To the editor:
Understanding of the tumor microenvironment has led to the development of novel agents for lymphoma, although few studies have combined these as a therapeutic strategy. We report unacceptable toxicity from such a biological triplet. Patients were treated in a multiarm phase 1 study using different combinations of chemotherapy, immunomodulators, and anti-CD20 monoclonal antibodies in combination with idelalisib (www.clinicaltrials.gov; #NCT01088048). We report on the cohort of patients treated with idelalisib, lenalidomide, and rituximab. The inclusion criteria were relapsed indolent lymphoma, age >18 years, measurable disease, and performance status ≤2. Patients were excluded if they had anticancer therapy within 4 weeks, prior allogeneic stem cell transplant, or active central nervous system involvement, were pregnant or breast-feeding, or had active serious infection, serum creatinine ≥2.0 mg/dL, neutrophils <1.0 × 109/L or platelets <75 × 109/L, bilirubin >2 mg/dL, aspartate aminotransferase or alanine aminotransferase (ALT) ≥2 times the upper limit of normal, Child-Pugh class B or C hepatic impairment, infection with HIV or hepatitis B or C virus, or prior treatment with idelalisib.
Treatment comprised lenalidomide 5 mg (days 8-21 cycle 1, days 1-21 thereafter), rituximab 375 mg/m2 day 1, and idelalisib 150 mg twice daily from day 1 (cycle 1, 35 days; subsequent cycles, 28 days). Seven patients enrolled in the initial cohort. The median age was 62 (range, 49-72) years. Five patients had follicular lymphoma (FL), 1 had small lymphocytic lymphoma, and 1 had marginal zone lymphoma. The median number of prior treatments was 1.5 (range, 1-5). None had liver disease or hepatic involvement with lymphoma. Three of the 6 patients (50%) who developed liver function test abnormalities were taking statins at study entry. The first 4 patients developed ALT elevation (2 grade 2 and 2 grade 4). Lenalidomide was held in all patients. Four patients (57%) had elevation in bilirubin (grade 1 in 2 patients, grade 2 in 1 patient, and grade 4 in 1 patient). ALT elevations of the first 4 subjects began on days 22, 25, 30, and 36, at which point patients had 15 to 21 days of concurrent lenalidomide. All therapy was interrupted until resolution of abnormalities. No patients developed renal impairment at the time of development of liver function test abnormalities. Three remaining patients had 1 week of concurrent lenalidomide before discontinuation. These patients continued on idelalisib and rituximab, but 2 developed subsequent grade 3 (days 50 and 98) ALT elevations (Figure 1A). All patients had imaging: 2 showed diffuse hypoechogenicity and 1 borderline fatty hepatomegaly. The median time to resolution of ALT and aspartate aminotransferase was 42 (range, 21-59) and 14 (range, 3-38) days, respectively. Four patients resumed idelalisib alone; patient 2 developed recurrent elevation in ALT on rechallenge. This arm of the study was closed to new patient enrollment. Two patients died due to toxicity and are described below.
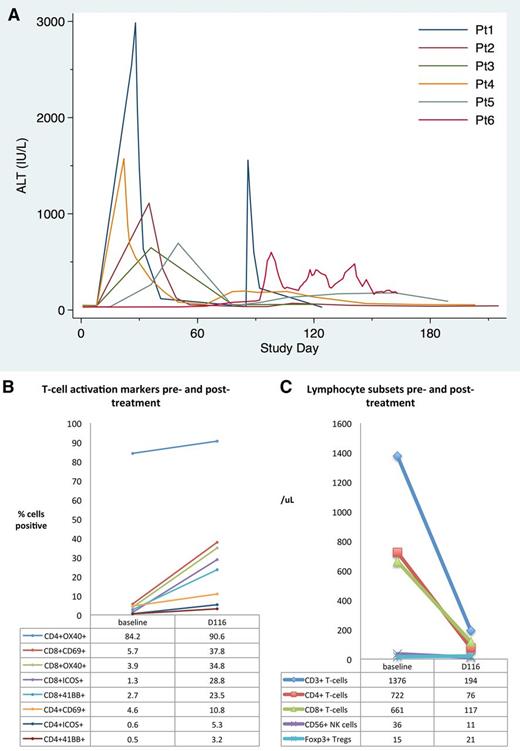
Biochemical and immunologic changes in patients treated with rituximab, lenalidomide, and idelalisib over time. (A) Kinetics of elevation of ALT (upper limit of normal, 56 IU/L) for the first 6 patients treated with rituximab, idelalisib, and lenalidomide (patient 7 did not develop abnormalities). (B) Change in peripheral blood lymphocyte numbers and (C) T-cell activation markers in patient 6, who died of hepatic failure, at study entry (baseline) and during acute liver failure (day 116). Elevation of CD69 and the costimulatory molecules ICOS, 4-1BB (CD137), and OX40 (CD134) on both CD4+ and CD8+ T cells suggested marked T-cell activation. Percentages shown are the percent of CD4+ or CD8+ T cells expressing the appropriate marker. NK, natural killer; Pt, patient; Tregs, regulatory T cells.
Biochemical and immunologic changes in patients treated with rituximab, lenalidomide, and idelalisib over time. (A) Kinetics of elevation of ALT (upper limit of normal, 56 IU/L) for the first 6 patients treated with rituximab, idelalisib, and lenalidomide (patient 7 did not develop abnormalities). (B) Change in peripheral blood lymphocyte numbers and (C) T-cell activation markers in patient 6, who died of hepatic failure, at study entry (baseline) and during acute liver failure (day 116). Elevation of CD69 and the costimulatory molecules ICOS, 4-1BB (CD137), and OX40 (CD134) on both CD4+ and CD8+ T cells suggested marked T-cell activation. Percentages shown are the percent of CD4+ or CD8+ T cells expressing the appropriate marker. NK, natural killer; Pt, patient; Tregs, regulatory T cells.
Patient 6 (62 years, male, FL) presented on day 90 with dyspnea and chest pain with computed tomography suggestive of pneumonia. Idelalisib was discontinued, and bronchoalveolar lavage isolated Fusarium species. Although the total bilirubin increased initially after commencing voriconazole, this trend continued after therapy was switched to liposomal amphotericin B. Quantitative cytomegalovirus and repeat hepatitis testing were negative; potentially hepatotoxic drugs were discontinued.
Liver biopsy on day 112 showed acute cholangitis with mixed portal inflammation and centrilobular cholestasis without viral inclusions, fungal organisms, or lymphoma. Immune dysregulation was suspected and peripheral blood lymphocytes were analyzed on samples collected prior to therapy and day 120. Analysis of T-cell subsets revealed reduced numbers of total T cells, CD4+ and CD8+ T cells, and natural killer cells at day 120, but Foxp3+ regulatory T-cell numbers were unchanged (Figure 1B). Interestingly, both CD4+ and CD8+ T cells showed broad expression of markers consistent with marked activation at day 116 (Figure 1C). To abrogate immunologic dysregulation, the patient was given pulse methylprednisolone on day 120 and continued prednisone at doses of >30 mg/day with transient stabilization of liver function. Mycophenolate mofetil and ursodeoxycholic acid were initiated but ineffective, and the patient died of hepatic failure.
Patient 5 (68 years, female, FL) experienced grade 3 ALT elevation requiring dose interruption during cycle 2, and she achieved complete response to therapy after cycle 3. Idelalisib and rituximab were recommenced from cycle 4. She had reported no significant gastrointestinal adverse events other than grade 1 constipation. During treatment cycle 7, she developed abdominal pain, nausea, vomiting, and hematochezia. Because of suspected idelalisib-induced colitis, high-dose IV corticosteroids were administered with symptomatic improvement. However, prior to discharge, she developed suspected septic shock with gram-positive bacteremia, acute kidney injury, and fatal respiratory failure.
Neither doublet (lenalidomide/rituximab, idelalisib/rituximab) has been associated with serious hepatotoxicity. Grade 3 ALT elevation was reported in 20% of patients treated with idelalisib monotherapy1 and 5% of patients treated with idelalisib and rituximab.2 Severe diarrhea/colitis has been reported in 14% of patients treated with idelalisib across clinical trials3 ; therefore, the colitis may not have been related to the addition of lenalidomide. We hypothesize that immune activation triggered by exposure to the biological triplet caused hepatotoxicity observed in this study, as lenalidomide is known to provide T-cell costimulation, suppress regulatory T-cell expansion, and enhance Th1 cytokine production,4 whereas phosphatidylinositol 3-kinase inhibition can impact both regulatory and effector arms of the immune system, potentially leading to both immune suppression and autoimmunity.5
Supporting our observation, Smith et al recently reported hepatotoxicity using a similar combination.6 All 3 agents are commercially available for the treatment of B-cell lymphoma; however, based on the available data, this combination is unacceptably toxic and should be avoided.
Authorship
Acknowledgments: This study was sponsored by Gilead Sciences.
Contribution: C.Y.C. analyzed data, created figures, and wrote the first draft of the manuscript; L.J.N., Y.O., and S.G.F. cowrote the manuscript; S.S.N. designed experiments and cowrote the manuscript; N.H.F. provided the research concept and cowrote the manuscript; and all authors had access to the complete data set for this safety analysis and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.S.N. is a consultant for Celgene Corporation, and has received research support and honorarium from Celgene Corporation. The remaining authors declare no competing financial interests.
Correspondence: Nathan H. Fowler, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd #429, Houston, TX 77030-4009; e-mail: nfowler@mdanderson.org.