Key Points
In a first randomized comparison of daunorubicin dose in induction (90 mg/m2 vs 60 mg/m2) for AML, no evidence of overall benefit was seen with the 90 mg/m2 dose.
There was no disease subgroup in which benefit could be shown.
Abstract
Modifying induction therapy in acute myeloid leukemia (AML) may improve the remission rate and reduce the risk of relapse, thereby improving survival. Escalation of the daunorubicin dose to 90 mg/m2 has shown benefit for some patient subgroups when compared with a dose of 45 mg/m2, and has been recommended as a standard of care. However, 60 mg/m2 is widely used and has never been directly compared with 90 mg/m2. As part of the UK National Cancer Research Institute (NCRI) AML17 trial, 1206 adults with untreated AML or high-risk myelodysplastic syndrome, mostly younger than 60 years of age, were randomized to a first-induction course of chemotherapy, which delivered either 90 mg/m2 or 60 mg/m2 on days 1, 3, and 5 combined with cytosine arabinoside. All patients then received a second course that included daunorubicin 50 mg/m2 on days 1, 3, and 5. There was no overall difference in complete remission rate (73% vs 75%; odds ratio, 1.07 [0.83-1.39]; P = .6) or in any recognized subgroup. The 60-day mortality was increased in the 90 mg/m2 arm (10% vs 5% (hazard ratio [HR] 1.98 [1.30-3.02]; P = .001), which resulted in no difference in overall 2-year survival (59% vs 60%; HR, 1.16 [0.95-1.43]; P = .15). In an exploratory subgroup analysis, there was no subgroup that showed significant benefit, although there was a significant interaction by FLT3 ITD mutation. This trial is registered at http://www.isrctn.com as #ISRCTN55675535.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3964.
Disclosures
Jorge Cortes, Associate Editor, has served as an advisor or consultant for Incyte, Novartis, and Gilead and received grants for clinical research from Incyte and Novartis. The article's authors and the CME questions author, Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Compare the overall efficacy of daunorubicin 90 mg/m2 vs 60 mg/m2 for induction in acute myeloid leukemia, based on findings of a randomized controlled trial.
Compare the overall safety and toxicity of daunorubicin 90 mg/m2 vs 60 mg/m2 for induction in acute myeloid leukemia.
Compare daunorubicin 90 mg/m2 vs 60 mg/m2 for induction in acute myeloid leukemia in various subgroups.
Release date: June 18, 2015; Expiration date: June 18, 2016
Introduction
Cytosine arabinoside and an anthracycline have been the standard induction therapy for acute myeloid leukemia (AML) for >3 decades. It is possible in younger adults (<60 years) to achieve remission rates of >80% with 1 or more courses.1-3 There is evidence that the improved remission rate is substantially attributable to improved supportive care, which has reduced the rate of early death.4,5 It is still important to improve induction chemotherapy because better initial cytoreduction can reduce or delay the risk of subsequent relapse,6,7 and thereby improve survival. The addition of a third drug, such as thioguanine8 or etoposide9,10 has not consistently achieved this, but some newer options such as the addition of the immunoconjugate, gemtuzumab ozogamicin (Mylotarg)11 or cladrabine7 show some promise in this respect. Meta-analyses of alternative anthracyclines did not suggest that there was a major difference between these agents.12,13 Several trials have tested cytosine arabinoside dose modification, again without convincing evidence of benefit.14-16 Our previous trial in younger patients (Medical Research Council [MRC] AML15) demonstrated that the fludarabine/cytosine arabinoside/granulocyte colony-stimulating factor and idarubicin (FLAG-Ida) regimen can deliver a higher number of remissions with the first course and also reduce the risk of relapse.6
Daunorubicin intensification, by either increasing the dose or the number of days of administration, has been suggested as a new standard of care. In adults under 60 years of age, 90 mg/m2 × 3 days improved the remission rate from 57% to 71% when compared with 45 mg/m2, with remission being achieved after course 1 in 59% of the 90-mg-dose patients and 41% of the 45-mg-dose patients.17 This also translated into a survival benefit (median survival 23.7 months vs 15.7 months) at a follow-up of 25 months. Although this has led to adoption as a standard of care by many investigators, there are some reservations. The overall survival (OS) of the 90-mg/m2 arm did not appear to be different from what many have reported with “conventional” dosing, and the control arm outcome in particular was suboptimal. The survival benefit was not seen in any defined subgroup and was limited to patients under 50 years. A subsequent analysis indicated that patients with mutations in NPM1 and DNMT3A also derive a survival benefit.18
In a second influential study in patients over 60 years, there was a better remission rate (64% vs 54%), but no significant improvement in OS with a median follow-up of living patients of 40 months, except in patients aged 60 to 65 years.19 In an additional exploratory subgroup analysis, survival was improved in the 33 patients with a core binding factor leukemia, but for no other subgroup. A similar trial was reported from Korea20 of a comparison of 90 mg/m2 vs 45 mg/m2, which has more mature follow-up (52 months) and an improved rate of remission (83% vs 72%) and a significant OS benefit (47% vs 35%). As in the ECOG1900 trial, the benefit was predominantly seen in intermediate-risk patients. None of these studies reported an excess of cardiotoxicity.
