Key Points
We conducted a phase-2 study in newly diagnosed PCNSL utilizing R-MPV and HDC with ASCT.
Excellent disease control and OS (2-year PFS: 79%) were observed, with an acceptable toxicity profile and minimal neurotoxicity.
Abstract
High-dose methotrexate-based chemotherapy is the mainstay of treatment of primary central nervous system lymphoma (PCNSL), but relapses remain frequent. High-dose chemotherapy (HDC) with autologous stem-cell transplant (ASCT) may provide an alternative to address chemoresistance and overcome the blood-brain barrier. In this single-center phase-2 study, newly diagnosed PCNSL patients received 5 to 7 cycles of chemotherapy with rituximab, methotrexate (3.5 g/m2), procarbazine, and vincristine (R-MPV). Those with a complete or partial response proceeded with consolidation HDC with thiotepa, cyclophosphamide, and busulfan, followed by ASCT and no radiotherapy. Primary end point was 1-year progression-free survival (PFS), N = 32. Median age was 57, and median Karnofsky performance status 80. Following R-MPV, objective response rate was 97%, and 26 (81%) patients proceeded with HDC-ASCT. Among all patients, median PFS and overall survival (OS) were not reached (median follow-up: 45 months). Two-year PFS was 79% (95% confidence interval [CI], 58-90), with no events observed beyond 2 years. Two-year OS was 81% (95% CI, 63-91). In transplanted patients, 2-year PFS and OS were 81%. There were 3 treatment-related deaths. Prospective neuropsychological evaluations suggested relatively stable cognitive functions posttransplant. In conclusion, this treatment was associated with excellent disease control and survival, an acceptable toxicity profile, and no evidence of neurotoxicity thus far. This trial was registered at www.clinicaltrials.gov as NCT00596154.
Introduction
More than 90% of patients with primary central nervous system lymphoma (PCNSL) display a diffuse large B-cell lymphoma (DLBCL) phenotypic subtype, but standard DLBCL regimens such as cyclophosphamide, doxorubicin, vincristine and prednisone and variations are ineffective in this disease.1,2 This has been explained by poor penetration of these agents across the blood-brain barrier (BBB), a problem that has been partially addressed with the development of high-dose methotrexate-based regimens (HD-MTX) that result in therapeutic central nervous system (CNS) and cerebrospinal fluid (CSF) levels after rapid infusions of 1.5 to 8 g/m2.3-5 Such high methotrexate doses are made possible with the concomitant use of leucovorin, which prevents bone marrow and systemic organ damage, while limiting rescue of lymphoma cells in the CNS because it has poor BBB penetration. This clever strategy, used with or without whole-brain radiotherapy (WBRT), has resulted in remarkable survival improvements, with recent studies reporting median overall survival (OS) of 31 to 79 months,6-13 as compared with 12 months observed with WBRT alone.14 In spite of these improvements, early and late relapses remain frequent, and the majority of patients still die of disease.15
High-dose chemotherapy (HDC) followed by autologous stem-cell transplant (ASCT) has been proposed as an alternative consolidation therapy in PCNSL.16 In addition to overcoming intrinsic chemoresistance of lymphoma cells, HDC-ASCT may improve disease control by affording higher CNS drug concentrations, circumventing chemoresistance mediated by the BBB, a similar “high-dose/ rescue” paradigm proved successful in the development of HD-MTX. We previously conducted a phase-2 study in newly diagnosed PCNSL testing an induction chemotherapy with HD-MTX and cytarabine, followed by consolidation HDC-ASCT utilizing carmustine (BCNU), etoposide, cytarabine, and melphalan (BEAM).17 Unfortunately, that treatment resulted in suboptimal disease control, with an intent-to-treat (ITT) 3-year event-free survival (EFS) of only 25%, and a 3-year OS of 60%. In the present study, we sought to optimize this strategy by utilizing an enhanced induction regimen consisting of rituximab, methotrexate, procarbazine, and vincristine (R-MPV),6 as well as a more aggressive, CNS penetrant HDC regimen consisting of thiotepa, busulfan, and cyclophosphamide (TBC).16
Patients and methods
Patients
Immunocompetent adult patients with histologically confirmed newly diagnosed PCNSL with brain involvement were enrolled in this prospective, single-arm, phase-2 study between June 2005 and September 2011. The supplemental Appendix (available on the Blood Web site) details inclusion/exclusion criteria and baseline evaluations. Patients were eligible if they had non-Hodgkin lymphoma involving the brain, as demonstrated by magnetic resonance imaging (MRI) and histologic confirmation by (1) positive CSF cytology for lymphoma or a monoclonal lymphocyte population defined by cell surface markers; (2) vitreous or uvea biopsy demonstrating non-Hodgkin lymphoma; or (3) brain biopsy. Other inclusion criteria consisted of age 18 to 72 years; negative HIV testing; left ventricular ejection fraction ≥50%; absence of systemic lymphoma on the chest, abdomen, and pelvis CT and bone marrow biopsy; leukocytes >3000/mm3; platelets >100 000/mm3; bilirubin <2 mg/dL; serum creatinine <1.5 mg/dL; or creatinine clearance >50 cc/min per 1.73 m2. Exclusion criteria consisted of prior cranial irradiation or chemotherapy for PCNSL, other active malignancy (exception: skin basal cell carcinoma and cervical carcinoma in situ), or immunodeficiency. Patients were eligible regardless of performance status. Pretreatment evaluations also included CSF sampling, slit lamp exam, electrolytes, liver enzymes, Epstein-Barr virus, cytomegalovirus, herpes simplex virus and hepatitis B/C serologies, urinalysis, 24-hour creatinine clearance, and chest radiograph.
