Key Points
Improved adaptive immune responses in humanized mice lacking murine MHC II and expressing human HLADR1.
NOD.PrkdcscidIl2rγ−/−H2-Ab1−/− Tg(HLA-DR1) mice reconstituted with hematopoietic stem cells from an IPEX syndrome patient develop fatal autoimmunity.
Abstract
Mice reconstituted with a human immune system provide a tractable in vivo model to assess human immune cell function. To date, reconstitution of murine strains with human hematopoietic stem cells (HSCs) from patients with monogenic immune disorders have not been reported. One obstacle precluding the development of immune-disease specific “humanized” mice is that optimal adaptive immune responses in current strains have required implantation of autologous human thymic tissue. To address this issue, we developed a mouse strain that lacks murine major histocompatibility complex class II (MHC II) and instead expresses human leukocyte antigen DR1 (HLA-DR1). These mice displayed improved adaptive immune responses when reconstituted with human HSCs including enhanced T-cell reconstitution, delayed-type hypersensitivity responses, and class-switch recombination. Following immune reconstitution of this novel strain with HSCs from a patient with immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, associated with aberrant FOXP3 function, mice developed a lethal inflammatory disorder with multiorgan involvement and autoantibody production mimicking the pathology seen in affected humans. This humanized mouse model permits in vivo evaluation of immune responses associated with genetically altered HSCs, including primary immunodeficiencies, and should facilitate the study of human immune pathobiology and the development of targeted therapeutics.
Introduction
Studies in mice have offered significant insight into the pathogenesis of human diseases; however, animal models have frequently failed to predict the efficacy and safety of novel therapeutics in humans.1-4 An experimental system allowing direct functional assessment of patient cells in vivo could serve as an invaluable intermediate step in the process of drug development that could increase safety while reducing overall cost of clinical trials. Over the past decade, advanced immunodeficient mouse models have been established to improve engraftment of human hematopoietic stem cells (HSCs) and leukocyte development facilitating in vivo mechanistic studies. Though several iterations of humanized mice have been described,5 most strains combine null mutations in Prkdc or Rag genes with Il2rγ−/− to impair de novo murine lymphocyte maturation and natural killer cell development respectively, while permitting xenogeneic thymopoiesis in the murine thymus.6 Transfer of human CD34+ HSCs in these mice leads to multilineage hematopoiesis with variable levels of reconstitution depending on the strain and age of recipient mice and the source of donor HSCs.7,8 Despite robust lymphoid reconstitution in most models, adaptive immune responses remain incomplete in both the CD34+ HSC model as well as advanced models incorporating concurrently implanted human fetal thymic and liver tissue and autologous HSCs (bone marrow liver thymic [BLT] mice).7,9,10 This impediment has been postulated to result from inefficient CD4+ T-cell selection on murine major histocompatibility complex class II (MHC II) in the mouse thymus.11 In support of this hypothesis, intravenous injection of human HSCs into adult NOD.Rag1−/−Il2rγ−/− mice expressing MHC II HLA-DR4 improves CD4+ T-cell development as well as B-cell function.12 One potential limitation of this model is that human CD4+ T cells can be restricted on either murine MHC II or HLA-DR4 molecules.
In this report, we developed a novel immunodeficient mouse strain lacking murine MHC II and instead express a human MHC II molecule to test whether adaptive immunity would be improved in this model. We show that these mice reconstituted with human HSCs exhibit adaptive immune responses and, when reconstituted using HSCs from a patient with immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, recapitulate many aspects of the patient’s disease. This humanized murine model has the potential to serve as a preclinical tool to screen therapeutic alternatives and ultimately facilitate precision medicine.
Materials and methods
Human HSC isolation and HLA typing
Human CD34+ HSCs were obtained by positive selection using CD34 microbeads (Miltenyi Biotec, San Diego, CA) on healthy human cord blood. Screening for HLA-DRA*0101, HLA-DRB*0101–matched donor samples was performed at the tissue typing laboratory of Brigham & Women’s Hospital using high-resolution LABType SSO kits (One Lambda, Canoga Park, CA). The IPEX patient sample was obtained from a bone marrow aspirate with parental consent and approval from the institutional review board at Boston Children’s Hospital before allogeneic HSC transplantation. Research was conducted in accordance with the Declaration of Helsinki.
Human immune reconstitution
One-day-old pups were preconditioned using 150 rads of 137Cs source γ-radiation. Pups were injected 5 hours later via the intrahepatic route with 3–5 × 104 human CD34+ HSCs in phosphate-buffered saline (PBS).
Human immunophenotyping and flow cytometry
Human immunophenotyping on reconstituted mice was performed at 20 weeks of age. Cells were blocked in 10% rat serum then incubated with fluorochrome-conjugated antibodies for 20 minutes at 4°C, washed 2X FACS buffer, and then analyzed using a 3-laser BD FACS Canto II (BD Biosciences, San Jose, CA).
Delayed-type hypersensitivity
Mice were injected subcutaneously with 200 μL emulsion containing 250 μg ovalbumin (OVA) (Sigma-Aldrich, St. Louis, MO) and 100 μL complete Freund’s adjuvant (Sigma-Aldrich) at the base of the tail. Seven days later, mice were challenged with 50 μL of 10 μgmL−1 OVA injected into the left footpad. The right footpad was injected with 50 μL of PBS as a control. Footpad swelling was measured at 24 hours using a digital caliper. Delayed-type hypersensitivity (DTH) was calculated as the difference in swelling between the left and the right footpad.
FOXP3 sequencing
A DNA fragment containing the C-terminal DNA binding domain of the FOXP3 gene was polymerase chain reaction amplified using forward: 5′-TAGTCCTGTCCCTGATTACCTGCCCC and reverse: 5′-TGTGCTTGTGTGTGATTGTGTGATGAT primers, gel purified, and sequenced using a forward: 5′-GTCTGGGCTCATAGGCACAT sequencing primer.