These studies adopted a 45 mg/m2 dose of daunorubicin as the control arm, but many investigators routinely use a 60 mg/m2 dose. In a retrospective nonrandomized study, the introduction of a 90 mg/m2 dose instead of 60 mg/m2 did not improve outcomes.21 Whereas the body of evidence suggests that a daunorubicin dose of 90 mg is superior to 45 mg, there has never been a prospective randomized trial comparing 90 mg/m2 with 60 mg/m2. For this reason, as part of the UK National Cancer Research Council [NCRI] AML17 trial, we prospectively addressed this question.
Patients and methods
The NCRI AML17 trial (ISRCTN55675535) is a large prospective trial undertaken in 136 centers in the United Kingdom, Denmark, and New Zealand. The trial addressed several questions. Patients with any form of AML (excluding acute promyelocytic leukemia) and high-risk myelodysplastic syndrome (MDS) (>10% marrow blasts) were eligible for this randomization, but they were required to have an ejection fraction of >45% to enter the daunorubicin dose comparison. From September 2011 to October 2013, 1206 patients were randomized to receive daunorubicin on days 1, 3, and 5 with cytosine arabinoside 100 mg/m2 every 12 hours on days 1 to 10 inclusive. This was repeated in course 2, but the cytosine arabinoside was give every 12 hours on days 1 to 8. In course 1, patients were randomized 1:1 to have each daunorubicin dose at 90 mg/m2 or 60 mg/m2. In course 2, the daunorubicin dose for all patients was 50 mg/m2.
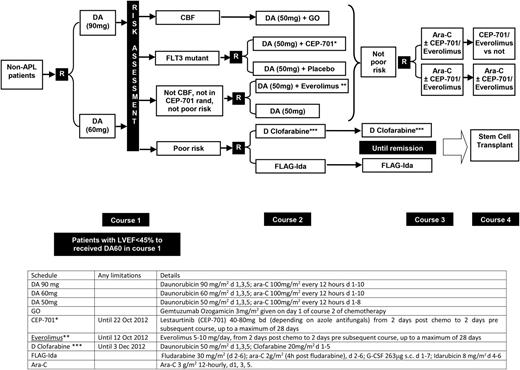
After course 1, patients were defined by risk of relapse, using a validated score that we have previously reported,22 which is based on age, presenting white blood cell count, presence of secondary disease, and the cytogenetic and morphologic response of the bone marrow after the first induction course. This marrow was in general assessed 21 to 25 days from the end of course 1. Patients designated as favorable or intermediate risk received the second daunorubicin/cytosine arabinoside course and were then randomized to receive either 1 or 2 courses of high-dose cytosine arabinoside (Figure 1). High-risk patients were allocated to a randomization between FLAG-Ida or daunorubicin/clofarabine for as many as 3 courses, with the intention of eventually undergoing allogeneic transplantation. After this randomization was fully recruited, all high-risk patients received FLAG-Ida.
Trial design of AML17. Patients allocated either CEP-701 or everolimus post–course 1 carried this molecule forward into subsequent courses. *After closure of the CEP-701 randomization, patients were guided by risk score to either poor risk or not poor risk options. **After closure of the everolimus randomization, patients in this group received daunorubicin (DA) 50 mg alone. ***After closure of the D clofarabine arm, patients were recommended to receive FLAG-Ida (which was also the case if renal criteria were not met).