The trial and informed consent were approved by the Institutional Review Board (www.clinicaltrials.gov: NCT00596154). Written informed consent was obtained from all patients or guardians in accordance with the Declaration of Helsinki. Data were analyzed by the authors at Memorial Sloan Kettering Cancer Center, and all authors had access to primary clinical trial data.
Induction chemotherapy
Treatment started with an induction R-MPV chemotherapy6 (1 cycle = 14 days), as follows: day 1, rituximab 500 mg/m2 IV; day 2, methotrexate 3.5 mg/m2 IV (over 2 hours) and vincristine 1.4 mg/m2 (capped at 2.8 mg). Procarbazine 100 mg/m2 per day was given on days 2 to 8 during odd cycles. Standard hydration and leucovorin rescue were given per institutional guidelines. To prevent febrile neutropenia and toxic deaths previously observed with R-MPV, prophylactic filgrastim was given to all patients.
An MRI of the brain was performed after 5 cycles. Patients with progressive disease (PD) were taken off-study. Patients with complete response (CR) proceeded directly to HDC-ASCT. Patients in partial response (PR) or stable disease (SD) received 2 additional cycles and proceeded with HDC-ASCT if PR/CR was observed on a repeat MRI or were taken off-study if SD or PD. After transplant, patients were followed radiographically, with no WBRT or further treatments offered until progression. Responses were assessed utilizing previously described criteria18 ; in addition to CR, PR, SD and PD, those criteria also characterize unconfirmed CR (CRu), defined by absence of contrast-enhancing disease in the setting of corticosteroids use, or minimal enhancing abnormalities of uncertain significance, typically corresponding to postoperative changes following biopsy.
Harvesting and HDC-ASCT
Peripheral blood stem cell harvesting was performed after the first or second R-MPV cycle, or at the discretion of treating physician, following institutional standard procedures. Cytapheresis was performed after bone marrow recovery and repeated daily up to day 7 until >5 × 106 CD34+ cells were collected (minimum acceptable total yield: 2 × 106 CD34+ cells per kg).
Patients in CR/PR as described previously underwent pretransplant evaluation (supplemental Appendix); those deemed clinically eligible proceeded with HDC-ASCT with thiotepa 250 mg/m2 IV on days −9, −8, and −7; busulfan 3.2 mg/kg IV on days −6, −5, and −4; and cyclophosphamide 60 mg/kg IV on days −3 and −2. Stem cell reinfusion occurred on day 0. Supportive therapy followed institutional guidelines, including granulocyte CSF, seizure and infection prophylaxis, hydration, antiemesis, vitamin K, and irradiated blood products.
Response was assessed after R-MPV and prior to transplant, and every 3 months thereafter (first 2 years), then every 4 months (third and fourth years) and every 6 months thereafter. Repeat CSF and/or ocular exam were done at those time points if CSF or ocular involvement was present at diagnosis.
Exploratory neuropsychological testing, quality of life, and radiographic evaluation of neurotoxicity
In addition to investigators’ clinical assessment of neurotoxicity,19 potential neurotoxic effects of disease, R-MPV, and HDC-ASCT were further characterized through prospective neuropsychological and quality of life (QoL) evaluations, as well as radiographic assessment of white matter abnormalities. Evaluations were conducted in progression-free patients at the following time points: pretreatment (baseline), after induction chemotherapy (before transplant), and at 6-month intervals following transplant. The present analysis reports on evaluations up to 24 months after transplant.
Neuropsychological evaluations were performed in 60-minute sessions. Raw test scores were compared with published normative values according to age and education, and converted into z scores to characterize presence and severity of cognitive difficulties. A z score ≤−1.5 represents impairment. The following tests were performed: Trail Making Test (TMT) Part A and TMT Part B; Brief Test of Attention; Controlled Oral Word Association Test; Hopkins Verbal Learning Test-Revised (HVLT-R)–Total Learning, HVLT-R–Delayed Recall, and HVLT-R–Discrimination Index; and Grooved Pegboard Test (GPT)–Dominant Hand and GPT–Non-Dominant Hand.
Self-reported QoL and mood were evaluated utilizing the Functional Assessment of Cancer Therapy–Brain Cancer (FACT-BR) and the Beck Depression Inventory (BDI), respectively.