Immunohistochemistry
Histopathology was carried out on formalin fixed paraffin–embedded tissue sections stained with hematoxylin and eosin. Detection of human T cells was performed using an anti-human CD3 antibody (catalog #A0452, Dako, Carpinteria, CA), whereas human macrophages were detected using anti-human CD68 (clone KP1, Dako). Images were acquired using an Olympus microscope mounted with an Olympus DP70 digital camera and DP-Manager software (Olympus, Melville, NY).
T-cell receptor CDR3 sequencing
Splenic human CD4+ T cells were enriched using negative selection (Miltenyi Biotec) and DNA isolated by ethanol precipitation following an overnight proteinase K digestion at 56°C. Purified DNA was subjected to next-generation sequencing of the complementarity determining region 3 (CDR3) region using immunoSEQ (Adaptive Biotechnology, Seattle, WA) and analyzed with the immunoSEQ Analyzer software (Adaptive Biotechnology).
Autoantibody detection
Detection of autoantibodies was performed on cryosections from lung and liver tissue of a nonreconstituted NOD.PrkdcscidIl2rγ−/−H2-Ab1−/− (NSGAb°DR1) mouse. Briefly, sections were warmed to room temperature (RT) for 5 minutes and fixed using precooled acetone for 10 minutes at RT. Samples were washed 3× in PBS and blocked using goat serum for 30 minutes at RT followed by incubation with sera from individual mice reconstituted with either IPEX or control HSCs diluted in PBS containing 0.1% Triton X-100 (PBST) overnight at 4°C. Sections were washed 3× for 5 minutes in PBST followed by an incubated with fluorescein isothiocyanate–conjugated goat anti-human immunoglobulin G (IgG; Santa Cruz Biotechnology, Dallas, TX) for 60 minutes at RT, washed 3× for 5 minutes in PBST, overlaid with Vectashield mounting medium containing 4,6 diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and detected by indirect immunofluorescence using an Olympus microscope mounted with an Olympus DP70 digital camera and DP-Manager software (Olympus).
Suppression assay
A leukocyte suspension was obtained from peripheral blood using Lympholyte (Cedarlane Laboratories, Burlington, NC). The CD4+ T cells were enriched by negative selection (Miltenyi Biotec) following the manufacture’s protocol. The flow-through was then subjected to CD25 -positive selection (Miltenyi Biotec) to isolate putative regulatory T cells (Tregs). CD25-depleted allogeneic responder Tnaive cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) and 5 × 104 cells were cocultured with or without an equal number of IPEX Treg or control Treg cells in the presence of 1 μg plate-bound αCD3 (clone: OKT3, eBioscience) and 1 μg soluble αCD28 (clone: CD28.6, eBioscience) in T-cell media. After 4 days of culture, proliferation of Tnaive cells was assessed by flow cytometry.
ELISAs
Total immunoglobulins in sera of humanized mice were quantified using standard enzyme-linked immunosorbent assay (ELISA) kits for human IgM (Bethyl Laboratories, Montgomery, TX) and human IgG (Bethyl Laboratories). Human IgE was measured using a capture antibody (clone: MHE-18, BioLegend) and detected using horseradish peroxidase–conjugated anti-human IgE (catalog #A9667, Sigma-Aldrich).
Statistical analysis
Statistical analysis was performed using Prism 6 (GraphPad Software, La Jolla, CA).
Results
NSG mice expressing human MHC class II permit enhanced T-cell development
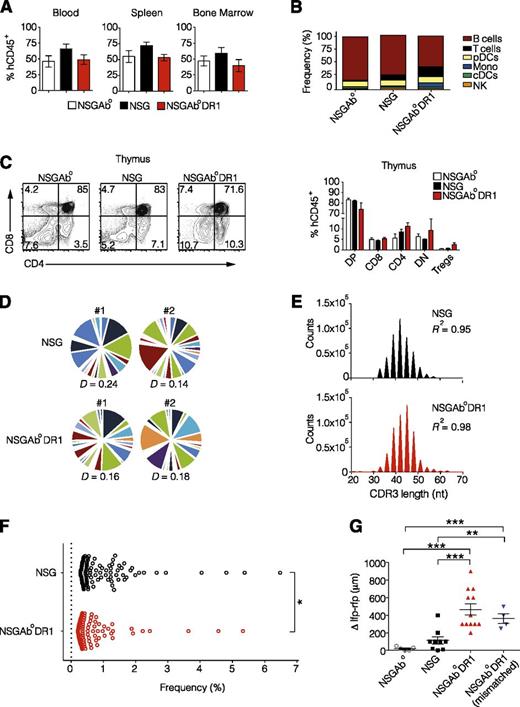
To investigate whether human CD4+ T cells can be positively selected and also promote adaptive immune responses in mice that express human MHC II in the absence of murine MHC II, we generated NOD.PrkdcscidIl2rγ−/− (NSG) H2-Ab1−/− (NSGAb°) mice harboring a human HLA-DR1 transgene (NSGAb°DR1) under the control of the murine MHC II promoter. We first confirmed cell-surface expression of HLA-DR1 on murine thymic epithelium and splenic antigen-presenting cells (supplemental Figure 1). Next, CD34+ cord blood human HSCs were obtained from a healthy allelically-matched DR1–positive donor and injected intrahepatically into radiation-conditioned 1-day-old NSG, NSGAb°, and NSGAb°DR1 pups. Human leukocyte chimerism at 20 weeks was found to be equivalent among the 3 strains in peripheral blood, spleen, and bone marrow (Figure 1A). Immunophenotyping of human leukocytes isolated from recipient strains revealed a slight but statistically significant increase in the proportion of T cells and monocytes with a concomitant/relative decrease in the proportion of B cells in NSGAb°DR1 mice compared with NSG and NSGAb° mice (Figure 1B; supplemental Table 1). There was a trend toward fewer CD4+ T cells in the thymus of NSGAb° mice, suggesting a requirement for either murine or human class II expression for proper development of human CD4+ T cells (Figure 1C). Further evaluation of the specific subtypes of CD4+ and CD8+ splenocytes in NSGAb°DR1 mice demonstrated no significant difference in the proportion of naive, effector, effector memory, or central memory cells compared with the other strains (supplemental Table 1). Most of the CD4+ and CD8+ T cells from NSGAb°DR1 mice were CD45RA−CD45RO+ (data not shown). Interestingly, the relative frequencies of thymic Tregs were slightly elevated in NSGAb°DR1 mice and may reflect higher avidity between human T-cell receptor (TCR) and HLA-DR1 (Figure 1C). T cells isolated from all reconstituted strains were equally adept in their ability to produce human cytokines in vitro following TCR-independent stimulation (supplemental Figure 2).
