined as having 1 U/mL activity.
Schematic transgenic construct and characterization of transgenic mice. (A) An expression cassette consists of a murine platelet glycoprotein 1b (mGP1b) proximal promoter sequence (∼2.6 kb), a part of its first exon, an intron, and a full-length human ADAMTS13 cDNA (∼4.3 kb) that is flanked by 5′ and 3′ untranslated regions (UTRs). Primer pairs used for genotyping and RT-PCR, respectively, are indicated in the arrows. (B) Genotyping shows 2 positive (∼0.18 kb) transgenic founders (#10 and #14). (C) RT-PCR demonstrates the platelet-specific expression of transgene mRNA (arrowhead, ∼0.4 kb) in the homozygous transgenic (TG1 and TG2) and heterozygous transgenic (Het) mice, but not in KO, mice. As a positive control, murine PF4 mRNA (open arrowhead, ∼0.3 kb) was amplified in all mice. (D) Western blotting with anti-ADAMTS13-Dis IgG demonstrates a full-length rADAMTS13 protein in duplet (∼195 kDa) (left panel, arrowhead) in transgenic platelets (lane 1), but not in nontransgenic WT platelets (lane 2). Lanes 3 and 4 contain only rADAMTS13 (rA13, arrowhead) and murine VWF (mVWF, arrowheads) for the control. **Fragments of IgG. (E) Western blotting of the same membrane in D with polyclonal anti-VWF IgG; therefore, murine VWF (arrowheads) are detected in lanes 1, 2, and 4, but not in lane 3. (F) ADAMTS13 activity in platelet lysate of KO and TG mice is determined by the cleavage of rF-VWF73 substrate. Normal human plasma is used for calibration, defined as having 1 U/mL activity.
By RT-PCR, platelet expression of transgene (the first exon of GP1b and rADAMTS13 mRNA, ∼0.4 kb) was detected in heterozygous Adamts13−/−PltA13+/− (Het) and homozygous Adamts13−/−PltA13+/+ (TG) mice, but not in KO mice (Figure 1C). As positive controls, murine PF4 mRNA (∼0.3 kb) was amplified in all samples (Figure 1C). Western blotting demonstrated ∼195-kDa rADAMTS13 in TG, but not in WT (Figure 1D) and KO (not shown), platelets. Endogenous murine VWF was detected in all platelets (Figure 1E). Functional analysis revealed that platelet-expressed rADAMTS13 in TG mice was proteolytically active (Figure 1F), with the specific activity of 0.7 U/109 platelets. Only a trace amount of ADAMTS13 activity (0.5% of normal murine plasma) was detected in platelet-poor plasma from TG mice, but not from KO (Figure 1F) and WT (not shown) mice. These results indicate that a functional rADAMTS13 enzyme is expressed in platelets.
Immunofluorescent studies
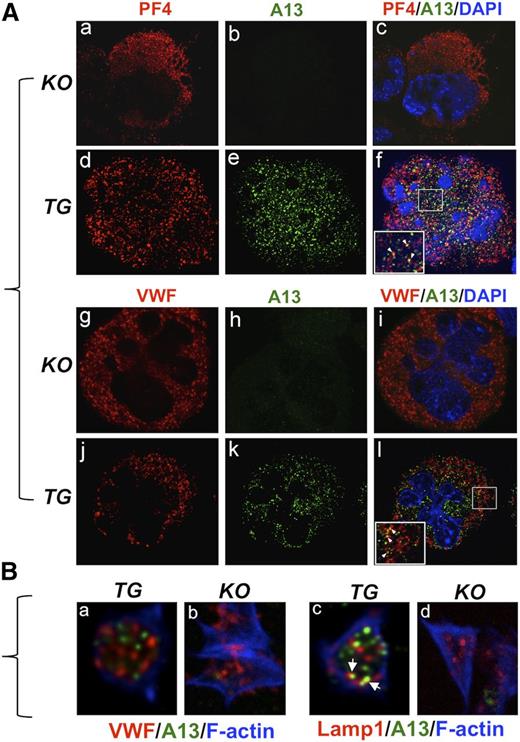
All platelets were identified by positive CD41 (supplemental Figure 1A,D,G, available on the Blood Web site). They all express rADAMTS13 protein (supplemental Figure 1H-I). No ADAMTS13 was detected in WT (supplemental Figure 1B-C) and KO (supplemental Figure 1E-F) platelets. Bone marrow-derived hematopoietic progenitor cells were cultured in the presence of thrombopoietin, and the matured megakaryocytes were fixed and stained with anti-PF4 or anti-VWF and anti-ADAMTS13 IgGs. ADAMTS13 was negative in all KO megakaryocytes (Figure 2Abh) but positive in all TG megakaryocytes (Figure 2Ae,k,n). Only partial colocalization of rADAMTS13 with PF4 (Figure 2Af) or with VWF (Figure 2Al) was detected in TG megakaryocytes. These results suggest that the expressed rADAMTS13 in megakaryocytes is only partially packaged into α-granules.