Trial design of AML17. Patients allocated either CEP-701 or everolimus post–course 1 carried this molecule forward into subsequent courses. *After closure of the CEP-701 randomization, patients were guided by risk score to either poor risk or not poor risk options. **After closure of the everolimus randomization, patients in this group received daunorubicin (DA) 50 mg alone. ***After closure of the D clofarabine arm, patients were recommended to receive FLAG-Ida (which was also the case if renal criteria were not met).
Diagnosis was confirmed locally and immunophenotyping and cytogenetics (20 metaphases) were performed in regional accredited laboratories and classified as previously published.23 Molecular characterization was undertaken in 2 reference labs. Supportive care policies were as determined by each center’s policy. Stem cell transplantation was undertaken in regional transplant centers. Response end point definitions were as described by Cheson et al.24
The trial was sponsored by Cardiff University and approved by the All Wales Research Ethics Committee on behalf of all UK investigators, by the Danish Medicines Agency for sites in Denmark, and by MEDSAFE for sites in New Zealand. The trial was conducted in accordance with the Declaration of Helsinki.
Statistical considerations and data analysis
All analyses are by intention-to-treat. Categorical end points (eg, complete remission [CR] rates) were compared using Mantel-Haenszel tests, giving Peto odds ratios and confidence intervals (CIs). Continuous/scale variables were analyzed by nonparametric (Wilcoxon rank-sum) tests. Time-to-event outcomes were analyzed using the log-rank test, with Kaplan-Meier survival curves. Odds ratio/hazard ratio (OR/HR) <1 indicate benefit for the investigational therapy (daunorubicin 90 mg/m2) vs standard therapy (daunorubicin 60 mg/m2). All survival percentages are at 2 years unless otherwise stated.
In addition to overall analyses, exploratory analyses were performed stratified by the randomization stratification parameters and other important variables, with suitable tests for interaction. Because of the well-known dangers of subgroup analysis, these were interpreted cautiously.
The trial was originally powered to recruit 1700 patients to the daunorubicin dose randomization. This would provide 90% power to detect a HR of 0.80, representing an improvement in 5-year survival from 45% to 53% at 5 years. The trial was closed early on the recommendation of the Data Monitoring Committee after the recruitment of 1206 subjects after a signal for early mortality was seen in the daunorubicin 90 mg/m2 arm of the trial, without any corresponding signal suggesting a later reduction in relapse.
Follow-up was complete by January 1, 2014, with a median follow-up for survival of 14.8 months (range, 2.5-27.6).
Results
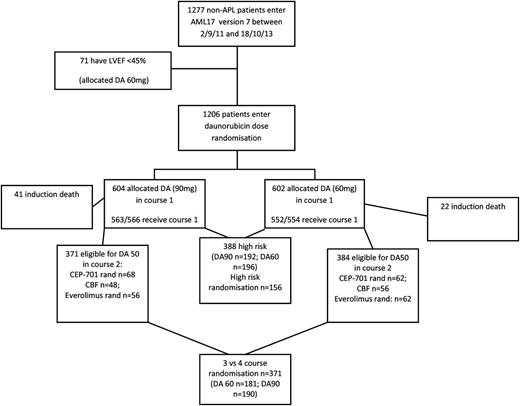
Between September 2011 and October 2013, 1206 patients entered the randomization from 122 centers. One thousand eighteen (84%) had de novo disease, 118 had secondary AML (10%; 23 t-AML, 86 antecedent hematologic disorder [51 MDS], 11 both, 20 not specified) and 70 (6%) had high-risk MDS. The median age was 53 years (range, 16-72), and 54% were male. The characteristics by treatment are shown in Table 1. Cytogenetic status was known on 1144 of 1206 (95%) patients, with 112 (10%) having favorable (core binding factor) disease, and 827 (72%) being defined as intermediate risk. The remaining 205 (18%) patients were designated as high risk. Seventy-one patients (5%) were excluded because the cardiac ejection fraction was <45%.