Normal T-cell development and function in NSGAb°DR1 mice. (A) Human chimerism in blood, spleen, and bone marrow determined using human CD45 staining 20 weeks after reconstitution from a single DR1 allelically matched cord blood donor. n ≥ 6 per group pooled from 3 independent experiments. (B) Human immune cell populations as a percent of human leukocytes in spleens of reconstituted mice. cDCs, classical dendritic cells; pDCs, plasmacytoid dendritic cells; NK, natural killer. (C) Representative flow cytometry dot plots of developing T cells (left) quantified (right) showing double-positive (DP), CD4+, CD8+, double-negative (DN) and CD4+CD25+FOXP3+ Tregs. n ≥ 5 per group from 2 independent experiments. (D) The CDR3 region of the TCR-β locus was profiled using next-generation ultrahigh-throughput sequencing. Pie segments are displayed in different colors to highlight unique V-gene segments for NSG and NSGAb°DR1 mice. TCR-β repertoire Diversity was calculated using normalized Shannon’s entropy (P > .05). (E) Histogram of CDR3 nucleotide length in CD4+ T cells isolated from spleens of reconstituted NSG or NSGAb°DR1 mice. (F) The relative frequency or “evenness” of the CD4+ TCRβ repertoire was plotted for the top 100 clonotypes in 2 representative animals in each genotype, with each circle depicting an individual clonotype. (G) Delayed-type hypersensitivity response in OVA-immunized mice displayed as the difference in swelling between OVA-injected left footpad (lfp) and PBS-injected right footpad (rfp). NSGAb°DR1 (mismatched) refers to reconstitution using HSCs from a donor that was negative for the DRB1*01:01 allele. Data are pooled from 2 independent experiments. Bars are the mean ± standard error of the mean (SEM). Statistical analysis performed using 1-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test (A), multiple Student t test corrected for multiple comparisons using Holm-Sidak method (C), nonlinear regression (E), unpaired Student t test (F-G). *P < .05, **P < .01, ***P < .001.
Normal T-cell development and function in NSGAb°DR1 mice. (A) Human chimerism in blood, spleen, and bone marrow determined using human CD45 staining 20 weeks after reconstitution from a single DR1 allelically matched cord blood donor. n ≥ 6 per group pooled from 3 independent experiments. (B) Human immune cell populations as a percent of human leukocytes in spleens of reconstituted mice. cDCs, classical dendritic cells; pDCs, plasmacytoid dendritic cells; NK, natural killer. (C) Representative flow cytometry dot plots of developing T cells (left) quantified (right) showing double-positive (DP), CD4+, CD8+, double-negative (DN) and CD4+CD25+FOXP3+ Tregs. n ≥ 5 per group from 2 independent experiments. (D) The CDR3 region of the TCR-β locus was profiled using next-generation ultrahigh-throughput sequencing. Pie segments are displayed in different colors to highlight unique V-gene segments for NSG and NSGAb°DR1 mice. TCR-β repertoire Diversity was calculated using normalized Shannon’s entropy (P > .05). (E) Histogram of CDR3 nucleotide length in CD4+ T cells isolated from spleens of reconstituted NSG or NSGAb°DR1 mice. (F) The relative frequency or “evenness” of the CD4+ TCRβ repertoire was plotted for the top 100 clonotypes in 2 representative animals in each genotype, with each circle depicting an individual clonotype. (G) Delayed-type hypersensitivity response in OVA-immunized mice displayed as the difference in swelling between OVA-injected left footpad (lfp) and PBS-injected right footpad (rfp). NSGAb°DR1 (mismatched) refers to reconstitution using HSCs from a donor that was negative for the DRB1*01:01 allele. Data are pooled from 2 independent experiments. Bars are the mean ± standard error of the mean (SEM). Statistical analysis performed using 1-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test (A), multiple Student t test corrected for multiple comparisons using Holm-Sidak method (C), nonlinear regression (E), unpaired Student t test (F-G). *P < .05, **P < .01, ***P < .001.