Immunofluorescent study of rADAMTS13 expression in transgenic megakaryocytes and platelets. (A) Isolated and cultured megakaryocytes from KO and TG mice were stained with rabbit anti-murine PF4 (a,d) or rabbit anti-VWF IgG (g,j) and mouse anti-ADAMTS13-Dis (A13) (b,e,h,k). The nuclei of megakaryocytes were counterstained with 4,6 diamidino-2-phenylindole (blue). The images were obtained under a confocal fluorescent microscope (magnification ×400). The merged images are shown in c, f, i, and l. The arrowheads in the insets in f and l indicate the potential granules that contain both PF4 (or VWF) and rADAMTS13. (B) Isolated platelets from KO and TG mice were spread on a fibrinogen-coated surface and stained with anti-VWF or anti-Lamp1 (red) and anti-ADAMTS13 (A13) (green), followed by Alexa350-labeled phalloidin (F-actin) (blue). The images were obtained by confocal microscope (magnification ×630). The arrowheads indicate the colocalization of rADAMTS13 and Lamp1.
Immunofluorescent study of rADAMTS13 expression in transgenic megakaryocytes and platelets. (A) Isolated and cultured megakaryocytes from KO and TG mice were stained with rabbit anti-murine PF4 (a,d) or rabbit anti-VWF IgG (g,j) and mouse anti-ADAMTS13-Dis (A13) (b,e,h,k). The nuclei of megakaryocytes were counterstained with 4,6 diamidino-2-phenylindole (blue). The images were obtained under a confocal fluorescent microscope (magnification ×400). The merged images are shown in c, f, i, and l. The arrowheads in the insets in f and l indicate the potential granules that contain both PF4 (or VWF) and rADAMTS13. (B) Isolated platelets from KO and TG mice were spread on a fibrinogen-coated surface and stained with anti-VWF or anti-Lamp1 (red) and anti-ADAMTS13 (A13) (green), followed by Alexa350-labeled phalloidin (F-actin) (blue). The images were obtained by confocal microscope (magnification ×630). The arrowheads indicate the colocalization of rADAMTS13 and Lamp1.
To localize ADAMTS13 in platelets, isolated platelets were spread on fibrinogen-coated surface, fixed, and stained with anti-VWF (or anti-Lamp1) and anti-ADAMTS13 IgGs. Unexpectedly, little or no colocalization of rADAMTS13 with VWF was observed (Figure 2Ba and supplemental Video 1). Interestingly, a partial colocalization of rADAMTS13 with Lamp1 was detected (Figure 2Bc; supplemental Video 2). These results indicate that rADAMTS13 expressed in platelets is not packed into the granules that store VWF or PF4 but, to some extent, is targeted for degradation. No ADAMTS13 staining was observed in KO platelets (Figure 2Bb-d), confirming the specificity of immunofluorescence study. Immunogold transmission electron microscopy may be necessary to determine the exact intracellular localization of rADAMTS13 in platelets.
Platelet counts and aggregation function in mice
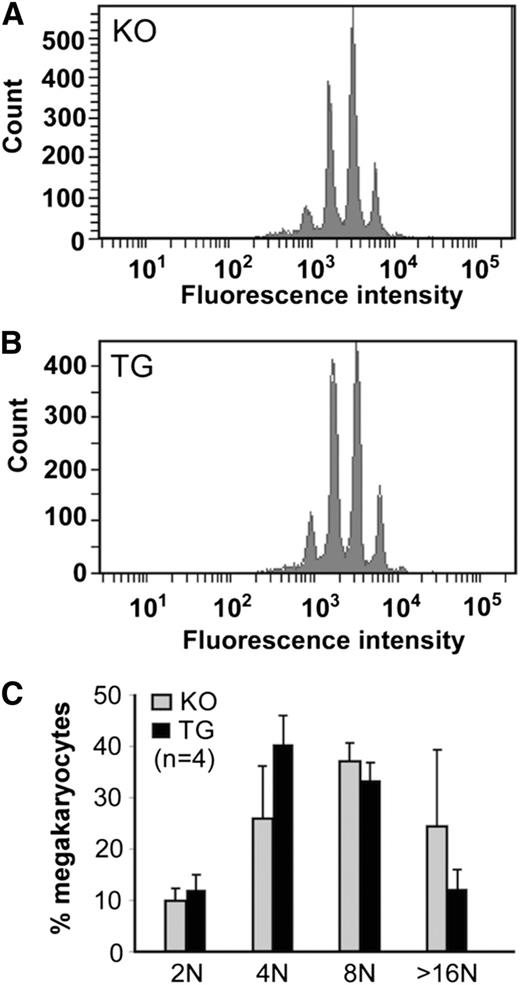
The baseline platelet counts (mean and standard deviation) in TG, KO, and WT mice were 896 ± 156, 631 ± 93, and 708 ± 179 × 109/L, respectively (Table 1). There was a statistically significant difference in platelet counts between TG and KO (or WT) mice (P < .001), but no difference in platelet counts between KO and WT mice of the same or different genetic backgrounds (P > .05). The increase in baseline platelet counts in TG mice was not associated with the increase in megakaryocyte differentiation, as the polyploidy of TG megakaryocyte after a 5-day culture in the presence of thrombopoietin was similar to that of KO and WT megakaryocytes (Figure 3).