Demographics
| Characteristic . | DA60 (n = 602) . | DA90 (n = 604) . |
|---|---|---|
| Age, y | ||
| 16-29 | 59 | 59 |
| 30-39 | 65 | 66 |
| 40-49 | 120 | 121 |
| 50-59 | 199 | 199 |
| 60+ | 159 | 159 |
| Median | 53 | 53 |
| Range | 16-72 | 16-72 |
| Sex | ||
| Female | 267 | 284 |
| Male | 335 | 320 |
| Diagnosis | ||
| De novo | 509 | 509 |
| Secondary | 59 | 59 |
| MDS | 34 | 36 |
| World Health Organization PS | ||
| 0 | 401 | 403 |
| 1 | 165 | 166 |
| 2 | 20 | 21 |
| 3 | 15 | 14 |
| 4 | 1 | 0 |
| White blood cell count | ||
| 0-9.9 | 320 | 312 |
| 10-49.9 | 175 | 175 |
| 50-99.9 | 65 | 65 |
| 100+ | 42 | 52 |
| Median | 8.0 | 9.3 |
| Range | 0.3-430.0 | 0.4-395.0 |
| Cytogenetics | ||
| Favorable | 60 | 52 |
| Intermediate | 410 | 417 |
| Adverse | 96 | 109 |
| Unknown | 36 | 26 |
| FLT3 ITD | ||
| WT | 458 | 462 |
| Mutant | 100 | 100 |
| Unknown | 44 | 42 |
| NPM1c | ||
| WT | 400 | 383 |
| Mutant | 153 | 167 |
| Unknown | 49 | 54 |
| ITD/NPM1c | ||
| ITD WT, NPM1c WT | 356 | 349 |
| ITD WT, NPM1c mutant | 97 | 102 |
| ITD mutant, NPM1c WT | 44 | 34 |
| ITD mutant, NPM1c mutant | 56 | 65 |
| Unknown | 49 | 54 |
| Post–course 1 risk score | ||
| Good risk | 80 | 63 |
| Standard risk | 255 | 257 |
| Poor risk | 219 | 214 |
| Not assessable* | 48 | 70 |
| Characteristic . | DA60 (n = 602) . | DA90 (n = 604) . |
|---|---|---|
| Age, y | ||
| 16-29 | 59 | 59 |
| 30-39 | 65 | 66 |
| 40-49 | 120 | 121 |
| 50-59 | 199 | 199 |
| 60+ | 159 | 159 |
| Median | 53 | 53 |
| Range | 16-72 | 16-72 |
| Sex | ||
| Female | 267 | 284 |
| Male | 335 | 320 |
| Diagnosis | ||
| De novo | 509 | 509 |
| Secondary | 59 | 59 |
| MDS | 34 | 36 |
| World Health Organization PS | ||
| 0 | 401 | 403 |
| 1 | 165 | 166 |
| 2 | 20 | 21 |
| 3 | 15 | 14 |
| 4 | 1 | 0 |
| White blood cell count | ||
| 0-9.9 | 320 | 312 |
| 10-49.9 | 175 | 175 |
| 50-99.9 | 65 | 65 |
| 100+ | 42 | 52 |
| Median | 8.0 | 9.3 |
| Range | 0.3-430.0 | 0.4-395.0 |
| Cytogenetics | ||
| Favorable | 60 | 52 |
| Intermediate | 410 | 417 |
| Adverse | 96 | 109 |
| Unknown | 36 | 26 |
| FLT3 ITD | ||
| WT | 458 | 462 |
| Mutant | 100 | 100 |
| Unknown | 44 | 42 |
| NPM1c | ||
| WT | 400 | 383 |
| Mutant | 153 | 167 |
| Unknown | 49 | 54 |
| ITD/NPM1c | ||
| ITD WT, NPM1c WT | 356 | 349 |
| ITD WT, NPM1c mutant | 97 | 102 |
| ITD mutant, NPM1c WT | 44 | 34 |
| ITD mutant, NPM1c mutant | 56 | 65 |
| Unknown | 49 | 54 |
| Post–course 1 risk score | ||
| Good risk | 80 | 63 |
| Standard risk | 255 | 257 |
| Poor risk | 219 | 214 |
| Not assessable* | 48 | 70 |
WT, wild-type.
Post–course 1 validated risk score22 is not available for patients who had induction death, had missing cytogenetics, or in whom a response to course 1 was not available.
Additional treatments
After course 1, 192 patients from the 90-mg dose arm and 196 from the 60-mg-dose arm were designated as high risk and were placed into the high-risk randomization arm (Figures 1 and 2). Of these 388 patients, 156 were randomized between FLAG-Ida and daunorubicin/clofarabine. When that randomization closed, all patients were scheduled to receive FLAG-Ida. One-hundred thirty patients who had a FLT3 mutation (either ITD or TKD) were randomized to the FLT3 inhibitor, CEP701 (lestaurtinib) or placebo, whereas 118 who were FLT3-ve were randomized to mTOR inhibitor (everolimus) or not. Each of these agents was given for as long as 28 days after each course of chemotherapy. Core binding factor patients were given gemtuzumab ozogamicin 3 mg/m2 with course 2. Patients who were not defined as high risk after course 1 were randomized between 1 and 2 courses of high-dose Ara-C (3 g/m2 on days 1, 3, and 5) as consolidation. The results of all these interventions will be reported in detail elsewhere, but any treatment effect in these interventions did not affect the daunorubicin dose comparison.
Two hundred seventy-seven patients were transplanted in CR 1 (DA60, n = 151; DA90, n = 126; 161 poor risk, 101 standard risk, 8 good, and 7 unknown risk), whereas 59 (DA90, n = 29; DA60, n = 30) were transplanted in CR 2. One-hundred seventy-one patients received a myeloablative and 71 received a reduced-intensity allograft from a matched sibling or matched unrelated donor in CR 1 (poor risk: 99 vs 41; standard risk: 62 vs 27; good risk: 7 vs 0; unknown risk: 3 vs 3).
Remission
Complete remission or CR with incomplete platelet recovery (CRi) was achieved in 82% of patients (60 mg 84% and 90 mg 81%; OR 1.21 [0.90-1.63]; P = .2). Of these, CR was achieved in 75% for DA60 and 73% for DA90 (OR, 1.07 [0.83-1.39]; P = .6). There was similarly no significant difference in the proportion who entered CR/CRi with the first induction course (66% vs 68%; OR, 0.89 [0.70-1.14]; P = .4; Table 2).
Trial outcomes and results of dose comparisons
| . | DA60 . | DA90 . | OR/HR and CI . | P . |
|---|---|---|---|---|
| CR | 75% | 73% | 1.07 (0.83-1.39) | .6 |
| CRi | 9% | 8% | ||
| CR/CRi | 84% | 81% | 1.21 (0.90-1.63) | .2 |
| Induction death | 4% | 6% | 1.63 (0.97-2.73) | .07 |
| Resistant disease | 12% | 13% | 1.04 (0.74-1.47) | .8 |
| CR/CRi post–course 1 | 66% | 68% | 0.89 (0.70-1.14) | .4 |
| 30-day mortality | 4% | 6% | 1.56 (0.94-2.61) | .09 |
| 60-day mortality | 5% | 10% | 1.98 (1.30-3.02) | .001 |
| 2-year OS | 60% | 59% | 1.16 (0.95-1.43) | .15 |
| 2-year RFS | 48% | 51% | 1.05 (0.85-1.30) | .7 |
| 2-year cumulative incidence of relapse | 43% | 39% | 1.00 (0.79-1.27) | 1.0 |
| 2-year cumulative incidence of death in CR | 8% | 10% | 1.27 (0.79-2.04) | .3 |
| 2-year OS from CR | 69% | 70% | 1.04 (0.79-1.38) | .8 |
| 2-year OS censored at SCT | 60% | 60% | 1.20 (0.96-1.51) | .11 |
| . | DA60 . | DA90 . | OR/HR and CI . | P . |
|---|---|---|---|---|
| CR | 75% | 73% | 1.07 (0.83-1.39) | .6 |
| CRi | 9% | 8% | ||
| CR/CRi | 84% | 81% | 1.21 (0.90-1.63) | .2 |
| Induction death | 4% | 6% | 1.63 (0.97-2.73) | .07 |
| Resistant disease | 12% | 13% | 1.04 (0.74-1.47) | .8 |
| CR/CRi post–course 1 | 66% | 68% | 0.89 (0.70-1.14) | .4 |
| 30-day mortality | 4% | 6% | 1.56 (0.94-2.61) | .09 |
| 60-day mortality | 5% | 10% | 1.98 (1.30-3.02) | .001 |
| 2-year OS | 60% | 59% | 1.16 (0.95-1.43) | .15 |
| 2-year RFS | 48% | 51% | 1.05 (0.85-1.30) | .7 |
| 2-year cumulative incidence of relapse | 43% | 39% | 1.00 (0.79-1.27) | 1.0 |
| 2-year cumulative incidence of death in CR | 8% | 10% | 1.27 (0.79-2.04) | .3 |
| 2-year OS from CR | 69% | 70% | 1.04 (0.79-1.38) | .8 |
| 2-year OS censored at SCT | 60% | 60% | 1.20 (0.96-1.51) | .11 |
RFS, relapse-free survival; SCT, stem cell transplant.