NSGAb°DR1 mice develop delayed-type hypersensitivity
Although there was no obvious difference in thymic selection or the development of the CD4+ T compartment when HLA-DR1 was expressed, we hypothesized that the TCR repertoire might exhibit improved diversity that would improve T-cell function. Development of a diverse population of antigen-specific T cells requires rearrangement of germline-encoded TCR gene segments13 and is largely mediated by the complementarity determining region 3 (CDR3) within variable (V) gene segments of the TCRα and TCRβ (TRB) genes.14 To assess if human CD4+ T cell selection on HLA-DR1 modulates T-cell clonotype diversity, we profiled the CDR3 region of TRB on splenic CD4+ T cells in reconstituted mice using high-throughput, next-generation sequencing.15 A high degree of combinatorial diversity in V gene segment usage was readily observed in splenic CD4+ T cells isolated from either NSGAb°DR1 or NSG mice with the CDR3 length following standard Gaussian distribution in both strains (Figure 1D-E). A few T-cell clonotypes were overrepresented in each of the reconstituted strains, but the NSG mice exhibited a higher degree of clonality compared with NSGAb°DR1 mice (Figure 1F). This diversity was achieved in the absence of exogenous interleukin-7, similar to a previous report in mice reconstituted as neonates.16 Although NSGAb°DR1 CD4+ displayed a greater frequency of unique clonotype sequences in the periphery compared with NSG mice (supplemental Table 2), the normalized TCR diversity index was similar between NSGAb°DR1 and NSG mice (Figure 1D). These data indicate that selection of human CD4+ T cells on HLA-DR1 does not alter TCR diversity relative to NSG mice, but exhibits reduced T-cell oligoclonality. Next, we assessed CD4+-dependent adaptive T-cell responses in vivo. One of the hallmarks of cell-mediated immunity is the ability to mount a rapid response to a previously encountered antigen. Though DTH responses to recall antigens have been reported in BLT mice,10 recall challenge inflammation-associated swelling is minimal in humanized mouse models in which there is absence of autologous human tissue graft or exogenous recombinant interleukin-7.8,17 We compared DTH responses to OVA in NSGAb°, NSG, and NSGAb°DR1 mice that were previously immunized subcutaneously against OVA. Although we observed low degree of footpad swelling in NSG mice, similar to a previous report,17 NSGAb°DR1 mice, when compared with NSG or NSGAb° mice, exhibited greater footpad swelling in response to the recall antigen following secondary challenge, indicating improved immunological memory responses are present (Figure 1G). This improved recall response also occurred when NSGAb°DR1 mice were reconstituted using donor HSCs that were not matched for the HLADR1*01:01 allele (Figure 2G). Although these data indicate that CD4+ T cells have functional capacity, CD8+ T cells and natural killer cells from NSGAb°DR1 mice also have functional properties at least in vitro, as evidenced by interferon-γ secretion (supplemental Figure 3A) and target cell lysis, respectively (supplemental Figure 3B-C).
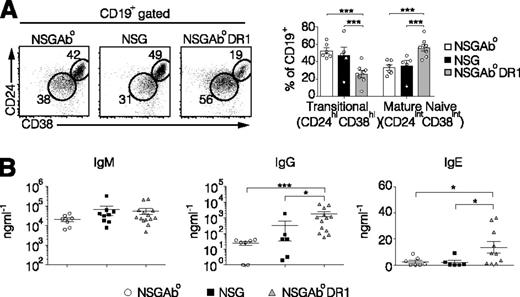
Increased B cell maturation and class switched immunoglobulins in reconstituted NSGAb°DR1 mice. (A) Representative flow cytometry dot plots of splenic B cells gated on CD45+CD19+ (left) and quantified (right) from 3 independent experiments with n ≥ 5 per group. (B) Serum immunoglobulin levels quantified by ELISA. Each data point represents an individual mouse from 3 independent experiments. Solid bars are the mean ± SEM. Statistical analysis was performed using multiple t test corrected for multiple comparisons using the Holm-Sidak method (A), Mann-Whitney test (B). *P < .05, **P < .01, ***P < .001.
Increased B cell maturation and class switched immunoglobulins in reconstituted NSGAb°DR1 mice. (A) Representative flow cytometry dot plots of splenic B cells gated on CD45+CD19+ (left) and quantified (right) from 3 independent experiments with n ≥ 5 per group. (B) Serum immunoglobulin levels quantified by ELISA. Each data point represents an individual mouse from 3 independent experiments. Solid bars are the mean ± SEM. Statistical analysis was performed using multiple t test corrected for multiple comparisons using the Holm-Sidak method (A), Mann-Whitney test (B). *P < .05, **P < .01, ***P < .001.
Human B-cell maturation and class switch recombination occurs in NSGAb°DR1 mice
T cells also play a critical role in the humoral arm of adaptive immunity because rodents and humans lacking T cells exhibit profound defects in B-cell maturation and antibody class switching.18,19 Previous data in humanized NSG mice show varying stages of human B-cell development; however, progression to mature naive B cells from immature transitional B cells remains poor, with low levels of class-switched antibodies.20-23 We analyzed the immature transitional splenic B-cell population in reconstituted NSGAb°, NSG, and NSGAb°DR1 mice by staining for CD45, CD19, CD24, and CD38. NSGAb°DR1 mice had a significant decrease in the proportion of CD45+CD19+CD24hiCD38hi immature transitional B cells, with a concomitant increase in CD45+CD19+CD24intCD38int mature naive B cells (Figure 2A).24,25 Consistent with an increase in mature naive B cells, serum IgG and IgE levels were also increased, indicating that immunoglobulin class switching was intact in NSGAb°DR1 mice (Figure 2B), as was previously seen in NOD.Rag1−/−Il2rγ−/− mice expressing both murine MHC II and human HLA-DR4 (DRAG mice).12 Immunization against OVA led to the development of OVA-specific IgG in some but not all NSGAb°DR1 mice (supplemental Figure 4). Collectively, these data indicate that adaptive humoral immune responses are present when human CD4+ T cells are restricted by human HLA-DR1 in the absence of murine MHC II.