Fluid-attenuated inversion recovery (FLAIR) MRI sequences obtained at the same time points were scored for white matter abnormalities utilizing the modified Fazekas scale.20
Statistics
The primary end point was 1-year progression-free survival (PFS) among all patients, defined as time from registration to tumor relapse, progression, or death of any cause, whichever comes first. For the purposes of PFS estimates, patients who withdrew consent or were removed from study because of reasons other than progression, toxicity, or death were censored at the date of last radiographic assessment confirming a progression-free status. With a sample size of 33 patients, an exact binomial test with a nominal 0.05 1-sided significance level has 90% power to detect the difference between the null hypothesis (0.5 observed in HD-MTX without radiotherapy13 ) and the alternative proportion of 0.75. The study was to be stopped if more than 2 toxic deaths occurred among the first 15 patients, or 4 toxic deaths at any time. If the true risk of toxic death is ≥16%, then the probability of seeing 4 toxic deaths (and stopping the trial) was at least 80%. Secondary end points consisted of OS, acute and chronic toxicities (CTCAEv3), and response rate after R-MPV and after HDC-ASCT. Exploratory end points consisted of evaluation of neuropsychological, QoL, and white matter abnormalities, as described previously.
Kaplan-Meier methodology was used for analysis of OS and PFS. Neuropsychological test scores were summarized using descriptive statistics, and longitudinal trajectories evaluated using linear mixed models (LMMs) controlling for age and estimated interquartile range (IQR). Exact follow-up assessment times from baseline were calculated, and both linear and quadratic terms were estimated by the LMMs. McNemar’s χ2 test was used to test for significant changes in MRI white matter abnormality scores between assessments.
Results
Patient characteristics
A total of 33 patients were enrolled and 32 analyzed; 1 registered patient was excluded because of presence of systemic lymphoma at diagnosis, discovered upon post hoc radiology review, deeming the patient ineligible. The median age was 57 (range: 23-67) and median Karnofsky performance status (KPS) was 80 (40-100). Table 1 shows detailed patient characteristics.
Patient characteristics (N = 32)
| Characteristic . | Value . |
|---|---|
| Median age (range) | 57 (23-67) |
| Age <60 | 21 (66%) |
| Age <50 | 11 (34%) |
| Median KPS (range) | 80 (40-100) |
| KPS <70 | 6 (19%) |
| KPS <50 | 1 (3%) |
| Women | 15 (47%) |
| Men | 17 (53%) |
| MSK RPA | |
| Class I | 11 (34%) |
| Class II | 15 (47%) |
| Class III | 6 (19%) |
| DLBCL | 32 (100%) |
| CSF cytology* | |
| Positive | 1 (3%) |
| Suspicious | 2 (6%) |
| Not performed | 1 (3%) |
| Ocular involvement | 3 (9%) |
| Median product of tumor diameters (range) | 6 cm2 (0-20 cm2) |
| Characteristic . | Value . |
|---|---|
| Median age (range) | 57 (23-67) |
| Age <60 | 21 (66%) |
| Age <50 | 11 (34%) |
| Median KPS (range) | 80 (40-100) |
| KPS <70 | 6 (19%) |
| KPS <50 | 1 (3%) |
| Women | 15 (47%) |
| Men | 17 (53%) |
| MSK RPA | |
| Class I | 11 (34%) |
| Class II | 15 (47%) |
| Class III | 6 (19%) |
| DLBCL | 32 (100%) |
| CSF cytology* | |
| Positive | 1 (3%) |
| Suspicious | 2 (6%) |
| Not performed | 1 (3%) |
| Ocular involvement | 3 (9%) |
| Median product of tumor diameters (range) | 6 cm2 (0-20 cm2) |
MSK RPA, Memorial Sloan-Kettering prognostic score determined by recursive partitioning analysis (I, age <50; II, age ≥50 and KPS ≥70; III, age ≥50 and KPS <70).
Conventional cytology; flow cytometry not performed.
Induction treatment
Induction R-MPV was well tolerated, with no treatment-related deaths and no treatment discontinuation because of toxicity; grades 3 and 4 toxicities are summarized in Table 2. Following 5 R-MPV cycles, 14 patients were in CR/CRu, 16 in PR, and 1 progressed (Table 3 and patient flowchart [supplemental Appendix]). One additional patient had undergone complete resection prior to enrollment and had no measurable disease at baseline; that patient was stable after 5 cycles of R-MPV. All patients in less than CR, in addition to 2 patients in CR/CRu with incomplete resolution of symptoms after 5 cycles, received 2 additional R-MPV cycles (N = 19). The objective response rate after R-MPV, defined as CR, CRu, or PR after 5 or 7 cycles in eligible patients with measurable disease (N = 31 evaluable) was 97% (95% confidence interval [CI], 83-100).