Day 30 mortality was 5% overall and did not differ significantly between arms (60 mg 4% and 90 mg 6%; HR, 1.56 [0.94-2.61]; P = .09), but day 60 mortality, which was 7% overall, was significantly greater in the 90-mg DA (90 mg 10%, 60 mg 5%; HR, 1.98 [1.30-3.02]; P = .001). The causes of death by day 60 for the 60-mg DA vs the 90-mg DA were reported as infection (11 vs 25), hemorrhage (3 vs 5), both (3 vs 1), resistant disease (2 vs 14), respiratory (4 vs 2), cardiac (2 vs 1), and multiple or unknown (4 vs 10).
Toxicity
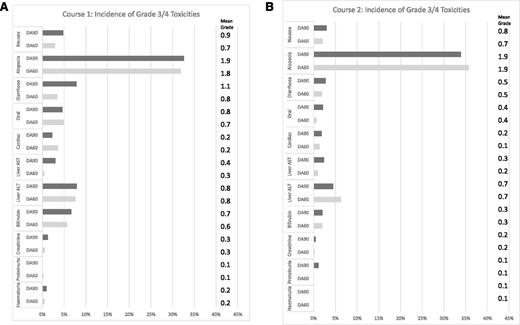
There was more grade 3 or 4 gastrointestinal toxicity (nausea and diarrhea) in the 90-mg DA in course 1 but not in course 2 (Figure 3A-B). There was no significant excess cardiac toxicity (with fewer events with DA90 in course1 being balanced by slightly more events in course 2) and no significant reports of excess toxicity so far recorded in follow-up. Median time to peripheral count recovery post–course 1 did not differ between arms (median time to neutrophil recovery 30 days from start of course in both arms, P = .12; median time to platelet recovery 28 days from start of course in both arms, P = .8), with similar lack of difference after course 2 (neutrophils 30 days in both arms, P = .6; platelets 60 mg 33 days vs 90 mg 36 days, P = .15). There were no significant differences in resource usage (blood, platelet transfusions, days on IV antibiotics, and time in hospital) between arms in either course.
Nonhematologic toxicity after courses 1 and 2. (A) Course 1: toxicity according to NCI CTCAE v.3.0. (B) Course 2.
Nonhematologic toxicity after courses 1 and 2. (A) Course 1: toxicity according to NCI CTCAE v.3.0. (B) Course 2.
Relapse and overall survival
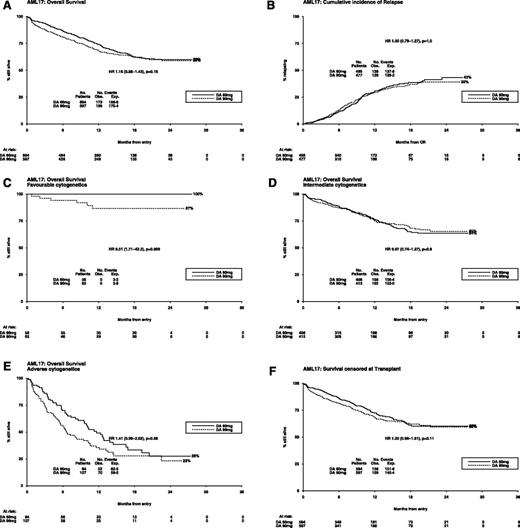
Overall survival at 24 months was 60% (60-mg DA) and 59% (90-mg DA; HR, 1.16 [0.95-1.43]; P = .15) (Figure 4A). However, there was evidence of a nonconstant hazard ratio, with a significant excess of deaths in the first 60 days, as noted before (HR, 1.98 [1.30-3.02]; P = .001) and no difference in mortality thereafter (HR, 0.98 [0.78-1.24]; P = .9); the test for difference in HR between these 2 periods gives P = .004 as significant (supplemental Figure 1). Consistent with this finding, there is no evidence of difference in relapse rate, with 43% of patients on the 60-mg DA relapsing compared with 39% on the 90-mg DA (HR, 1.00 [0.79-1.27]; P = 1.0) (Figure 4B). Taken altogether, the mean survival, truncated at 24 months, was 16.5 months for DA90 and 16.0 months for DA60.
Outcomes after daunorubicin dose randomization. (A) Overall survival (OS); (B) cumulative incidence of relapse; (C) OS—favorable cytogenetics; (D) OS—intermediate cytogenetics; (E) OS—adverse cytogenetics; (F) survival censored at transplant.
Outcomes after daunorubicin dose randomization. (A) Overall survival (OS); (B) cumulative incidence of relapse; (C) OS—favorable cytogenetics; (D) OS—intermediate cytogenetics; (E) OS—adverse cytogenetics; (F) survival censored at transplant.
There was no evidence of benefit for the 90-mg DA in any cytogenetic subgroup (favorable 87% vs 100%, P = .009; intermediate 65% vs 64%, P = .8; adverse 23% vs 28%, P = .06; Figure 4C-E; P = .01 for heterogeneity, P = .5 for trend) (supplemental Figure 2). The OS at 2 years of the 101 standard-risk patients who received a stem cell transplant in CR 1 was 87%, and for the 161 poor-risk patients it was 72%. When patients are censored at the time of transplant, the effect of treatment on survival was unaffected either overall (HR, 1.20 [0.96-1.51]; P = .11; Figure 4F) or in the standard-risk patients (70% vs 72%; HR, 1.00 [0.62-1.58]; P = 1.0) or poor-risk patients (49% vs 33%; HR, 0.99 [0.70-1.40]; P = 1.0). There was no significant difference between the arms in survival 12 months after relapse (HR, 0.94 [0.67-1.32]; P = .7).
Exploratory subgroup analysis
In addition to the predefined risk groups, the outcome in the demographic subgroups described in Table 1 was examined. No subgroup was associated with a differential benefit from either the 60-mg or the 90-mg dose level, with the exception of a significant interaction between dose and FLT3-ITD (P = .03 for heterogeneity). In FLT3-ITD, wild-type patients at the 90-mg dose level were associated with significantly worse outcome (HR, 1.30 [1.02-1.66]; P = .03); conversely, outcomes with 90 mg were better in FLT3-ITD–mutant patients emerging after 12 months, although this did not reach statistical significance (HR, 0.74 [0.47-1.17]; P = .2; supplemental Figure 2), although longer follow-up is required. No benefit was seen in patients aged 60 to 65 years. Additional exploration of interaction with center size showed no evidence that any excess mortality was confined to smaller centers (survival, P = .8; 60-day mortality, P = .3).
Discussion
In this large study, we aimed to resolve the issue of whether daunorubicin at a dose of 90 mg/m2 should be regarded as a new standard of care, particularly in the context of the widely-used 60 mg/m2 dose. Although the overall median follow-up was 14.8 months and therefore shorter than the other 3 trials discussed here, 529 randomized patients had a minimum follow-up of 12 months. We failed to show any significant benefit overall or in any subgroup that was examined. The previous trials suggest that 90 mg/m2 is superior to 45 mg/m2. This may be so, but in younger patients in 1 study, the outcome of the control arm was inferior to what we have regularly reported using a 50 mg/m2 dose level.2,6 However, the more mature Korean trial20 resulted in a survival benefit when a 90 mg/m2 dose was compared with 45 mg/m2, which had a survival in line with what is usually expected. There are other differences between these trials. In our protocols, patients routinely received 2 induction daunorubicin-containing courses, which in this AML17 trial delivered a total dose of 420 mg/m2 over 2 courses for favorable and intermediate-risk patients who received the 90 mg/m2 dose, whereas in our previous protocols where 50 mg/m2 was routinely used, the total dose delivered was 300 mg/m2 over 2 courses. This was augmented by the immunoconjugate, gemtuzumab ozogamicin (GO) in some cases. It has been suggested that the beneficial effect of GO in favorable and intermediate-risk disease represented an anthracycline-type dose escalation.25 Our data refute this speculation. In the ECOG 1900 and HOVON trials, only patients not reaching remission with the first induction course received a second course containing daunorubicin (which was ∼10% of patients in the E1900 trial), which delivered a total dose of 415 mg/m2. The total exposure in the HOVON trial was capped at 270 mg/m2. The Korean trial20 had a similar design with longer follow-up. The comparison was 90 mg/m2 vs 45 mg/m2 for 3 days in 383 patients. In this study, the control arm delivered an expected survival, but it was significantly inferior to the outcome of the 90 mg/m2 arm. No further daunorubicin-containing courses were given. In our studies, we have given the daunorubicin on days 1, 3, and 5 and the cytosine arabinoside over 10 days in course 1 as a twice-daily infusion. Whether this differs from daunorubicin administration on 3 consecutive days or cytarabine by continuous infusion is not clear.
Based on the data from these trials, there is no evidence that 90 mg/m2 is harmful overall, with the exception of the increase in early death within 60 days in the AML17 trial, although one cannot rule out the possibility that a higher dose of daunorubicin is detrimental in favorable cytogenetic and FLT3-unmutated patients. However, no long-term follow-up data have so far been presented, and in particular whether early dose escalation compromises tolerability if reinduction is required. In this study, 327 patients progressed to transplant (CR 1, 271, CR 2, 56) for whom we have no reported excess toxicity. It is of interest that when the patients were censored at transplant, the survival did not deteriorate.
In the initial subgroup analysis in the ECOG1900 trial, no benefit was seen for patients in any defined subgroup except for those <50 years and nonadverse cytogenetics, whereas in the HOVON trial, there was the overall benefit for the 60- to 65-year-old patients and a strong trend for benefit in the core binding factor (favorable) subgroup. In the Korean trial, the overall benefit was attributable to intermediate-risk patients. In the AML17 trial, there was no evidence of a subgroup benefit for the 90 mg/m2 dose. However, in a recent analysis of the E1900 trial,26 with a median follow-up of 80 months, the OS benefit was maintained and a late benefit has emerged in every cytogenetic group and in patients with mutations of FLT3, as well as in NPM1 and DNMT3A, and also patients >50 years. This may reflect a genuine personalized direction for these patients, with the challenge of identifying them before commencing treatment, or it may reflect the poor performance of a 45 mg/m2 dose. In the subgroup analysis of the AML17 trial, there was no evidence of a clear heterogeneity of effect in any subgroup examined, although there is a nonsignificant trend for benefit in FLT3 mutations beyond 12 months. It is therefore of importance to examine molecular subgroups when longer follow-up is available, which is the intention.
In conclusion, it is clear that overall escalation of the daunorubicin dose to 90 mg/m2 produces superior outcomes to 45 mg/m2, with emerging evidence that it is applicable in identifiable subgroups. However, in this the only randomized trial comparing 90 mg/m2 with 60 mg/m2, there was no evidence that 90 mg/m2 was superior to 60 mg/m2 overall or in any identified subgroup.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Cancer Research UK for research support and the Cardiff University Haematology Trials Unit staff for supervision of the trial.
Authorship
Contribution: A.K.B., N.H.R., and D.M. designed the study; R.K.H. provided statistical input to the design and analyzed data; J.K., J.C., L.K., N.H.R., M.-F.M., P.C., M.D., L.F., and R.E.C. provided patients to the study; I.F.T. provided trial coordination for the study; and A.K.B. drafted the paper, which was revised and approved by all authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the investigators from the UK NCRI AML Study Group who recruited patients for this study appears in the online data supplement.
Correspondence: Alan K. Burnett, Department of Haematology, School of Medicine, Cardiff University, Heath Park, Cardiff CF4 14XN, United Kingdom; e-mail; akburnett719@gmail.com.