Multiorgan inflammation and mortality in NSGAb°DR1 mice reconstituted with IPEX CD34+ HSCs
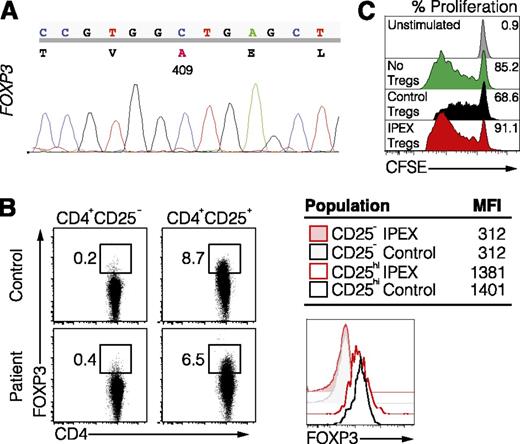
One inherent limitation of the BLT humanized mouse system is the requirement for an autologous setting comprising human thymus, liver, and HSCs from the same donor source, which prohibits assessment of immune dysfunction associated with patient-specific genetic abnormalities. The improved adaptive immune responses in humanized NSGAb°DR1 mice led us to test whether immune reconstitution using HSCs isolated from a patient with a genetically defined immunological disorder would transfer manifestations of the human disease to recipient mice. Given that CD4+ T-cell function is relatively intact in NSGAb°DR1 mice, we hypothesized that transplantation of HSCs from a patient in which CD4+ T cells, especially Tregs, are dysfunctional and directly involved in disease pathogenesis would permit assessment of the utility of this model system. We identified a 7-month-old male patient who presented with secretory watery nonbloody diarrhea, eczema, and enteropathy and was found to have a nonsynonymous single-nucleotide polymorphism at position c.A1226C in the FOXP3 gene (Figure 3A). This mutation resulted in a p.D409A amino acid change in the C-terminal DNA-binding domain, the same location previously reported in IPEX syndrome.26 IPEX syndrome is a rare and often fatal X-linked recessive disorder caused by loss-of-function mutations in FOXP3, the master transcriptional regulator of Tregs that is essential for establishment and maintenance of central tolerance.27-30 Patients with IPEX syndrome develop enteropathy, eczema, autoantibodies, and sometimes insulin-dependent diabetes mellitus.31 Mice harboring a loss-of-function mutation in Foxp3 (Foxp3sf) display a similar phenotype with multiorgan inflammation attributed to autoreactive CD4+ T cells.32,33 Although FOXP3 expression was intact in CD4+CD25+ Tregs isolated from this patient (Figure 3B), which has also been described for other FOXP3 mutations associated with IPEX syndrome,34 suppression of allogeneic naive T-cell proliferation by the patient’s Tregs was profoundly impaired (Figure 3C).
Identification of a novel variant in FOXP3 associated with IPEX syndrome. (A) Sequence of the FOXP3 gene in the IPEX patient. (B) FOXP3 expression in Tregs (CD4+CD25hi) and Tnaive (CD4+CD25−) from the IPEX patient and healthy control shown by flow cytometry (left) and histogram the mean fluorescence intensity (right). (C) In vitro suppression assay using Tregs from a healthy control or IPEX patient and carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled allogeneic responder T cells.
Identification of a novel variant in FOXP3 associated with IPEX syndrome. (A) Sequence of the FOXP3 gene in the IPEX patient. (B) FOXP3 expression in Tregs (CD4+CD25hi) and Tnaive (CD4+CD25−) from the IPEX patient and healthy control shown by flow cytometry (left) and histogram the mean fluorescence intensity (right). (C) In vitro suppression assay using Tregs from a healthy control or IPEX patient and carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled allogeneic responder T cells.
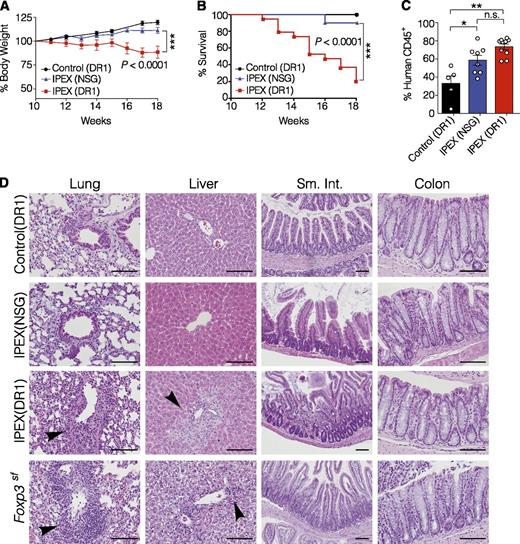
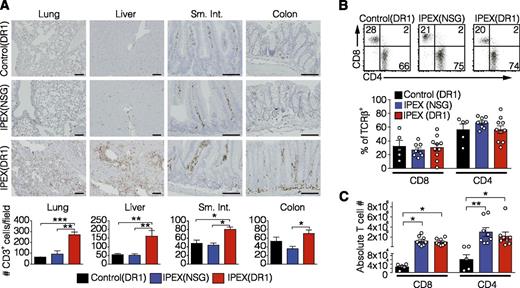
To test whether transfer of HSCs from this IPEX patient would cause a spontaneous IPEX-like disease in NSGAb°DR1 and NSG mice, bone marrow–derived CD34+ HSCs were purified and used to engraft 1-day-old NSG and NSGAb°DR1 recipient mice, hereafter referred to as IPEX(NSG) and IPEX(DR1), respectively. In parallel, bone marrow–derived CD34+ HSCs from a healthy pediatric donor were injected into NSGAb°DR1 mice as controls, referred to as control(DR1) mice. Because the patient was not an allelic match with the DR1 allele expressed in the mice, we also selected mismatched bone marrow as a control to account for any potential pathology resulting from HLA mismatch. Mice were monitored for the development of clinical manifestations characteristic of patients with IPEX syndrome and Foxp3sf mice. IPEX(DR1) mice exhibited significant weight loss and increased mortality by 18 weeks posttransfer, which was rarely observed in IPEX(NSG) mice and never observed in control(DR1) mice (Figure 4A-B). Similar to the absence of diabetes in this patient, IPEX(DR1) mice also did not exhibit signs or symptoms of insulitis. At necropsy, IPEX(NSG) and IPEX(DR1) mice exhibited splenomegaly (not shown) with increased human immune cells in the spleen compared with control(DR1) mice (Figure 4C). Because IPEX patients, Foxp3sf mice, and mice with targeted depletion of FOXP3-expressing cells develop multiorgan inflammation,35 we analyzed organs known to be affected by Treg deficiency. Similar to observations in Foxp3sf mice,36 inflammation was consistently observed by immunohistochemistry in the lung and liver of IPEX(DR1) mice, with mild leukocyte infiltration also observed in the small intestine (Figure 4D). Given the inflammation detected in these organs, we screened whole tissue to quantify human inflammatory cytokines which were broadly elevated in both lung and liver tissue of IPEX(DR1) mice compared with IPEX(NSG) and control(DR1) mice as anticipated (supplemental Figure 5). This inflammatory infiltrate consisted of human CD3+ T cells, and to a lesser extent human CD68+ macrophages, in the lung and liver tissue of IPEX(DR1) mice but not IPEX(NSG) mice or control(DR1) mice (Figure 5A; supplemental Figure 6). We assessed the T-cell compartment and found similar proportions of CD4+ and CD8+ T cells in the spleens of all 3 groups (Figure 5B). The absolute numbers of CD4+ and CD8+ T cells were increased in both IPEX(NSG) and IPEX(DR1) mice, with a normal CD4+/CD8+ ratio, reminiscent of the lymphoproliferation observed in Foxp3sf mice (Figure 5C).37 We tested the function of IPEX human Tregs by isolating the CD4+CD25+ fraction from several pooled spleens of IPEX(DR1) mice and found, analogous to the patient’s Treg suppression defects, that the ability to suppress autologous CD4+CD25− T cells was impaired (supplemental Figure 7).