Baseline platelet counts in various mice
| Genotype . | Number of mice . | Platelet count (×109/L) . |
|---|---|---|
| TG | 20 | 896 ± 156** |
| KO | 20 | 631 ± 93 |
| WT | 16 | 708 ± 179 |
| Genotype . | Number of mice . | Platelet count (×109/L) . |
|---|---|---|
| TG | 20 | 896 ± 156** |
| KO | 20 | 631 ± 93 |
| WT | 16 | 708 ± 179 |
The data presented are the mean ± SD.
TG, Adamts13−/−PltA13 mice; KO, Adamts13−/− mice; WT, wild-type mice.
P < .001 when compared with KO and WT mice, respectively.
Analysis of murine megakaryocyte ploidy. (A-B) The numbers and distribution of megakaryocytes with various amounts of DNA content after 7-day culture in the presence of thrombopoietin from KO and TG mice, as indicated, are shown. (C) The quantitation of the ploidy distribution (ie, the percentage of cells with 2N, 4N, 8N, and 16N) is shown as the means and standard deviation from 4 independent experiments. ANOVA was performed and revealed no statistical significance among the groups (P > .05).
Analysis of murine megakaryocyte ploidy. (A-B) The numbers and distribution of megakaryocytes with various amounts of DNA content after 7-day culture in the presence of thrombopoietin from KO and TG mice, as indicated, are shown. (C) The quantitation of the ploidy distribution (ie, the percentage of cells with 2N, 4N, 8N, and 16N) is shown as the means and standard deviation from 4 independent experiments. ANOVA was performed and revealed no statistical significance among the groups (P > .05).
Platelet function study demonstrated that fibrillar collagen (2 µg/mL) (Figure 4A), α-thrombin (0.1 U/mL) (Figure 4B), and 2MesADP (30 nM) (Figure 4C) or botrocetin (1 µg/mL) (not shown) induced agglutination and aggregation in all platelets from TG, KO, and WT mice, indicating that the overexpression of rADAMTS13 in platelets does not affect their in vitro function.
Agonist-induced platelet aggregation and release of rADAMTS13 from transgenic platelets. (A-C) Aggregation tracing recorded using a light scattering aggregometer for washed platelets isolated from various mice, as indicated, after being stimulated with soluble type 1 collagen (2 µg/mL), α-thrombin (0.1 U/mL), and 2MesADP (30 nM), respectively. (D) Western blotting demonstrates the rADAMTS13 (arrowheads) released from TG platelets after stimulation with a buffer alone (CON), type 1 collagen [Coll.(10 µg/ml)], α-thrombin [Throm. (1 U/ml)], and 2MesADP (ADP, 100 nM) as indicated in each lane. (E) The residual rADAMTS13 (top) and control β-actin (bottom) are detected in platelet lysate after aggregation. (F) The ratios of the residual rADAMTS13 (top band) to β-actin in platelet lysate (the means ± standard deviation) (n = 3) are shown. ANOVA was used to determine statistical significance of the differences among various groups. *P < .05; **P < .01.
Agonist-induced platelet aggregation and release of rADAMTS13 from transgenic platelets. (A-C) Aggregation tracing recorded using a light scattering aggregometer for washed platelets isolated from various mice, as indicated, after being stimulated with soluble type 1 collagen (2 µg/mL), α-thrombin (0.1 U/mL), and 2MesADP (30 nM), respectively. (D) Western blotting demonstrates the rADAMTS13 (arrowheads) released from TG platelets after stimulation with a buffer alone (CON), type 1 collagen [Coll.(10 µg/ml)], α-thrombin [Throm. (1 U/ml)], and 2MesADP (ADP, 100 nM) as indicated in each lane. (E) The residual rADAMTS13 (top) and control β-actin (bottom) are detected in platelet lysate after aggregation. (F) The ratios of the residual rADAMTS13 (top band) to β-actin in platelet lysate (the means ± standard deviation) (n = 3) are shown. ANOVA was used to determine statistical significance of the differences among various groups. *P < .05; **P < .01.
ADAMTS13 is released from TG platelets on stimulation
Western blotting analysis showed that a full-length rADAMTS13 (doublet) was detected in the releasate after being stimulated with fibrillar collagen and α-thrombin, but little with 2MesADP (Figure 4D). The release of rADAMTS13 coincided with the reduction of intraplatelet mature form of rADAMTS13 (Figure 4E). Quantitative results by densitometry showed that the ratios of intracellular matured rADAMTS13 to β-actin were significantly reduced after stimulation with collagen and thrombin (Figure 4F). Only a trace amount of rADAMTS13 was found bound to platelet surface by surface biotinylation (data not shown). These results indicate that the expressed rADAMTS13 in murine platelets may be released on stimulation at the site of injury.
VWF multimer distribution in plasma and platelets
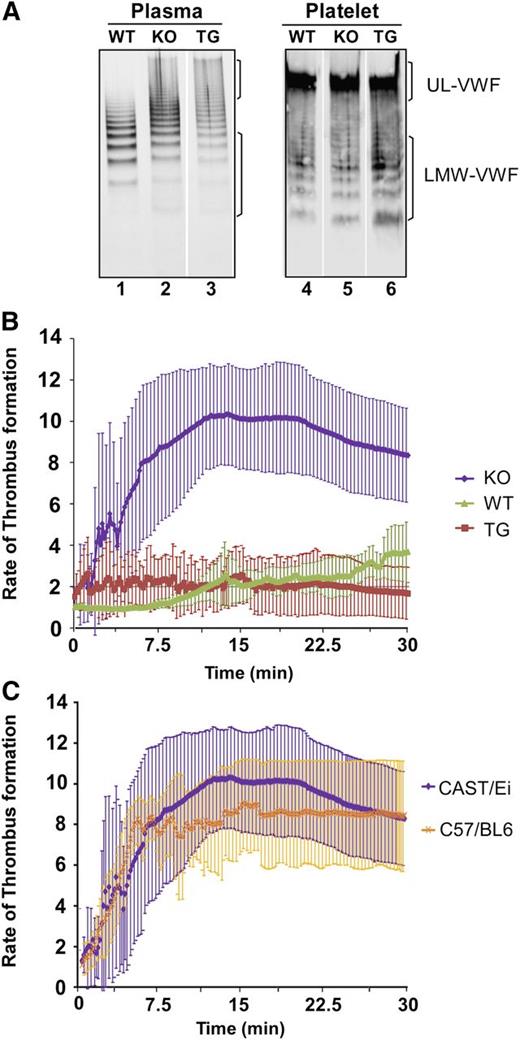
ADAMTS13 coexpressed with VWF in HeLa cells results in intracellular degradation of VWF.43 VWF in plasma and platelet lysate remained UL in size, with no increase in the cleavage products in TG mice compared with that in KO and WT mice (Figure 5A). The results suggest that rADAMTS13 expressed in platelets has no effect on their VWF multimer distribution.
VWF multimer distribution in plasma and platelets and the rate of thrombus formation in mesenteric arterioles after injury. (A) The multimer distribution in plasma (lanes 1-3) and in platelet lysate (lanes 4-6) of WT, KO, and TG mice, as indicated. (B) The rate of thrombus formation measured by the accumulation of rhodamine 6G-labeled platelets and leukocytes as a function of time (minutes:seconds) in the mesenteric arterioles of WT, KO, and TG mice (CAST/Ei) after being injured with a topical application of 10% FeCl3. (C) Comparison of the rate of thrombus formation in the mesenteric arterioles between Adamts13−/− CAST/Ei and Adamts13−/− C57BL/6 mice. The data represent the means and standard errors of the means of 3-5 mice in each group, as indicated.
VWF multimer distribution in plasma and platelets and the rate of thrombus formation in mesenteric arterioles after injury. (A) The multimer distribution in plasma (lanes 1-3) and in platelet lysate (lanes 4-6) of WT, KO, and TG mice, as indicated. (B) The rate of thrombus formation measured by the accumulation of rhodamine 6G-labeled platelets and leukocytes as a function of time (minutes:seconds) in the mesenteric arterioles of WT, KO, and TG mice (CAST/Ei) after being injured with a topical application of 10% FeCl3. (C) Comparison of the rate of thrombus formation in the mesenteric arterioles between Adamts13−/− CAST/Ei and Adamts13−/− C57BL/6 mice. The data represent the means and standard errors of the means of 3-5 mice in each group, as indicated.