Grades 3 and 4 toxicities reported during R-MPV (N = 32)
| Toxicity . | Grade 3 . | Grade 4 . | ||
|---|---|---|---|---|
| Hemoglobin | 6 | 18% | ||
| Neutrophils | 6 | 18% | 1 | 3% |
| ALT and/or AST | 14 | 42% | 3 | 9% |
| Creatinine | 5 | 15% | ||
| Infection | 4 | 12% | ||
| Peripheral neuropathy | 2 | 6% | ||
| Fatigue | 2 | 6% | ||
| Thrombosis/embolism | 1 | 3% | 2 | 6% |
| Constipation | 1 | 3% | ||
| Syncope | 1 | 3% | ||
| Encephalopathy | 1 | 3% | ||
| Toxicity . | Grade 3 . | Grade 4 . | ||
|---|---|---|---|---|
| Hemoglobin | 6 | 18% | ||
| Neutrophils | 6 | 18% | 1 | 3% |
| ALT and/or AST | 14 | 42% | 3 | 9% |
| Creatinine | 5 | 15% | ||
| Infection | 4 | 12% | ||
| Peripheral neuropathy | 2 | 6% | ||
| Fatigue | 2 | 6% | ||
| Thrombosis/embolism | 1 | 3% | 2 | 6% |
| Constipation | 1 | 3% | ||
| Syncope | 1 | 3% | ||
| Encephalopathy | 1 | 3% | ||
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Response status after R-MPV and following transplant
| . | CR/CRu . | PR . | SD . | PD . |
|---|---|---|---|---|
| Response after 5 R-MPV cycles (N = 32) | 14 (44%) | 16 (50%) | 1* (3%) | 1 (3%) |
| Best response to R-MPV induction chemotherapy (5 or 7 cycles) (N = 32) | 21 (66%) | 9 (28%) | 1* (3%) | 1 (3%) |
| Pretransplant response status in the transplanted patients (N = 26) | 18 (69%) | 7 (27%) | 1* (4%) | 0 (0) |
| Best response after transplant (N = 26) | 21 (81%) | 3 (11%) | 1* (4%) | 1 (4%) |
| . | CR/CRu . | PR . | SD . | PD . |
|---|---|---|---|---|
| Response after 5 R-MPV cycles (N = 32) | 14 (44%) | 16 (50%) | 1* (3%) | 1 (3%) |
| Best response to R-MPV induction chemotherapy (5 or 7 cycles) (N = 32) | 21 (66%) | 9 (28%) | 1* (3%) | 1 (3%) |
| Pretransplant response status in the transplanted patients (N = 26) | 18 (69%) | 7 (27%) | 1* (4%) | 0 (0) |
| Best response after transplant (N = 26) | 21 (81%) | 3 (11%) | 1* (4%) | 1 (4%) |
This 1 patient had no measurable disease at start of R-MPV because of complete resection, remained stable after 7 cycles of R-MPV and underwent HDC-ASCT, and was considered nonevaluable for objective response rate assessment.
HDC-ASCT
Thirty-one (97%) patients were deemed eligible for transplant based on response, which includes the completely resected patient who remained stable following 7 R-MPV cycles. A total of N = 26 (81%) patients eventually received HDC-ASCT. The other 5 patients did not undergo transplant because of refusal/consent withdrawal (N = 2) or physician’s decision (N = 3) and were removed from study. There were no harvesting failures.
Table 4 summarizes toxicities observed with TBC and median times to hematologic recovery. There was no venoocclusive disease. Two patients died of transplant-related acute complications: a 57-year-old patient developed a Stevens-Johnson syndrome, and a 59-year-old patient developed septic shock. Another patient aged 61 developed a fatal chronic colitis of undetermined etiology, potentially representing graft-versus-host disease. In that patient, symptoms started 3 weeks following transplant and responded poorly to corticosteroids. To date, no patient has developed secondary malignancies.
Number of patients experiencing grades 3 to 5 toxicities from transplant, and time to hematologic recovery (N = 26)
| Nonhematologic . | Grade 3 . | Grade 4 . | Grade 5 . | |||
|---|---|---|---|---|---|---|
| Febrile neutropenia | 11 | 42% | ||||
| Infection | 4 | 15% | 2 | 8% | 1 | 4% |
| Skin rash | 1 | 4% | 1 | 4% | 1 | 4% |
| Chronic colitis | 1 | 4% | ||||
| Encephalopathy | 1 | 4% | ||||
| Dehydration | 1 | 4% | ||||
| Cardiac failure | 1 | 4% | ||||
| Weight loss | 1 | 4% | ||||
| Nausea | 1 | 4% | ||||
| Diarrhea | 1 | 4% | ||||
| Mucositis | 1 | 4% | ||||
| Engraftment | ||||||
| Median time to neutrophil recovery (>500/mm3): 9 d (range: 7-15) | ||||||
| Median time to platelet recovery (>25 000/mm3): 15 d (range: 10-124) | ||||||
| Median duration of hospital admission for HDC-ASCT: 26 d (range 20-90) | ||||||
| Nonhematologic . | Grade 3 . | Grade 4 . | Grade 5 . | |||
|---|---|---|---|---|---|---|
| Febrile neutropenia | 11 | 42% | ||||
| Infection | 4 | 15% | 2 | 8% | 1 | 4% |
| Skin rash | 1 | 4% | 1 | 4% | 1 | 4% |
| Chronic colitis | 1 | 4% | ||||
| Encephalopathy | 1 | 4% | ||||
| Dehydration | 1 | 4% | ||||
| Cardiac failure | 1 | 4% | ||||
| Weight loss | 1 | 4% | ||||
| Nausea | 1 | 4% | ||||
| Diarrhea | 1 | 4% | ||||
| Mucositis | 1 | 4% | ||||
| Engraftment | ||||||
| Median time to neutrophil recovery (>500/mm3): 9 d (range: 7-15) | ||||||
| Median time to platelet recovery (>25 000/mm3): 15 d (range: 10-124) | ||||||
| Median duration of hospital admission for HDC-ASCT: 26 d (range 20-90) | ||||||
PFS and OS
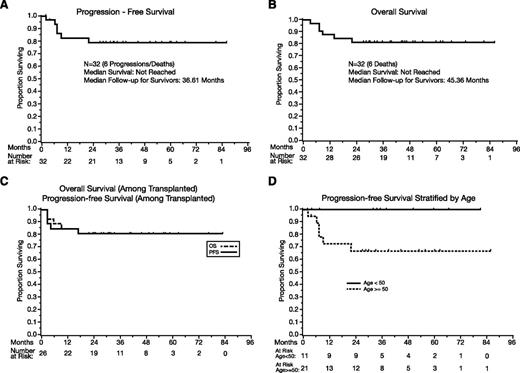
Among all patients (N = 32), the median PFS has not been reached (Figure 1A), and the 1-year PFS estimate is 82% (95% CI, 62-92). There have been no events after 2 years, and the 2-year, 3-year, and 5-year PFS estimates are 79% (95% CI, 58-90). Aside from the toxic deaths, there was no treatment discontinuation because of toxicities, and EFS and PFS are identical. The events were toxic deaths (N = 3), progression during R-MPV (N = 1), and progression after transplant (N = 2).
PFS and OS. (A) PFS, all patients (N = 32). (B) OS, all patients (N = 32). (C) PFS and OS in transplanted patients. (D) PFS according to age (above 50 vs 50 and under). P = .05.
PFS and OS. (A) PFS, all patients (N = 32). (B) OS, all patients (N = 32). (C) PFS and OS in transplanted patients. (D) PFS according to age (above 50 vs 50 and under). P = .05.
The median OS (Figure 1B) has not been reached, and the 1-year OS is 88% (95% CI, 70-95). No deaths were observed beyond 2 years, with a 2-year, 3-year, and 5-year OS of 81% (95% CI, 63-91). The median follow-up of survivors is 45 months (range: 27-86).
Among the 26 transplanted patients, the 1-year PFS is 85% (95% CI, 64-94), and the 2-year, 3-year, and 5-year PFS estimates are 81% (95% CI, 60-92); the median PFS was not reached (Figure 1C). The 1-year OS is 88% (95% CI, 68-96), and the 2-year, 3-year, and 5-year OS estimates are 81% (60% to 92%); the median OS was not reached (Figure 1C).
The effects of age were analyzed according the MSK RPA class cutoff of 50.21 Patients age ≤50 tended to achieve superior PFS (P = .05, Figure 1D) and OS (P = .06), with no observed progression or death of any cause.
The outcomes of patients removed from study in spite of being eligible for transplant (N = 5) were as follows: 2 patients chose to receive high-dose cytarabine and no further treatment; 1 of those relapsed and received salvage chemotherapy and HDC-ASCT with TBC. The remainder 3 patients chose to receive WBRT (2 patients: 23.4 Gy; 1 patient: 45 Gy) and have never relapsed. All 5 patients remain alive, but as described previously, they were censored at the time of last on-study radiographic assessment for the purposes of PFS calculation, and therefore the reported PFS end points do not reflect these additional treatments.
Neuropsychological testing, QoL, and radiographic evaluation of neurotoxicity
No clinical neurotoxicity, defined as neurologic deterioration in the absence of disease progression, was reported by treating physicians. In addition, 16 progression-free patients participated in the neuropsychological and QoL evaluations (Figure 2 and supplemental Appendix).
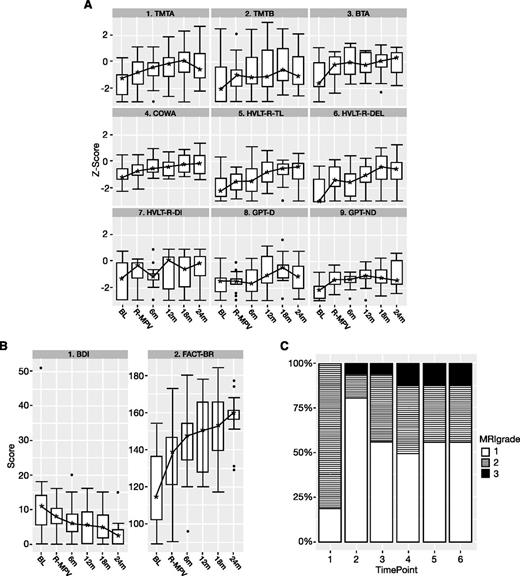
Neuropsychological testing, mood/QoL scores, and evaluation of white matter changes on MRI over time. Time points: 1, baseline; 2, after R-MPV and before transplant; 3, 4, 5, and 6, every 6 months after transplant. (A) Neuropsychological testing (z scores) boxplot. BL, baseline; BTA, Brief Test of Attention; COWA, Controlled Word Association Test; GPT-D, Grooved Pegboard Test–Dominant Hand; GPT-ND, Grooved Pegboard Test–Non-Dominant Hand; HVLT-R-DEL, Hopkins Verbal Learning Test-Revised–Delayed Recall; HVLT-R-DI, Hopkins Verbal Learning Test-Revised–Discrimination Index; HVLT-R-TL, Hopkins Verbal Learning Test-Revised–Total Learning; R-MPV, after induction chemotherapy; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B. The z scores ≤1.5 represent impairment. The lower and upper boundaries of each box represent the first and third quartiles (ie, the 25th and 75th percentiles), respectively, at the indicated time point, and the IQR is the distance between the lower and upper boundaries. The error bars (“whiskers”) extend from the first and third quartiles to the lowest and highest scores that are within 1.5 × IQR of their respective quartile. Any scores beyond the error bars are considered outliers and are represented as individual points. The asterisks represent the median scores. The lines connecting the medians over time for each test have no statistical interpretation and are intended to serve as visual aids. (B) BDI and FACT-BR (raw scores) boxplot. See panel A for boxplot explanation. (C) White matter abnormality scores (modified Fazekas scale) over time (N = 16). Fazekas scores: 0, no white matter abnormality; 1, minimal patchy white matter foci; 2, start of confluence of white matter disease; 3, large confluent areas; 4, confluence of white matter abnormalities with cortical and subcortical involvement; 5, diffuse leukoencephalopathy. No patient developed white matter abnormality scores of 4 or 5.
Neuropsychological testing, mood/QoL scores, and evaluation of white matter changes on MRI over time. Time points: 1, baseline; 2, after R-MPV and before transplant; 3, 4, 5, and 6, every 6 months after transplant. (A) Neuropsychological testing (z scores) boxplot. BL, baseline; BTA, Brief Test of Attention; COWA, Controlled Word Association Test; GPT-D, Grooved Pegboard Test–Dominant Hand; GPT-ND, Grooved Pegboard Test–Non-Dominant Hand; HVLT-R-DEL, Hopkins Verbal Learning Test-Revised–Delayed Recall; HVLT-R-DI, Hopkins Verbal Learning Test-Revised–Discrimination Index; HVLT-R-TL, Hopkins Verbal Learning Test-Revised–Total Learning; R-MPV, after induction chemotherapy; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B. The z scores ≤1.5 represent impairment. The lower and upper boundaries of each box represent the first and third quartiles (ie, the 25th and 75th percentiles), respectively, at the indicated time point, and the IQR is the distance between the lower and upper boundaries. The error bars (“whiskers”) extend from the first and third quartiles to the lowest and highest scores that are within 1.5 × IQR of their respective quartile. Any scores beyond the error bars are considered outliers and are represented as individual points. The asterisks represent the median scores. The lines connecting the medians over time for each test have no statistical interpretation and are intended to serve as visual aids. (B) BDI and FACT-BR (raw scores) boxplot. See panel A for boxplot explanation. (C) White matter abnormality scores (modified Fazekas scale) over time (N = 16). Fazekas scores: 0, no white matter abnormality; 1, minimal patchy white matter foci; 2, start of confluence of white matter disease; 3, large confluent areas; 4, confluence of white matter abnormalities with cortical and subcortical involvement; 5, diffuse leukoencephalopathy. No patient developed white matter abnormality scores of 4 or 5.
As shown in Figure 2A, at baseline, there was evidence of impairment in several cognitive domains, characterizing baseline disease burden. Results of LMM analysis showed significant positive linear time components (months since baseline) for the HVLT-R–Delayed Recall and HVLT-R–Discrimination Index (P < .05), indicating continuous improvement in scores from baseline over time. Likewise, all of the other tests had significant positive linear components up to 12 to 18 months posttransplant. However, a significant (TMT Part A, TMT Part B, Brief Test of Attention, HVLT-R–Total Learning; P < .05) or marginally significant (Controlled Word Association Test, GPT–Dominant Hand, GPT–Non-Dominant Hand; P < .10) quadratic time component was observed, suggesting that the rate of cognitive improvement slowed by 12 to 18 months posttransplant.
Self-reported QoL largely mirrored the improvement of cognitive function in the LMM analysis (Figure 2B). FACT-BR scores significantly improved from baseline, with slowed improvement by 12 to 18 months posttransplant; BDI scores significantly and linearly decreased over time.
Analysis of white matter abnormalities (Figure 2C) showed an improvement after R-MPV, with 81% of patients displaying scores 2 to 3 at baseline, compared with 19% after R-MPV (McNemar’s χ2P = .002). Following transplant, there was an increase in white matter abnormalities, with 44% of patients with scores 2 to 3 (McNemar’s χ2P = .046), which then remained stable over time. No scores above 3 were seen at any time.
Discussion
In this phase-2 study, patients with newly diagnosed PCNSL were treated with R-MPV chemotherapy, followed by consolidation HDC-ASCT with TBC in responding patients and no further treatment until progression. Favorable disease control was observed (2-year PFS, 75%; 2-year OS, 81%), far exceeding results of our previous experience with HDC-ASCT in PCNSL utilizing HD-MTX/cytarabine and BEAM.17 Importantly, no clinically significant neurotoxicity has developed to date, and QoL continuously improved over time.
A number of factors contributed to the favorable results observed in this trial, in comparison with our previous study (Table 5).17 The induction chemotherapy performed better, with a higher response rate (97%) that increased the number of patients undergoing HDC-ASCT (81%) and minimized disease burden prior to transplant (69% transplanted in CR). This excellent performance confirms our previous experience with R-MPV,6 which seems superior to results with MPV, although it remains difficult to determine the contribution of rituximab, currently being investigated in an ongoing randomized trial. Moreover, the TBC regimen, although more toxic, resulted in improved, durable disease control, likely reflecting higher CNS drug levels in comparison with BEAM.
Trials or case series (20 or more patients) reporting outcomes in newly diagnosed PCNSL patients treated with consolidation HDC-ASCT after methotrexate-based initial treatment
| Reference . | N . | Induction regimen . | ORR to induction . | HDC-ASCT regimen . | Received consolidation HDC-ASCT . | Received consolidation WBRT* . | ORR to HDC-ASCT . | Med F/u (mo) . | ITT 3-y PFS . | ITT 3-y OS . |
|---|---|---|---|---|---|---|---|---|---|---|
| Regimens including WBRT to all patients | ||||||||||
| 29 | 30 | MTX 8 g/m2, Ara-C, thiotepa | 80% | BCNU, thiotepa + WBRT | 23 (77%) | 21 (70%) | 100% | 63 | na | 69% |
| 33 | 25 | MTX 3 g/m2, BCNU, VP16, methylpred | 84% | BEAM + WBRT | 17 (68%) | 24 (96%) | 100% | 34 | 58% | 64% |
| Regimens with WBRT to patients achieving less than CR | ||||||||||
| 34 | 28 | MTX 8 g/m2 | 61% | Busulfan, thiotepa + WBRT if no CR | 16 (70%) | 8 (29%) | 69% | 15 | 45% | 48% (2 y) |
| Regimens without WBRT | ||||||||||
| 17 | 28 | MTX 3.5 g/m2, Ara-C | 57% | BEAM | 14 (50%) | 0 | 77% | 28 | 25% | 60% |
| This study | 32 | MTX 3.5 g/m2, rituximab, procarbazine, vincristine | 97% | Thiotepa, busulfan, CTX | 26 (81%) | 0 | 92% | 45 | 79% | 81% |
| Reference . | N . | Induction regimen . | ORR to induction . | HDC-ASCT regimen . | Received consolidation HDC-ASCT . | Received consolidation WBRT* . | ORR to HDC-ASCT . | Med F/u (mo) . | ITT 3-y PFS . | ITT 3-y OS . |
|---|---|---|---|---|---|---|---|---|---|---|
| Regimens including WBRT to all patients | ||||||||||
| 29 | 30 | MTX 8 g/m2, Ara-C, thiotepa | 80% | BCNU, thiotepa + WBRT | 23 (77%) | 21 (70%) | 100% | 63 | na | 69% |
| 33 | 25 | MTX 3 g/m2, BCNU, VP16, methylpred | 84% | BEAM + WBRT | 17 (68%) | 24 (96%) | 100% | 34 | 58% | 64% |
| Regimens with WBRT to patients achieving less than CR | ||||||||||
| 34 | 28 | MTX 8 g/m2 | 61% | Busulfan, thiotepa + WBRT if no CR | 16 (70%) | 8 (29%) | 69% | 15 | 45% | 48% (2 y) |
| Regimens without WBRT | ||||||||||
| 17 | 28 | MTX 3.5 g/m2, Ara-C | 57% | BEAM | 14 (50%) | 0 | 77% | 28 | 25% | 60% |
| This study | 32 | MTX 3.5 g/m2, rituximab, procarbazine, vincristine | 97% | Thiotepa, busulfan, CTX | 26 (81%) | 0 | 92% | 45 | 79% | 81% |
Number of patients who received WBRT as part of initial treatment, in the absence of tumor progression.
Ara-C, cytarabine; CTX, cyclophosphamide; Med F/u, median follow-up; methylpred, methylprednisolone; MTX, methotrexate; na, not available; ORR, objective response rate; VP-16, etoposide.
The TBC regimen was chosen based on favorable results observed in recurrent PCNSL.16,22-25 In a multicenter phase-2 study (N = 43),16 TBC was used following a cytarabine/etoposide (CYVE) salvage chemotherapy, achieving PFS of 12 months and OS of 18 months. Among transplanted patients (N = 27), PFS was 41 months. Three patients died of CYVE, but there were no transplant-related deaths, which is in line with other studies on TBC in CNS lymphomas24,25 and other malignancies,26-28 all reporting transplant-related mortality under 5%. In our study, treatment-related mortality appeared higher, but it is difficult to determine if this could be because of a higher susceptibility specific to PCNSL patients, or if it could reflect a less selected patient population, as compared with recurrent disease patients receiving TBC after surviving highly toxic salvage chemotherapies, such as CYVE. Also of interest is the fact that some patients in Soussain’s study16 were transplanted in spite of no response to CYVE and still survived longer (OS: 9 months) than CYVE-refractory patients who were not transplanted (OS: 5 months). This raises the intriguing question of whether the chemoresistance observed in recurrent CNS lymphoma after induction chemotherapies can be at least in part a consequence of the BBB reducing drug access, which could be overcome by the high chemotherapy doses afforded by transplant-based strategies. This question was not addressed in our study, given that only 1 patient progressed on R-MPV, and as per protocol design, she did not receive HDC-ASCT.
In addition to our previous study with BEAM, a number of trials have examined the use of HDC-ASCT in newly diagnosed PCNSL (Table 5). However, interpreting results is difficult because, unlike our trials, a significant proportion of patients in those studies also received WBRT, given either as adjuvant treatment post-ASCT to all patients or to patients who did not achieve a CR to induction. In a study of 30 patients, methotrexate, cytarabine, and thiotepa induction was followed by HDC-ASCT with BCNU/thiotepa, and hyperfractionated WBRT (45 Gy for CR and 50 Gy for PR).29,30 The 3-year OS was 69% in all patients, and 87% in transplanted patients. There was no neuropsychological evaluation, but 5 patients developed clinically defined neurotoxicity. That same group subsequently reported a small series (N = 13) using a similar strategy, but without WBRT if a CR was achieved, and the 3-year OS was 77%31 ; that study has been expanded,32 and final results are awaited. In another phase-2 study (N = 25), induction with MTX 3 g/m2, BCNU, etoposide, and methylprednisolone followed by HDC-ASCT (BEAM) and WBRT (30 Gy) achieved 3-year EFS of 58% and 3-year OS of 64%. There was no neuropsychological follow-up, but at least 1 patient developed neurotoxicity.33 A study in 28 patients used 2 doses of single-agent HD-MTX, followed by HDC-ASCT (busulfan/thiotepa), and WBRT 45 Gy if less than CR. Three of the 9 irradiated patients died of neurotoxicity, for a 2-year OS of 48%.34 Another study focusing on patients with primary and secondary CNS lymphoma of various histologies and previous treatments selected for transplant used rituximab combined with TBC as myeloablative regimen and found a favorable toxicity profile, with a 2-year PFS of 81%.35 Additional retrospective studies have been reported, adding to anecdotal experience with HDC-ASCT in CNS lymphoma.36-39
The lack of clinically detectable neurotoxicity is a favorable aspect of our regimen, but comprehensive neuropsychological evaluations are essential to fully characterize neurotoxic effects that may impair QoL in long-term survivors. In line with previous reports of R-MPV in PCNSL,6 results showed marked improvements in cognitive function and QoL following induction MTX chemotherapy, reflecting a decrease in disease burden. Following transplant, neuropsychological test scores remained overall stable, with self-reported QoL continuously improving. However, we found some indications of acute neurotoxicity following TBC, as exemplified by the increase in white matter abnormalities, and a mild, transient decrease in some neurocognitive scores at the first posttransplant evaluation (Figure 2A and supplemental Appendix). Moreover, the rate of cognitive improvement slowed after 12 to 18 months, and long-term follow-up is warranted to determine whether this could represent a trend toward development of late-delayed neurotoxicity.
Our study has some limitations. Inclusion criteria allowed for enrollment of patients regardless of performance status, and up to 72 years old, but our oldest patient was 67, and median age was 57, which is younger than the typical PCNSL population enrolled in non-ASCT trials; only 19% of patients had an MSK RPA class III. Therefore, results must be compared against other PCNSL transplant trials (Table 5), rather than nontransplant treatment strategies that are inclusive of a wider population of patients. Moreover, the R-MPV regimen has not been formally tested without a consolidation strategy such as WBRT or HDC-ASCT. Therefore, it is difficult to assess whether such patients with a more favorable prognosis40 could have achieved similar results with R-MPV alone and without any consolidation treatment. It is noteworthy that salvage treatments including TBC-based HDC-ASCT16,24 or even WBRT41,42 are also effective in this population and will require further investigation. Ongoing study Radiation Therapy Oncology Group 1114 is investigating R-MPV with and without reduced-dose WBRT and will provide further data on the relevance of consolidation treatments in newly diagnosed PCNSL. Finally, this was a single-institution trial, and even though we are reporting on the largest number of transplanted patients published to date (Table 5), the sample size remains small, and results need verification in a multicenter, randomized environment.
In summary, we report a prospective trial of HDC-ASCT in newly diagnosed PCNSL that was associated with excellent disease control, reflecting an optimized induction chemotherapy and a more effective myeloablative regimen. However, acute toxicities, including 3 toxic deaths, were observed, and longer neurocognitive follow-up is warranted for evaluation of late-delayed neurotoxicity. This treatment should still be considered experimental, and enrollment in randomized trials43 encouraged to establish the ultimate role and optimal timing of HDC-ASCT in the care of PCNSL patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank participants of the American Association for Cancer Research (AACR)/American Society of Clinical Oncology (ASCO) Vail Workshop on Methods in Clinical Cancer Research where the protocol was developed. Presented at the ASCO Annual Meeting 2012 (platform presentation).
This work was an investigator-initiated clinical trial sponsored by Memorial Sloan Kettering Cancer Center Department of Neurology Research and Development Funds and was conducted with no industry support. K.S.P. received research funding from National Institutes of Health, National Cancer Institute (grant P30 CA008748).
Authorship
Contribution: A.O., C.H.M., D.D.C., K.S.P., and L.E.A. conceived of and designed the trial; A.O. and G.F. performed collection and assembling of data; all authors performed analysis and interpretation of data; A.O. and G.F. wrote the manuscript; and all authors performed final approval of the manuscript.
Conflict-of-interest disclosure: L.E.A. is currently an employee at Roche. The remaining authors declare no competing financial interests.
The current affiliation for L.E.A. is Roche, Basel, Switzerland.
Correspondence: Antonio Omuro, Department of Neurology, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: omuroa@mskcc.org.