NSGAb°DR1 mice reconstituted with IPEX hematopoietic stem cells causes mortality and multiorgan inflammation. (A) Longitudinal assessment of body weight change compared with weight at 10 weeks from 2 independent experiments. (B) Kaplan-Meier survival curve comparing reconstituted mice. The 18-week cutoff was selected based on 100% lethality in IPEX(DR1) mice by this time point in the first experimental cohort to compare variability across 2 independent experiments. Control(DR1) n = 10, IPEX(NSG) n = 10, IPEX(DR1) n = 19. (C) Human chimerism in spleen of reconstituted mice assessed by flow cytometry pooled from 2 experiments. Open circles are individual mice. Bars are the mean ± SEM. (D) Hematoxylin and eosin–stained sections from the lung, liver, small intestine (sm. int.), and colon of NSGAb°DR1 mice reconstituted with control bone marrow CD34+ HSCs or IPEX CD34+ HSCs, NSG mice reconstituted with IPEX CD34+ HSCs, and Foxp3sf mice. Black arrowheads denote leukocyte infiltrate. Scale bars are 100 μm. Statistical analysis performed using 1-way ANOVA with Tukey’s multiple comparisons test (A,C), curve comparison using log-rank Mantel-Cox test (B). *P < .05, **P < .01.
NSGAb°DR1 mice reconstituted with IPEX hematopoietic stem cells causes mortality and multiorgan inflammation. (A) Longitudinal assessment of body weight change compared with weight at 10 weeks from 2 independent experiments. (B) Kaplan-Meier survival curve comparing reconstituted mice. The 18-week cutoff was selected based on 100% lethality in IPEX(DR1) mice by this time point in the first experimental cohort to compare variability across 2 independent experiments. Control(DR1) n = 10, IPEX(NSG) n = 10, IPEX(DR1) n = 19. (C) Human chimerism in spleen of reconstituted mice assessed by flow cytometry pooled from 2 experiments. Open circles are individual mice. Bars are the mean ± SEM. (D) Hematoxylin and eosin–stained sections from the lung, liver, small intestine (sm. int.), and colon of NSGAb°DR1 mice reconstituted with control bone marrow CD34+ HSCs or IPEX CD34+ HSCs, NSG mice reconstituted with IPEX CD34+ HSCs, and Foxp3sf mice. Black arrowheads denote leukocyte infiltrate. Scale bars are 100 μm. Statistical analysis performed using 1-way ANOVA with Tukey’s multiple comparisons test (A,C), curve comparison using log-rank Mantel-Cox test (B). *P < .05, **P < .01.
Human T-cell inflammation in IPEX(DR1) mice. Immunohistochemical analysis detecting human immune cell infiltration using (A) anti-hCD3 staining on lung, liver, sm. int., and colon tissue sections (top) quantified by counting the number of CD3+ cells/20× field for 3 mice per group (bottom). (B) Representative flow cytometry dot plots of splenic T cells gated on TCR-β+ (left) with the frequency of CD4+/CD8+ T cells reported (right). Open circles are individual mice. (C) The absolute numbers of splenic T cells were quantified. Open circles are individual mice. All values are mean ± SEM. Statistical analysis performed using 1-way ANOVA with Tukey’s multiple comparisons test (A), 1-way ANOVA using Kruskal-Wallis multiple comparisons test (B-C). *P < .05, **P < .01, ***P < .001.
Human T-cell inflammation in IPEX(DR1) mice. Immunohistochemical analysis detecting human immune cell infiltration using (A) anti-hCD3 staining on lung, liver, sm. int., and colon tissue sections (top) quantified by counting the number of CD3+ cells/20× field for 3 mice per group (bottom). (B) Representative flow cytometry dot plots of splenic T cells gated on TCR-β+ (left) with the frequency of CD4+/CD8+ T cells reported (right). Open circles are individual mice. (C) The absolute numbers of splenic T cells were quantified. Open circles are individual mice. All values are mean ± SEM. Statistical analysis performed using 1-way ANOVA with Tukey’s multiple comparisons test (A), 1-way ANOVA using Kruskal-Wallis multiple comparisons test (B-C). *P < .05, **P < .01, ***P < .001.