Rates of thrombus formation
Intravital microscopic imaging analysis demonstrated that the rate of thrombus formation in the mesenteric arterioles after FeCl3 injury in TG mice was significantly lower than that in KO mice (P < .001), but similar to that in WT mice (P > .05). Zero (0%) of 5 TG and 4 (80%) of 5 KO mice showed occlusive thrombi within 10 minutes (Figure 5B). No difference in the rate of thrombus formation between CAST/Ei and C57BL/6 mice in Adamts13−/− background was observed (Figure 5C), suggesting the strain difference does not account for the significant difference observed in the rates of thrombus formation between KO and TG mice. These results demonstrate that platelet-delivered rADAMTS13 is efficacious in mitigating arterial thrombosis after vascular injury.
Murine models of hereditary TTP
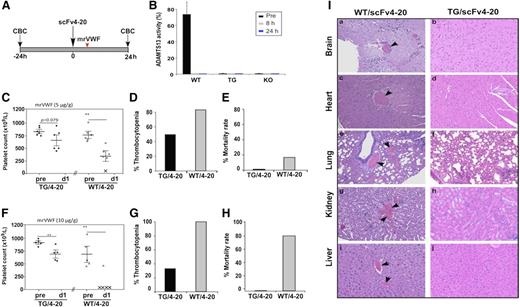
Animals that received an intravenous bolus of a low-dose Stx-2 developed thrombocytopenia, as defined by a 30% drop in platelet count from baseline, as previously described,34,42 in 39% (24 hours) and 50% (72 hours) of KO mice after injection. However, no persistent thrombocytopenia was observed in TG (Figure 6A-B) and WT (not shown) mice with the same challenge. Animals given a high dose of Stx-2 developed more severe thrombocytopenia, defined by a 50% drop in platelet count from baseline, in 3 (100%) of 3 KO mice, but only in 1 (20%) of 5 TG mice 24 to 72 hours after injection (Figure 6C-D) (P < .01). Overall, there was no statistically significant difference in the platelet counts before and after low (Figure 6A) and high doses (Figure 6C) of Stx-2 injection in TG mice (P = .261) within 72 hours. Histologic analysis demonstrated the presence of occlusive thrombi in the heart, lungs, kidneys, and liver in mice who died after the high dose of Stx-2 challenge (Figure 6E), but absence in the same tissues in WT and TG mice after similar challenges (not shown). Together, these results demonstrate that platelet-delivered rADAMTS13 protects mice from bacterial toxin-induced TTP despite lack of plasma ADAMTS13 activity.
Platelet counts and histology analysis of major organs of mice after being challenged with Stx-2 or rmVWF. Platelet counts in TG and KO before (pre) and 24 (d1) and 72 (d3) hours after being challenged with Stx-2 at the dose of 50 pg/g (A) and 250 pg/g body weight (C), or with rmVWF at the dose of 5 μg/g body weight (E). ANOVA was used to determine the statistical significance of the differences among various groups. **P < .01. (B,D,F) Percentages of mice in A, C, and E having a 30% reduction of platelet counts from the baseline. (G-H) Representative images of hematoxylin and eosin staining demonstrating the occlusive thrombi in the brain, heart, lung, kidneys, and liver obtained from KO mice that died after being challenged by high doses of Stx-2 and rmVWF. The digital images were taken under light microscope with magnification ×200.
Platelet counts and histology analysis of major organs of mice after being challenged with Stx-2 or rmVWF. Platelet counts in TG and KO before (pre) and 24 (d1) and 72 (d3) hours after being challenged with Stx-2 at the dose of 50 pg/g (A) and 250 pg/g body weight (C), or with rmVWF at the dose of 5 μg/g body weight (E). ANOVA was used to determine the statistical significance of the differences among various groups. **P < .01. (B,D,F) Percentages of mice in A, C, and E having a 30% reduction of platelet counts from the baseline. (G-H) Representative images of hematoxylin and eosin staining demonstrating the occlusive thrombi in the brain, heart, lung, kidneys, and liver obtained from KO mice that died after being challenged by high doses of Stx-2 and rmVWF. The digital images were taken under light microscope with magnification ×200.
To determine whether the ability of platelet-delivered rADAMTS13 to cleave circulating VWF confers protection against TTP, mice were challenged with a single intravenous bolus of rmVWF. Within 24 hours, thrombocytopenia (30% drop from the baseline) was observed in 6 (100%) of 6 of KO mice but only in 1 (20%) of 5 TG mice (P < .001) (Figure 6F-G). The platelet counts recovered 72 hours after the challenge (Figure 6F-G), consistent with a transient effect of injected VWF in this model.44 When challenged with the high-dose rmVWF, 4 (50%) of 8 KO mice died the next day, but all 6 TG mice survived (data not shown). Histological analysis demonstrated the formation of disseminated arterial thrombi involving brain, heart, lung, liver, and kidneys in the KO mice that died (Figure 6H). In contrast, rare microthrombi were detected in euthanized TG mice after being challenged with the same rmVWF dosage (not shown). These results suggest that sufficient amount of rADAMTS13 is released from activated platelets at the site of vascular injury, which protects against the onset and progression of “TTP-like” syndrome in mice despite lack of plasma ADAMTS13 activity.
Murine models of antibody-mediated TTP
Factor VIII ectopically expressed in platelets offers limited protection against hemophilia in the presence of inhibitors.27 To determine whether platelet-delivered rADAMTS13 ameliorates the phenotype of acquired TTP with inhibitors, we administered a monoclonal antibody against human ADAMTS13 (scFv4-20) cloned from patients with acquired TTP, according to the scheme illustrated (Figure 7A). A single bolus of scFv4-20 completely inhibited plasma Adamts13 activity in WT mice between 8 and 24 hours (Figure 7B). Six to 8 hours after scFv4-20 injection, mice were challenged with a single bolus of rmVWF (Figure 7A); 5 (83%) of 6 WT mice with scFv4-20 developed thrombocytopenia (a 30% drop in platelet counts from baseline)34 within 24 hours compared with 3 (50%) of 6 TG mice (P < .01) (Figure 7C-D). One (15%) of 6 WT but 0 (0%) of 6 TG mice with scFv4-20 died 24 hours after this low dose of rmVWF (Figure 7E). More significantly, 4 (80%) of 5 WT but none of 6 TG mice with scFv4-20 died after being challenged with the high-dose rmVWF (P < .001) (Figure 7H), although thrombocytopenia was still observed in 33% of the TG mice with scFv4-20 (Figure 7F-G). Histologic analysis revealed disseminated arterial and microvascular thrombi in the major organs of the deceased WT mice with scFv4-20, including brain, heart, lung, liver, and kidneys (Figure 7Ia,c,e,g,i), but not spleen (not shown). TG mice with scFv4-20 that were challenged with rmVWF showed a minimal number of occlusive thrombi (Figure 7Ib,d,f,h,j). These results further support the hypothesis that platelet-delivered rADAMTS13 may be efficacious in ameliorating thrombus formation and consumptive thrombocytopenia, thereby reducing the mortality rate associated with acquired TTP, even in the face of persistent inhibitors.
Platelet counts, mortality rates, and histological changes in mice with antibody-mediated inhibition of ADAMTS13 after being challenged with rmVWF. (A) Schematic representation of an animal protocol used to induce acquired deficiency of plasma ADAMTS13 activity and the time points for blood collection and complete blood count analysis in mice. (B) Plasma ADAMTS13 activity in WT after a single bolus infusion of scFv4-20 (5 µg/g body weight). Plasma from TG and KO mice also receiving scFv4-20 was used as a negative control. (C-E) Platelet counts, percentage of mice with thrombocytopenia (defined by greater than 30% reduction in platelet count from their baseline), and mortality rate, respectively, in TG (closed) and WT (open) mice that received an intraperitoneal injection of scFv4-20 (100 µg) before (pre) and 24 hours (d1) after being challenged with rmVWF at the dosage of 5 µg/g body weight. (F-H) Platelet counts, percentage of thrombocytopenia, and the mortality rate, respectively, in TG (closed) and WT (open) mice with scFv4-20 after being challenged with rmVWF at the doses of 10 µg/g body weight. ANOVA determined the statistical significance of the differences among various groups. **P < .01. (I) Occlusive thrombi were commonly found in histology analysis of the major organs including brain, heart, lung, kidneys, and liver in WT mice with scFv4-20 and rmVWF challenge at the dosage of 10 µg/g body weight (a,c,e,g,i). Such occlusive thrombi were rarely seen in TG mice receiving similar treatment (b,d,f,h,j).
Platelet counts, mortality rates, and histological changes in mice with antibody-mediated inhibition of ADAMTS13 after being challenged with rmVWF. (A) Schematic representation of an animal protocol used to induce acquired deficiency of plasma ADAMTS13 activity and the time points for blood collection and complete blood count analysis in mice. (B) Plasma ADAMTS13 activity in WT after a single bolus infusion of scFv4-20 (5 µg/g body weight). Plasma from TG and KO mice also receiving scFv4-20 was used as a negative control. (C-E) Platelet counts, percentage of mice with thrombocytopenia (defined by greater than 30% reduction in platelet count from their baseline), and mortality rate, respectively, in TG (closed) and WT (open) mice that received an intraperitoneal injection of scFv4-20 (100 µg) before (pre) and 24 hours (d1) after being challenged with rmVWF at the dosage of 5 µg/g body weight. (F-H) Platelet counts, percentage of thrombocytopenia, and the mortality rate, respectively, in TG (closed) and WT (open) mice with scFv4-20 after being challenged with rmVWF at the doses of 10 µg/g body weight. ANOVA determined the statistical significance of the differences among various groups. **P < .01. (I) Occlusive thrombi were commonly found in histology analysis of the major organs including brain, heart, lung, kidneys, and liver in WT mice with scFv4-20 and rmVWF challenge at the dosage of 10 µg/g body weight (a,c,e,g,i). Such occlusive thrombi were rarely seen in TG mice receiving similar treatment (b,d,f,h,j).
Discussion
Our present study provides evidence that platelet-delivered rADAMTS13 is able to protect against arterial thrombosis and TTP. The antithrombotic and anti-TTP effects of platelet-derived rADAMTS13 suggest that the enzyme can be released from platelets at the sites of vascular injury. The locally released rADAMTS13 appears to be sufficient to cleave either endothelium-anchored ULVWF or circulating large VWF or both, which play a central role in initiation of arterial thrombosis and TTP syndrome.
As shown in our results, rADAMTS13 can be expressed in the developing megakaryocytes without detrimental effect on megakaryocyte differentiation (Figure 3) and platelet production (Table 1). Approximately 0.35 U of rADAMTS13 is present in 1 mL whole-blood-derived platelets (0.5-1.0 × 109), equivalent to ∼35% of normal plasma ADAMTS13 activity. Only a trace amount (less than 0.5%) of expressed rADAMTS13 is detected in murine platelet-poor plasma under nonstimulating conditions (Figure 1). These results suggest that the synthesized rADAMTS13 in megakaryocytes is likely stored in intracellular organelles including α-granules or other intracellular structures. However, rADAMTS13 in megakaryocytes is only partially colocalized with murine PF4 or VWF, which is known to reside in the α-granules of megakaryocytes and mature platelets (Figure 2).45 The ability to express rADAMTS13 in megakaryocytes/platelets comes as no surprise because they normally produce ADAMTS13, albeit at extremely low levels,4,5 and therefore, normal platelets are hemostatic/prothrombotic, but not antithrombotic. Overexpression of rADAMTS13 in murine platelets does not appear to have any detrimental effect on the aggregability of platelets, at least under the experimental conditions (Figure 4). In contrast, transgenic mice expressing rADAMTS13 in their megakaryocytes appear to have higher baseline platelet counts than nontransgenic Adamts13−/− counterparts, and even wild-type mice, suggesting that expression of rADAMTS13 in megakaryocytes may either enhance platelet production or reduce its clearance, although the data available to date do not provide a definitive answer to this question.
Although only small amount of the expressed rADAMTS13 in transgenic platelets is detected in the releasate after stimulation by strong agonists including collagen and α-thrombin in vitro (Figure 4), significant antithrombotic effect of platelet-derived rADAMTS13 in the murine model of mesenteric arterial thrombosis (Figure 5) suggests platelets may be an ideal carriers for antithrombotic rADAMTS13, allowing its release at high concentrations at the site of thrombus formation without being inactivated by the potential circulating anti-ADAMTS13 inhibitors. Therefore, it is conceivable that platelet-delivered rADAMTS13 may be efficacious in preventing many arterial thrombotic diseases associated with a deficiency of plasma ADAMTS13, including TTP, myocardial infarction, and ischemic cerebral stroke. Each has been shown to benefit from intravenous infusion of rADAMTS13.46,47 The rADAMTS13 delivered by platelets may have greater advantages than that delivered by plasma because ∼99.5% of therapeutic rADAMTS13 protein is stored inside platelets, which is available for release locally at high concentration on physiological stimulation. This strategy may potentially prolong the duration of rADAMTS13 action because of the longer half-life of platelets than soluble rADAMTS13 (3 days vs 20-30 minutes).28
Most important, platelet-delivered rADAMTS13 is shown to prevent thrombocytopenia and microvascular thrombosis in a murine model of TTP, which is induced by bacterial toxin or rmVWF with or without circulating anti-ADAMTS13 antibody. Stx-2 or its B-subunit stimulates release of ULVWF from endothelial cells,48 which triggers a “TTP-like” syndrome in Adamts13-deficient mice.34,42,48 When challenged with a similar dose of Stx-2, the TG mice expressing rADAMTS13 in their platelets are largely protected from thrombocytopenia (Figure 6). A similar protective effect is also observed when the TG mice are challenged with rmVWF (Figure 6). However, when challenged with high dose of rmVWF, 4 (80%) of 5 KO mice died the next day, whereas none of 6 TG mice died (data not shown). Unfortunately, platelet counts were not immediately measured in KO mice before the unpredictable time of death in these mice. Autopsy results confirmed that disseminated thrombi in small arterioles of the major organs were the most likely cause of death (Figure 6).
Finally, platelet-delivered rADAMTS13 also protects against acquired TTP in the face of inhibitor, demonstrated by the reduced rate of thrombocytopenia and mortality rate after low- and high-dose rmVWF challenge (Figure 7). Disseminated arterial thrombi were evident in the small arterioles and microvasculature of the brain, heart, lung, liver, and kidneys in the WT mice injected with scFv4-20 that died (Figure 7), consistent with the diagnosis of acquired TTP after rmVWF challenge. These findings are particularly important because most acquired TTP patients have circulating inhibitory antibodies at the time treatment is instituted. There are still a number of obstacles to be overcome before translation of these findings into patient care can be expected: how to pack a sufficient amount of rADAMTS13 into platelets for therapeutic purpose and how many rADAMTS13-loaded platelets must to be transfused to achieve antithrombotic and anti-TTP effects in patients with severe plasma ADAMTS13 deficiency resulting from circulating inhibitors.
In summary, the current study demonstrates the high levels of expression of human rADAMTS13 in mouse megakaryocytes/platelets; the expressed rADAMTS13 is packed into distinct intracellular granules that appear to be different from those storing VWF and PF4, but can be released on agonist stimulation. The released rADAMTS13 offers protection against arterial thrombosis and acquired TTP resulting from either constitutional or acquired deficiency of plasma ADAMTS13 activity, followed by a trigger. Together, our findings provide proof of concept that platelet-delivered rADAMTS13 may be explored as a novel therapeutic strategy for arterial thrombotic diseases, including hereditary and acquired TTP with inhibitors.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr David Ginsburg at the University of Michigan, Ann Arbor, Michigan, for providing KO mice.
This study was supported in part by the grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (NIH NHLBI) HL115187-01A1, the American Society of Hematology Bridge Fund, and the Answering TTP Foundation to X.L.Z.; NIH NHLBI grants HL099656, HL110860, and HL64190 to M.P.; and the PennPort Program, The University of Pennsylvania to B.P.
Authorship
Contribution: B.P., Y.M., D.L., and X.L.Z. designed experiments, performed experiments, analyzed the data, and wrote the manuscript. D.L.S., D.B.C., and M.P. helped design the research, contributed critical reagents, and helped prepare the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for D.L. is Department of Hematology, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China.
Correspondence: X. Long Zheng, Division of Laboratory Medicine, Department of Pathology, The University of Alabama at Birmingham, WP/P230, 619 19th St South, Birmingham, AL 35249-7331; e-mail: zhengl@uab.edu or longzheng01@gmail.com.
References
Author notes
B.P. and Y.M. contributed equally to this study.

![Figure 4. Agonist-induced platelet aggregation and release of rADAMTS13 from transgenic platelets. (A-C) Aggregation tracing recorded using a light scattering aggregometer for washed platelets isolated from various mice, as indicated, after being stimulated with soluble type 1 collagen (2 µg/mL), α-thrombin (0.1 U/mL), and 2MesADP (30 nM), respectively. (D) Western blotting demonstrates the rADAMTS13 (arrowheads) released from TG platelets after stimulation with a buffer alone (CON), type 1 collagen [Coll.(10 µg/ml)], α-thrombin [Throm. (1 U/ml)], and 2MesADP (ADP, 100 nM) as indicated in each lane. (E) The residual rADAMTS13 (top) and control β-actin (bottom) are detected in platelet lysate after aggregation. (F) The ratios of the residual rADAMTS13 (top band) to β-actin in platelet lysate (the means ± standard deviation) (n = 3) are shown. ANOVA was used to determine statistical significance of the differences among various groups. *P < .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/21/10.1182_blood-2014-07-587139/4/m_3326f4.jpeg?Expires=1765038309&Signature=eP~XlkWmo-Z4GLqAqBZbNfm8IFkag23cMriDycdHmoNWeUTcW6dPXRqOl~YCs0hLUcg2L-LPlpPeT8zy9ENM0a~6c6WsOxcXBJDhFVpB9~8Kvw4as0nPUJonvc00ky7EmQ-MZz-CvX-doO02kLyAMOur0YvRHHtveiWHW8Ty4566aKaNx0YxBZ1ucKNlc5IIsiPJWoWOQ6QTn6MwTKKFRXS8Lh-eobn3KG4T05MO-rlbnMkOypLTAkDt7-nP7IYhVtDkwMt-m3nGgJWnUDfZuivXRyapyCm3o3fjSBRcoj91hl~tdJ3ht2SGT2SnvPBp0CLBfrYXVV-qf1h0OHlJVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)