Engraftment of IPEX CD34+ HSCs leads to autoantibody production
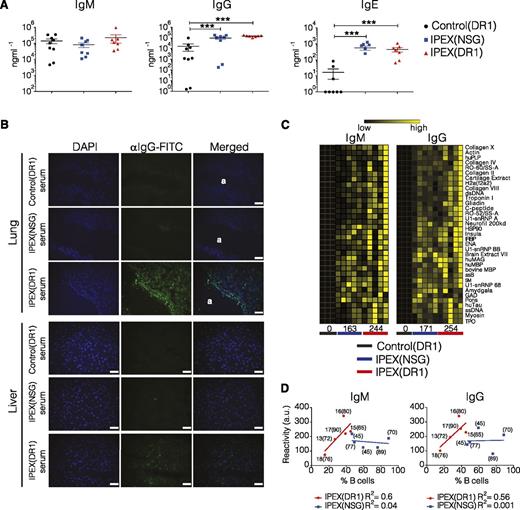
Defective Treg function in both Foxp3sf mice and IPEX patients is also associated with hyperimmunoglobulinemia, loss of peripheral B-cell tolerance, and the development of autoantibodies.38-41 Similar to observations in patients with IPEX syndrome and in Foxp3sf mice,39,40,42 serum IgG and IgE levels were elevated in both IPEX(NSG) and IPEX(DR1) mice compared with control(DR1) mice (Figure 6A). Patients with IPEX syndrome develop autoantibodies, and our patient tested positive for several, including antibodies against glutamic acid decarboxylase, thyroperoxidase, and nuclear antigens.39,43 We investigated whether human mouse-specific autoantibodies were produced in mice reconstituted with HSCs from the IPEX patient. Indirect immunofluorescence on cryosectioned lung and liver tissue revealed the presence of autoreactive IgG in serum from IPEX(DR1) mice that colocalized with DAPI-stained nuclei and was not observed in sera from IPEX(NSG) or control(DR1) mice (Figure 6B). Using a more global approach, sera from control(DR1), IPEX(NSG), and IPEX(DR1) mice were screened using an autoantigen microarray spotted with 500 murine lipid and peptide antigens. Broad IgM and IgG autoreactivity was detected in IPEX(DR1) mice, and to a lesser extent in IPEX(NSG) mice, with several autoantibodies commonly observed in human autoimmune diseases including gliadin, double-stranded DNA, and collagen (Figure 6C; supplemental Figures 8 and 9). Although there was a clear correlation between autoantibody reactivity and the percentage of human B cells in IPEX(DR1) mice, this was not the case for IPEX(NSG) mice (Figure 6D). This suggests that the increased autoreactivity is likely attributed to T-cell help provided by DR1-restricted CD4+ T cells. It is noteworthy that the higher levels of autoantibodies also did not appear to correspond to early mortality in IPEX(DR1) mice, suggesting that additional factors are likely dominant (Figure 6D).
Loss of B-cell tolerance in IPEX mice. (A) Serum immunoglobulin levels quantified by ELISA from 2 independent experiments. (B) Autoantibody detection by indirect immunofluorescence on cryosections of frozen lung and liver tissue from a nonhumanized NSGAb°DR1 mouse. Frozen sections were incubated independently with sera from 4 individual reconstituted mice per group. Fluorescein isothiocyanate (FITC)-conjugated anti-human IgG was used as a secondary antibody combined with DAPI to label nuclei. (a) Denotes lung airway. Scale bars are 50 μm. (C) Heat map of antibody response to select murine antigens determined using an antigen microarray. Each column is the IgM and IgG serum reactivity from an individual mouse in each group with the averaged reactivity score for all antigens per group noted at the bottom. IgM P = .06, IgG P = .09 (D) The normalized cumulative autoreactive IgM and IgG antibody reactivity values for all 500 mouse antigens in arbitrary units (a.u.) were plotted against the % human B cells in each mouse (n = 5). Solid line marks the best-fit linear regression for each cohort with the R2 value calculated using Pearson correlation coefficients. For each data point, the % of human chimerism is shown in parenthesis and for IPEX(DR1) mice, the age in weeks when mice became moribund and were killed. Statistical analysis was performed using Mann-Whitney test (A) and nonlinear regression correlation using Pearson correlation coefficients (D). ***P < .001.
Loss of B-cell tolerance in IPEX mice. (A) Serum immunoglobulin levels quantified by ELISA from 2 independent experiments. (B) Autoantibody detection by indirect immunofluorescence on cryosections of frozen lung and liver tissue from a nonhumanized NSGAb°DR1 mouse. Frozen sections were incubated independently with sera from 4 individual reconstituted mice per group. Fluorescein isothiocyanate (FITC)-conjugated anti-human IgG was used as a secondary antibody combined with DAPI to label nuclei. (a) Denotes lung airway. Scale bars are 50 μm. (C) Heat map of antibody response to select murine antigens determined using an antigen microarray. Each column is the IgM and IgG serum reactivity from an individual mouse in each group with the averaged reactivity score for all antigens per group noted at the bottom. IgM P = .06, IgG P = .09 (D) The normalized cumulative autoreactive IgM and IgG antibody reactivity values for all 500 mouse antigens in arbitrary units (a.u.) were plotted against the % human B cells in each mouse (n = 5). Solid line marks the best-fit linear regression for each cohort with the R2 value calculated using Pearson correlation coefficients. For each data point, the % of human chimerism is shown in parenthesis and for IPEX(DR1) mice, the age in weeks when mice became moribund and were killed. Statistical analysis was performed using Mann-Whitney test (A) and nonlinear regression correlation using Pearson correlation coefficients (D). ***P < .001.
Discussion
The main goal of this study was to develop a humanized mouse strain that would support human adaptive immune function and enable in vivo modeling of a monogenic human immune-mediated disease using patient HSCs. Because CD4+ T cells are known to play critical roles in maintaining immune homeostasis, we reasoned that a setting in which human CD4+ T cells were selected on a HLA class II molecule in the absence of murine MHC II might improve thymic positive selection and, consequently, adaptive immune responses in humanized mice. The NSGAb°DR1 mice described in this study were an attractive strain to test this hypothesis, especially given the frequency of the HLA-DR1 allele in the population.44
The improved CD4+ T-cell recall response in NSGAb°DR1 mice may result from more effective interactions between DR1-restricted CD4+ T cells and HLA-DR1/peptide complexes presented by DR1+ antigen-presenting cells resulting from coevolution of the human TCR and human MHC II molecules.45,46 Although we observed a slight but significant increase in T-cell frequency in NSGAb°DR1 mice compared with NSG mice, we did not observe an increase in the frequency of CD4+ T cells, which is in contrast to the increase in CD4+ T cells reported in DRAG mice that also express a human class II molecule.12 This difference may result from expression of both human and murine class II in DRAG mice, which permits interspecies α/β MHC II pairing and enhanced positive selection.
Humoral immunity in humanized mice has generally been poor, with B cells exhibiting an immature phenotype and yielding lower levels of serum immunoglobulins compared with humans.47,48 In our studies, B-cell maturation and antibody class switch recombination was increased in reconstituted NSGAb°DR1 mice compared with NSG and NSGAb° mice. This increase may be a result of improved cognate interactions between HLA-DR1–restricted CD4+ T cells and HLA-DR1+ B cells triggering cytokine secretion and antibody class switching.49 The observation that B cells in NSG mice exhibited a more immature phenotype and relatively lower serum IgG and IgE levels suggests that MHC II-restricted CD4+ T cells in NSG mice are less efficient at providing T-cell help to B cells.
The most significant finding of our study was that HSCs from a patient with IPEX syndrome caused immunodysregulation in NSGAb°DR1 mice. To date, human HSCs with a causative mutation of a monogenic disease triggering a similar disease phenotype in humanized mice has not been reported. Interestingly, the disease phenotype was not readily observed when these HSCs were transferred into the NSG strain despite NSG mice having increased T-cell numbers, elevated serum immunoglobulins, and autoantibodies compared with control bone marrow HSCs. This is likely attributed to the enhanced adaptive immune responses seen in reconstituted NSGAb°DR1 mice. Similar to the phenotype in our IPEX(DR1) mice, a recent report also demonstrated a role for human Tregs in immune homeostasis in humanized mice by showing that administration of antibodies blocking CTLA-4, a molecule critical for Treg suppressive function,50-52 also caused weight loss, liver inflammation, and antinuclear antibodies.53 One possibility for the multiorgan inflammation is that the IPEX mice developed allogeneic graft versus host disease (GVHD) because the IPEX donor was not matched for the DR1*01:01 allele; a previous report using NSGAb° mice expressing human DR4 injected with human peripheral blood mononuclear cells resulted in allogeneic GVHD.54 However, human stem cell reconstitution in 1-day-old NSGAb°DR1neonates gives rise to human T cells selected on murine (and likely human) antigens in the mouse thymus, where autoreactive T cells are likely deleted. In this regard, using this method, we have never observed characteristic xeno-GVHD symptoms (weight loss or alopecia) following reconstitution, even in NSG mice. Moreover, control HSCs that were negative for the DR1*01:01 allele did not result in systemic T-cell inflammation following reconstitution in NSGAb°DR1 mice.
In summary, genetic studies in mice have advanced our understanding of gene/protein function in health and disease giving rise to new therapeutic targets. Even so, disparities persist between validated therapeutics using rodent models and improved clinical outcomes, necessitating the development of new model systems.1,3 Humanized mice provide the opportunity to study the function of human immune cells in an in vivo setting without risk to human subjects. The model described here exhibits enhanced adaptive immune function, which circumvents the requirement of surgically implanted autologous thymic and liver tissue and enables use of HSCs from genetically defined patients.53,55 These mice will likely be most useful for studies pertaining to T-cell responses and immune-mediated diseases resulting from Treg dysfunction and/or aberrant effector CD4+ T-cell activation. One preclinical utility of this model would permit assessment of the efficacy and safety of novel genome editing or vector-based gene therapy strategies aimed at correcting monogenic immunodeficiencies; early trials using retroviral-based vectors had complications resulting from vector-mediated insertional activation of cellular oncogenes.24,56,57 Overall, we propose that NSGAb°DR1 mice, alone or in combination with additional HLA molecules and/or other human cytokines,58 will further the utility and usefulness of humanized mice to study human immune responses in health and disease and the development of emerging immunotherapies.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Emanuele Bosi and Vito Lampasona for their assistance with patient serum and the BIDMC Research Histology core.
This work was supported by the National Institutes of Health (NIH) National Research Service Award (F32DK097894) (J.A.G.); grants from the NIH National Institute of Allergy and Infectious Diseases (AI075037) (E.F.) and (AI50950), the NIH National Heart, Lung, and Blood Institute (HL59561), and the NIH National Institute of Diabetes and Digestive and Kidney Diseases (DK034854); the Helmsley Charitable Trust, and the Wolpow Family Chair in IBD Treatment and Research (S.B.S.).
Authorship
Contribution: J.A.G., S.B., W.S.L., A.Y., S.Y., J.S., W.A.M., D.M., E.F., F.J.Q., B.H.H., C.K., A.M.M., and S.B.S. designed the experiments and collected and analyzed data; J.O., D.S.S., K.J.M., L.D.N., L.P., M.-G.R., R.B., S.-Y.P., C.K., and S.B.S. assisted with acquisition of patient samples; E.L.M. performed HLA typing; B.P. analyzed autoantigen array data; and J.A.G. and S.B.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott B. Snapper, 300 Longwood Ave, Enders 670, Boston, MA 02115; e-mail: scott.snapper@childrens.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal