Key Points
Activated factor XI binds and proteolyzes tissue factor pathway inhibitor.
Activated factor XI promotes factor X activation generation and fibrin formation through the inactivation of tissue factor pathway inhibitor from platelets and on endothelial cells.
Abstract
Activation of coagulation factor XI (FXI) may play a role in hemostasis. The primary substrate of activated FXI (FXIa) is FIX, leading to FX activation (FXa) and thrombin generation. However, recent studies suggest the hemostatic role of FXI may not be restricted to the activation of FIX. We explored whether FXI could interact with and inhibit the activity of tissue factor pathway inhibitor (TFPI). TFPI is an essential reversible inhibitor of activated factor X (FXa) and also inhibits the FVIIa-TF complex. We found that FXIa neutralized both endothelium- and platelet-derived TFPI by cleaving the protein between the Kunitz (K) 1 and K2 domains (Lys86/Thr87) and at the active sites of the K2 (Arg107/Gly108) and K3 (Arg199/Ala200) domains. Addition of FXIa to plasma was able to reverse the ability of TFPI to prolong TF-initiated clotting times in FXI- or FIX-deficient plasma, as well as FXa-initiated clotting times in FX-deficient plasma. Treatment of cultured endothelial cells with FXIa increased the generation of FXa and promoted TF-dependent fibrin formation in recalcified plasma. Together, these results suggest that the hemostatic role of FXIa may be attributed not only to activation of FIX but also to promoting the extrinsic pathway of thrombin generation through inactivation of TFPI.
Introduction
Congenital deficiency of coagulation factor (F) XI is associated with postoperative or posttraumatic bleeding, especially in tissues with robust fibrinolytic activity.1 When blood is exposed to negatively charged surfaces in vitro, plasma FXII is converted to the protease FXIIa, which catalyzes the activation of FXI to FXIa, leading to the activation of FIX and subsequent thrombin generation. FXI can also be activated by thrombin or FXIa.2 Because FXII deficiency does not affect hemostasis, the hemostatic function of FXI may well be manifested through feedback activation by thrombin generated by the exposure of blood to tissue factor (TF).3 In addition to activation of FIX, FXIa can also promote thrombin generation through direct activation of FX, FV, and FVIII,4-6 suggesting it can support hemostasis even in the absence of FIX.
TF pathway inhibitor (TFPI), a Kunitz-type protease inhibitor, is the primary inhibitor of the TF/FVIIa/FXa complex and is essential for life.7 Endothelial cells and platelets are the primary cells producing TFPI.8,9 TFPI is also present in monocytes10 and circulates in plasma.11 The TFPI gene encodes several alternatively spliced products. TFPI-α has 3 Kunitz-type inhibitor domains (K1, K2, K3) and a basic C-terminal region. TFPI-β comprises the K1 and K2 domains attached to a glycosylphosphatidyl inositol-anchored C-terminal moiety. Endothelial cells secrete TFPI-α and express TFPI-β, whereas platelets only present the isoform TFPI-α. TFPI-β and TFPI-α reversibly inhibit FXa through the K2 domain and, in a FXa-dependent manner, the TF-FVIIa complex through the K1 domain,12,13 whereas the K3 domain has no known inhibitory function. TFPI-mediated inhibition of the procoagulant activity of TF is required for proper embryonic development and hemostasis. Reduced TFPI levels reversed the hemorrhagic defect and prolonged survival of TF-null mice expressing a low level of human TF.14 On the basis of the observation that members of the Kunitz-type class of inhibitors, such as protease nexin 2 and bovine pancreatic trypsin inhibitor (aprotinin), inhibit FXIa activity,15,16 and the fact that aprotinin is a close analog of TFPI, we hypothesized that TFPI interacts with FXIa. Here we demonstrate that TFPI binds FXIa and that FXIa proteolyzes recombinant TFPI and TFPI derived from platelets and on endothelial cells. Neutralization of endothelial cell TFPI by FXIa results in enhanced FXa and fibrin generation independent of the role of FXIa in the intrinsic pathway of blood coagulation. Neutralization of TFPI may represent a novel mechanism by which FXIa contributes to thrombin generation during hemostasis, as well as pathologic processes such as thrombosis.
Methods
Reagents
A list of reagents is included in the supplemental Methods, available on the Blood Web site.
Anti-FXI antibodies
The anti-factor XI antibody, 10C9, binds near the FXIa active site and inhibits FXIa cleavage of a chromogenic substrate (supplemental Figure 1). The anti-factor XI antibody, 12F5, also binds near the FXIa active site and inhibits FXIa cleavage of a chromogenic substrate.5 The anti-factor XI antibodies, 1A6 and 14E11, were generated as previously described.3,17
Cell surface immunoassays and western blotting
A detailed description can be found in the supplemental Methods.
Purification of human washed platelets
Human venous blood was drawn in accordance with an institutional review board-approved protocol from healthy donors and platelets purified as previously described.18 Platelets were stimulated with 0.5 U/mL thrombin and 2 µM A23187 for 15 minutes at 37°C. Subsequently, 10 U/mL hirudin was added to neutralize thrombin.
Characterization of FXIa binding and cleavage of recombinant full-length TFPI (rTFPI)
A detailed description can be found in the supplemental Methods.
FXa generation and activity
Innovin (1:100) was incubated with 50 pM FVIIa and 100 nM FX in the presence of 20 µL supernatant from 3 × 107 quiescent or activated platelets in 25 mM Hepes, pH 7.4, 150 mM NaCl (HBS) with 5 mM CaCl2, 25 µM ZnCl2 (HBS-Ca2+), and 0.3% bovine serum albumin (BSA) for 15 minutes at 37°C (final volume, 100 µL). HBS containing 100 mM EDTA was added to stop the reaction. Spectrozyme Xa was added, and the rate of substrate hydrolysis was measured at 405 nm and converted to FXa concentrations, using a standard curve. Alternatively, FXa (0.5 nM) in the presence of 20 µL supernatant from quiescent or activated platelets was added to Spectrozyme Xa (0.5 mM) in HBS and 0.3% BSA; hydrolysis was monitored at 405 nm for 1 hour.
Plasma clotting assay
Human platelet-poor plasma was prepared and clotting time measured as previously described.4 In selected experiments, samples were pretreated at room temperature for 10 minutes with the anti-FXI antibodies 1A6 (20 µg/mL), 10C9 (50 µg/mL), or 14E11 (20 µg/mL). Plasma (33% final) was incubated for 3 minutes in the presence of vehicle, activated partial thromboplastin time (aPTT) reagent (1:600 final), or FXIa (5 nM) in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered saline at 37°C. Subsequently, CaCl2 (8.3 mM final) and diluted Innovin (1:600 final) were added, and time to clot formation was measured. In selected experiments, FXI-, FIX-, or FX-depleted plasma was used.
Fibrin generation assay
Human umbilical vein endothelial cells (HUVECs) were grown to confluence in 96-well plates and incubated for 3 hours in serum-free medium with 0.3% BSA and 25 µM ZnCl2 containing 0.5 nM tumor necrosis factor α. HUVECs were then incubated for 2 hours with FXIa in serum-free medium and incubated for 10 minutes at 37°C with 50 µM aprotinin to stop the reaction. Alternatively, HUVECs were incubated with citrated plasma for 30 min, and reactions were stopped with aprotinin and corn trypsin inhibitor (CTI) (50 µg/mL). HUVECs were washed with HBS before a solution of citrated plasma (33% final) in the presence of 1A6 (20 µg/mL) was added and fibrin formation initiated with 8.3 mM CaCl2. Fibrin formation was measured as change in turbidity at 405 nm. The time interval required for the solution turbidity to reach the half-maximal value was defined as Thalf-max. In selected experiments, a solution of citrated plasma (33% final) and the chromogenic FXIIa and kallikrein substrate S-2302 (0.5 mM) was added in Ca2+-free HBS, and hydrolysis was measured at 405 nm.
Results
FXIa binds and cleaves TFPI
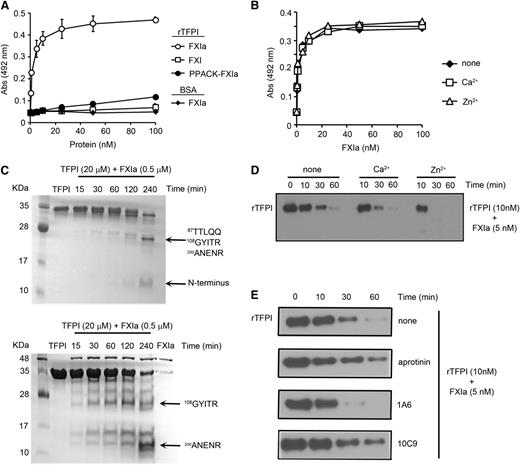
Binding studies were performed to characterize the interaction between FXIa and TFPI. FXIa bound to immobilized rTFPI in a FXIa concentration-dependent manner (Figure 1A). In contrast, neither active site-blocked FXIa (Phe-Pro-Arg chloromethylketone (PPACK)-FXIa) nor zymogen FXI bound immobilized rTFPI. FXIa binding to TFPI was unaffected by the presence of Ca2+ or Zn2+ (Figure 1B).
Characterization of the interaction between FXIa and rTFPI. (A) Ninety-six-well plates were coated with 5 µg/mL rTFPI or BSA (♦), and increasing concentrations of FXI (◱), FXIa (○,♦), or PPACK-FXIa (●) were added to selected wells. Binding was detected with a specific antibody against FXI. (B) Ninety-six-well plates were coated with 5 µg/mL rTFPI and increasing concentrations of FXIa in the absence (♦) or presence of 25 µM Zn2+ (△) or 1 mM Ca2+ (◱). Binding was detected with a specific antibody against FXI. (C) rTFPI (10 µg) was incubated with FXIa (500 nM) for selected times at 37°C before being separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under nonreducing (upper) or reducing (lower) conditions and analyzed by Coomassie blue staining. Protein bands were subjected to aminoterminal sequencing, using an automated Edman sequencer. Numbers refer to position of amino acid in full-length TFPI protein. (D) rTFPI (10 nM) was incubated with 5 nM FXIa for 10, 30, or 60 minutes in the presence or absence of 25 µM Zn2+ or 1 mM Ca2+. rTFPI was analyzed by western blotting with a polyclonal anti-TFPI antibody. (E) rTFPI (10 nM) was incubated with 5 nM FXIa for 10, 30, or 60 minutes in the presence or absence of 1A6 (20 µg/mL), 10C9 (50 µg/mL), or aprotinin (50 µM). rTFPI was analyzed by western blotting with a polyclonal anti-TFPI antibody.
Characterization of the interaction between FXIa and rTFPI. (A) Ninety-six-well plates were coated with 5 µg/mL rTFPI or BSA (♦), and increasing concentrations of FXI (◱), FXIa (○,♦), or PPACK-FXIa (●) were added to selected wells. Binding was detected with a specific antibody against FXI. (B) Ninety-six-well plates were coated with 5 µg/mL rTFPI and increasing concentrations of FXIa in the absence (♦) or presence of 25 µM Zn2+ (△) or 1 mM Ca2+ (◱). Binding was detected with a specific antibody against FXI. (C) rTFPI (10 µg) was incubated with FXIa (500 nM) for selected times at 37°C before being separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under nonreducing (upper) or reducing (lower) conditions and analyzed by Coomassie blue staining. Protein bands were subjected to aminoterminal sequencing, using an automated Edman sequencer. Numbers refer to position of amino acid in full-length TFPI protein. (D) rTFPI (10 nM) was incubated with 5 nM FXIa for 10, 30, or 60 minutes in the presence or absence of 25 µM Zn2+ or 1 mM Ca2+. rTFPI was analyzed by western blotting with a polyclonal anti-TFPI antibody. (E) rTFPI (10 nM) was incubated with 5 nM FXIa for 10, 30, or 60 minutes in the presence or absence of 1A6 (20 µg/mL), 10C9 (50 µg/mL), or aprotinin (50 µM). rTFPI was analyzed by western blotting with a polyclonal anti-TFPI antibody.
Incubation of rTFPI with FXIa in solution led to the disappearance of the TFPI band on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel and the appearance of lower molecular mass bands under nonreducing or reducing conditions (Figure 1C). N-terminal sequencing of the bands under nonreducing conditions revealed that the cleavages occurred between K1 and K2 domain (Lys86/Thr87) and within the active sites of the second and third K domains (K2-Arg107/Gly108 and K3-Arg199/Ala200). These sites are identical to those previously reported to be cleaved by plasmin.19
Western blot analyses of rTFPI under nonreducing conditions with a polyclonal anti-TFPI antibody demonstrated the disappearance of the broad band with increasing time in the presence of FXIa (Figure 1D). We found that the presence of Zn2+, but not Ca2+, increased the rate of TFPI proteolysis by FXIa, leading to the complete disappearance of TFPI after 30 minutes. The presence of aprotinin and the anti-FXI antibody 10C9, which binds near the active site of the FXIa, reduced the ability of FXIa to proteolyze TFPI (Figure 1E). In contrast, proteolysis of TFPI by FXIa was unaffected by the anti-FXI antibody 1A6, which binds the FXI A3 domain and inhibits FXI activation by FXIIa and FIX and FV activation by FXIa, or by anti-FXI antibody14 E11 (data not shown), which binds the FXI A2 domain and inhibits FXI activation by FXIIa (supplemental Figure 2).
FXIa inhibits the anticoagulant effect of rTFPI in plasma
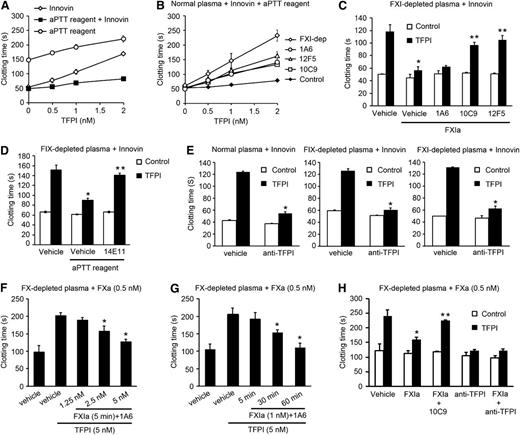
We tested the capacity of FXIa to inhibit the anticoagulant function of rTFPI in plasma. The addition of rTFPI increased the TF-induced clotting time of normal plasma from ∼50 seconds to ∼170 seconds (Figure 2A). Interestingly, when we added an aPTT reagent (ellagic acid) and TF to plasma, the subsequent addition of rTFPI only increased the clotting time from ∼50 seconds to ∼80 seconds, suggesting the activation of the contact pathway reduced the anticoagulant effect of rTFPI. To determine whether this effect was dependent on the activation of FXI by FXIIa, we performed a plasma clotting assay in the presence of 1A6. Under these conditions, the inhibitor activity of rTFPI was fully recovered (Figure 2B). An equivalent effect was observed when we used FXI-depleted plasma in the presence of the active-site blocking anti-FXIa antibodies, 10C9 or12F5, or in the presence of aprotinin (data not shown).
FXIa inhibits the anticoagulant role of TFPI in plasma. (A) Clotting times were measured for recalcified plasma after addition of TF (Innovin) or/and an aPTT reagent in the presence of increasing concentrations of rTFPI. (B) TF-induced clotting times were measured in recalcified plasma in the presence of an aPTT reagent and increasing concentrations of rTFPI. Plasma was pretreated with vehicle (♦), 20 µg/mL 1A6 (○), 50 µg/mL 10C9 (◱), or 50 µg/mL 12F5 (△). Selected experiments were performed in FXI-depleted plasma (FXI-dep) (♢). (C) TF-induced clotting times were measured in FXI-deficient plasma in the presence of vehicle (white bars) or 2 nM rTFPI (black bars). In selected experiments, FXIa (5 nM) was added to plasma in the presence of vehicle or 20 µg/mL 1A6, 50 µg/mL 10C9, or 50 µg/mL 12F5. *P < .05 with respect to vehicle in the presence of rTFPI. **P < .05 with respect to vehicle in the presence of rTFPI and FXIa. (D) TF-induced clotting times were measured in FIX-deficient plasma in the presence of vehicle (white bars) or 2 nM rTFPI (black bars). Selected experiments were performed in the presence of an aPTT reagent and/or 20 µg/mL 14E11. *P < .05 with respect to vehicle in the presence of rTFPI. **P < .05 with respect to vehicle in the presence of rTFPI and aPTT reagent. (E) TF-induced clotting times were measured in normal plasma, FIX-depleted plasma, or FXI-depleted plasma in the presence of vehicle or 2 nM rTFPI. Selected experiments were performed in the presence of blocking anti-TFPI K1 and K2 antibodies (10 µg/mL). *P < .05 with respect to vehicle in the presence of rTFPI. (F) FXa (0.5 nM)-induced clotting time was measured in FX-depleted plasma in the presence of vehicle or 5 nM rTFPI pretreated with 1.25, 2.5, or 5 nM FXIa for 5 minutes. (G) FXa (0.5 nM)-induced clotting time was measured in FX-depleted plasma in the presence of vehicle or 5 nM rTFPI pretreated with 1 nM FXIa for 5, 30, or 60 minutes. *P < .05 with respect to vehicle in the presence of rTFPI. (H) FXa (0.5 nM)-induced clotting time was measured in FX-depleted plasma in the presence of vehicle (white bars) or 5 nM rTFPI (black bars). Experiments were performed in the presence of 5 nM FXIa pretreated with 20 µg/mL 1A6. Selected experiments were performed in the presence of blocking anti-TFPI K1 and K2 antibodies (10 µg/mL) or 50 µg/mL 10C9. Data are mean ± standard error (n = 3). *P < .05 with respect to vehicle in the presence of rTFPI. **P < .05 with respect to FXIa in the presence of rTFPI. Mann-Whitney U test was used for statistical comparisons.
FXIa inhibits the anticoagulant role of TFPI in plasma. (A) Clotting times were measured for recalcified plasma after addition of TF (Innovin) or/and an aPTT reagent in the presence of increasing concentrations of rTFPI. (B) TF-induced clotting times were measured in recalcified plasma in the presence of an aPTT reagent and increasing concentrations of rTFPI. Plasma was pretreated with vehicle (♦), 20 µg/mL 1A6 (○), 50 µg/mL 10C9 (◱), or 50 µg/mL 12F5 (△). Selected experiments were performed in FXI-depleted plasma (FXI-dep) (♢). (C) TF-induced clotting times were measured in FXI-deficient plasma in the presence of vehicle (white bars) or 2 nM rTFPI (black bars). In selected experiments, FXIa (5 nM) was added to plasma in the presence of vehicle or 20 µg/mL 1A6, 50 µg/mL 10C9, or 50 µg/mL 12F5. *P < .05 with respect to vehicle in the presence of rTFPI. **P < .05 with respect to vehicle in the presence of rTFPI and FXIa. (D) TF-induced clotting times were measured in FIX-deficient plasma in the presence of vehicle (white bars) or 2 nM rTFPI (black bars). Selected experiments were performed in the presence of an aPTT reagent and/or 20 µg/mL 14E11. *P < .05 with respect to vehicle in the presence of rTFPI. **P < .05 with respect to vehicle in the presence of rTFPI and aPTT reagent. (E) TF-induced clotting times were measured in normal plasma, FIX-depleted plasma, or FXI-depleted plasma in the presence of vehicle or 2 nM rTFPI. Selected experiments were performed in the presence of blocking anti-TFPI K1 and K2 antibodies (10 µg/mL). *P < .05 with respect to vehicle in the presence of rTFPI. (F) FXa (0.5 nM)-induced clotting time was measured in FX-depleted plasma in the presence of vehicle or 5 nM rTFPI pretreated with 1.25, 2.5, or 5 nM FXIa for 5 minutes. (G) FXa (0.5 nM)-induced clotting time was measured in FX-depleted plasma in the presence of vehicle or 5 nM rTFPI pretreated with 1 nM FXIa for 5, 30, or 60 minutes. *P < .05 with respect to vehicle in the presence of rTFPI. (H) FXa (0.5 nM)-induced clotting time was measured in FX-depleted plasma in the presence of vehicle (white bars) or 5 nM rTFPI (black bars). Experiments were performed in the presence of 5 nM FXIa pretreated with 20 µg/mL 1A6. Selected experiments were performed in the presence of blocking anti-TFPI K1 and K2 antibodies (10 µg/mL) or 50 µg/mL 10C9. Data are mean ± standard error (n = 3). *P < .05 with respect to vehicle in the presence of rTFPI. **P < .05 with respect to FXIa in the presence of rTFPI. Mann-Whitney U test was used for statistical comparisons.
To determine whether the effect of FXIa on the inhibition of TFPI in plasma was independent of the ability of FXIa to activate FIX, 5 nM FXIa was added to FXI-depleted plasma, and coagulation was initiated by the addition of TF. We found that FXIa blocked the anticoagulant activity of 1 nM rTFPI on TF-initiated coagulation (Figure 2C). The presence of the FXI antibody, 1A6, which inhibits FIX activation, did not block the effect of FXIa, whereas addition of either of the FXIa active site domain-neutralizing antibodies, 10C9 or 12F5, inhibited the rTFPI-neutralizing effect of FXIa. In control experiments, in the absence of TF and rTFPI, the addition of FXIa to FXI-depleted plasma decreased the clotting time to ∼150 seconds; this effect was partially reversed by the addition of 1A6, 10C9, or12F5 (supplemental Figure 3). These results indicate that the inhibition of TFPI by FXIa is independent of the generation of FIX, based on the fact that 1A6 was not able to block the ability of FXIa to reverse the effect of TFPI. Moreover, the addition of an aPTT reagent to FIX-depleted plasma blocked the anticoagulant effect of rTFPI on TF-initiated coagulation (Figure 2D). Importantly, in the presence of 14E11, the anticoagulant activity of rTFPI was recovered. An inhibitory effect of blocking anti-TFPI antibodies was only observed when rTFPI was exogenously added to normal or FXI- or FIX-depleted plasma (Figure 2E) or when clotting was initiated with Innovin at a dilution lower than 1:10 000 (supplemental Figure 4), suggesting that at high TF concentrations, FXa generation is sufficiently fast to escape from inhibition by the TFPI pathway.7
FXIa is able to activate FX and FV.2,3 The anti-FXI antibody, 1A6, which inhibits the activation of FV by FXIa, is not able to block the generation of FX by FXIa. To determine whether the inhibitory effect of FXIa on the anticoagulant activity of TFPI in plasma was independent of the ability of FXIa to activate FX or FV, 0.5 nM FXa was added to FX-depleted plasma in the presence of 1A6. We found that the addition of FXIa blocked the anticoagulant activity of 5 nM rTFPI in this system (Figure 2F-G). This effect was reversed by the addition of a blocking anti-TFPI antibody or the presence of the FXIa active site domain-neutralizing antibodies, 10C9 (Figure 2H).
Protein S is a cofactor for TFPIα, serving to accelerate the inhibition of FXa.20 Our results show that the ability of FXIa to inhibit the anticoagulant activity of TFPI was not affected by the immunodepletion of protein S from plasma (supplemental Figure 5), suggesting the anti-TFPI activity of FXIa is independent of the function of protein S in plasma in this system.
FXIa inhibits the function of TFPI from platelets
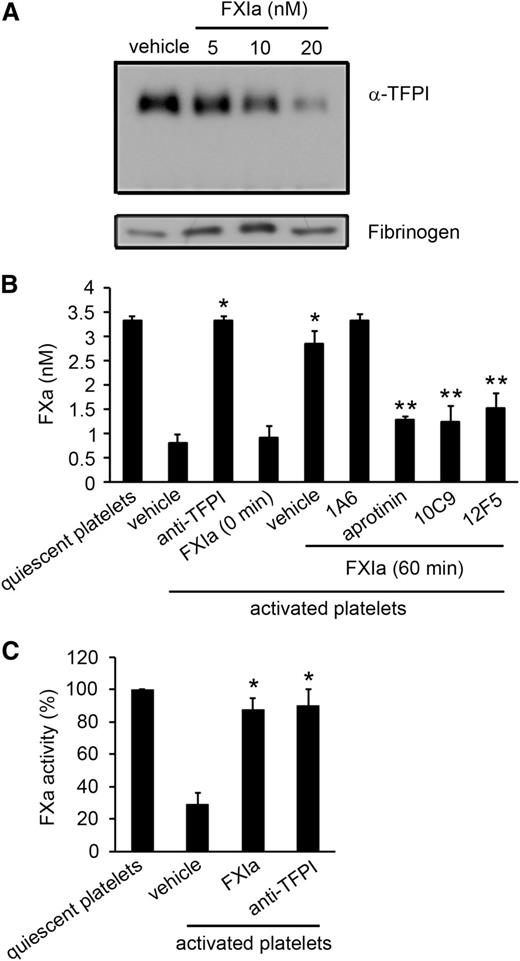
To determine whether FXIa was able to inhibit platelet-derived TFPI-α, we activated platelets with thrombin and a Ca2+ ionophore to induce degranulation and TFPI-α release. After incubation of platelet release with FXIa for 1 hour, we observed that the TFPI-α in the platelet supernatant decreased in a FXIa concentration-dependent manner (Figure 3A). We next established that supernatant from activated platelets decreased the activation of FX by the TF–FVIIa complex (Figure 3B). This effect was completely reversed after the incubation of platelet supernatant with FXIa. Equivalent results were observed after the incubation of the platelet supernatant with antibodies to the TFPI K1 and K2 domains. The addition of the active-site blocking anti-FXIa antibodies 10C9 or 12F5 eliminated the ability of FXIa to promote FXa generation. Equivalent results were observed in the presence of aprotinin. In contrast, the addition of 1A6 had no effect on the ability of FXIa to potentiate FXa generation.
FXIa inhibits the anticoagulant role of platelet TFPI. (A) Supernatant from activated platelets (2.5 × 108) was incubated with increasing concentrations of FXIa for 1 hour. The extent of TFPI present in the supernatant was analyzed by western blotting with a polyclonal anti-TFPI antibody, with levels of fibrinogen serving as a loading control. (B) FXa generation after initiation with tissue factor was determined in the absence or presence of supernatant from activated platelets. In selected experiments, platelet supernatant (3 × 107) was pretreated with 5 nM FXIa for 60 minutes in the presence of 1A6 (20 µg/mL), 10C9 (50 µg/mL), 12F5 (50 µg/mL), or aprotinin (50 µM). In separate experiments, platelet supernatant was incubated with blocking anti-TFPI K1 and K2 antibodies (10 µg/mL) ,or 5 nM FXIa was added immediately before assaying FXa generation (0 minutes). Aprotinin (50 µM) was added to stop the reaction. *P < .05 with respect to vehicle in the presence of supernatant from activated platelets. **P < .05 with respect to vehicle in the presence of supernatant from activated platelets and FXIa. (C) FXa activity was determined in the presence of supernatant from quiescent or activated platelets pretreated with or without 5 nM FXIa or blocking anti-TFPI K1 and K2 antibodies (10 µg/mL). After 1 hour of incubation with FXIa, aprotinin (50 µM) was added to stop the reaction. Data are mean ± standard error (n = 3). *P < .05 with respect to vehicle in the presence of supernatant from activated platelets. Mann-Whitney U test was used for statistical comparisons.
FXIa inhibits the anticoagulant role of platelet TFPI. (A) Supernatant from activated platelets (2.5 × 108) was incubated with increasing concentrations of FXIa for 1 hour. The extent of TFPI present in the supernatant was analyzed by western blotting with a polyclonal anti-TFPI antibody, with levels of fibrinogen serving as a loading control. (B) FXa generation after initiation with tissue factor was determined in the absence or presence of supernatant from activated platelets. In selected experiments, platelet supernatant (3 × 107) was pretreated with 5 nM FXIa for 60 minutes in the presence of 1A6 (20 µg/mL), 10C9 (50 µg/mL), 12F5 (50 µg/mL), or aprotinin (50 µM). In separate experiments, platelet supernatant was incubated with blocking anti-TFPI K1 and K2 antibodies (10 µg/mL) ,or 5 nM FXIa was added immediately before assaying FXa generation (0 minutes). Aprotinin (50 µM) was added to stop the reaction. *P < .05 with respect to vehicle in the presence of supernatant from activated platelets. **P < .05 with respect to vehicle in the presence of supernatant from activated platelets and FXIa. (C) FXa activity was determined in the presence of supernatant from quiescent or activated platelets pretreated with or without 5 nM FXIa or blocking anti-TFPI K1 and K2 antibodies (10 µg/mL). After 1 hour of incubation with FXIa, aprotinin (50 µM) was added to stop the reaction. Data are mean ± standard error (n = 3). *P < .05 with respect to vehicle in the presence of supernatant from activated platelets. Mann-Whitney U test was used for statistical comparisons.
We next determined whether FXIa degradation of platelet-derived TFPI-α decreased the FXa-inhibitory activity of TFPI. We measured the cleavage of Spectrozyme Xa by FXa in the presence of supernatant from either quiescent or activated platelets. The supernatant from activated platelets decreased the activity of FXa (Figure 3C). The effect was reversed in the presence of FXIa or anti-TFPI antibodies.
FXIa enhances the ability of HUVECs to initiate FXa generation and fibrin formation in plasma
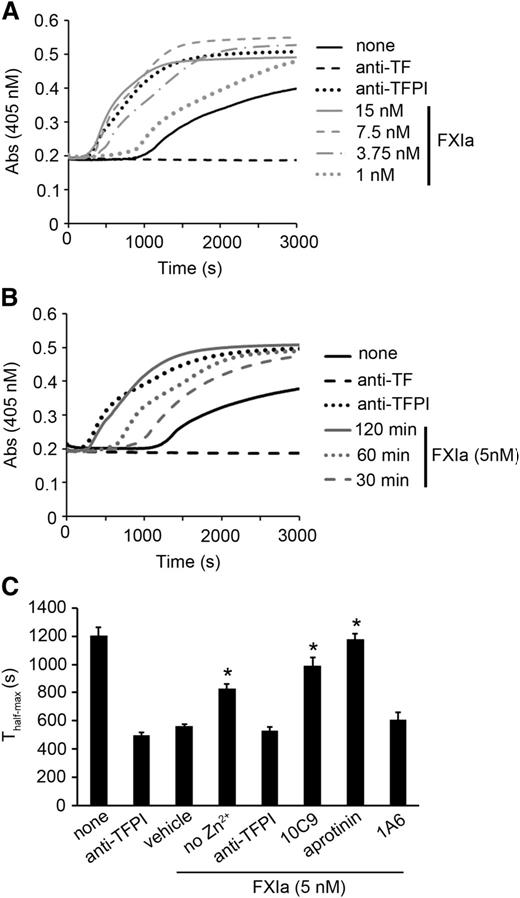
We tested the ability of FXIa to promote fibrin formation on HUVECs via inhibition of TFPI-β. HUVECs were preincubated with tumor necrosis factor α to induce TF expression, and fibrin generation was measured. In all experiments, plasma was pretreated with 1A6 to block the activity of the intrinsic pathway. We observed FXa generation and fibrin formation in plasma after ∼1000 seconds (Figure 4A; supplemental Figure 5B). The presence of a blocking anti-TF mAb delayed detectable FXa generation and fibrin formation beyond 3000 seconds. The addition of a blocking anti-TFPI mAb increased FXa generation and concurrently decreased the initiation time for fibrin generation from ∼1000 seconds to ∼100 seconds. The treatment of HUVECs with FXIa led to a similar increase in FX generation and a decrease in the initiation time for fibrin generation, while simultaneously increasing the rate of fibrin formation in a FXIa-concentration- and time-dependent manner (Figure 4A-B). The presence of FXIa did not affect the TF expression of level (supplemental Figure 6A) or EC confluence (supplemental Figure 7).
FXIa potentiates fibrin formation on HUVECs. (A) HUVECs were pretreated with increasing concentrations of FXIa for 2 hours; reactions were stopped with aprotinin (50 µM). Subsequently, recalcified plasma was added to HUVECs, and fibrin generation was measured in plasma pretreated with 20 µg/mL 1A6. (B) HUVECs were pretreated with vehicle or 5 nM FXIa for 30, 60, or 120 minutes; reactions were stopped with aprotinin (50 µM). Subsequently, recalcified plasma was added to HUVECs, and fibrin generation was measured in plasma pretreated with 20 µg/mL 1A6. Selected experiments were performed in the presence of blocking anti-TFPI K1 and K2 antibodies (50 µg/mL) or a blocking anti-TF antibody (10 µg/mL). (C) HUVECs were pretreated with vehicle or 5 nM FXIa for 2 hours in the presence or absence of 25 µM Zn2+, 50 µg/mL 10C9, 10 µg/mL anti-TFPI K1 and K2 antibodies, 20 µg/mL 1A6, or 50 µM aprotinin; reactions were stopped with aprotinin (50 µM). Subsequently, recalcified plasma pretreated with 20 µg/mL 1A6 was added to HUVECs, and the time for fibrin generation to reach half-maximal (Thalf-max) was measured. P < .05 with respect to vehicle in the presence of FXIa. Mann-Whitney U test was used for statistical comparisons.
FXIa potentiates fibrin formation on HUVECs. (A) HUVECs were pretreated with increasing concentrations of FXIa for 2 hours; reactions were stopped with aprotinin (50 µM). Subsequently, recalcified plasma was added to HUVECs, and fibrin generation was measured in plasma pretreated with 20 µg/mL 1A6. (B) HUVECs were pretreated with vehicle or 5 nM FXIa for 30, 60, or 120 minutes; reactions were stopped with aprotinin (50 µM). Subsequently, recalcified plasma was added to HUVECs, and fibrin generation was measured in plasma pretreated with 20 µg/mL 1A6. Selected experiments were performed in the presence of blocking anti-TFPI K1 and K2 antibodies (50 µg/mL) or a blocking anti-TF antibody (10 µg/mL). (C) HUVECs were pretreated with vehicle or 5 nM FXIa for 2 hours in the presence or absence of 25 µM Zn2+, 50 µg/mL 10C9, 10 µg/mL anti-TFPI K1 and K2 antibodies, 20 µg/mL 1A6, or 50 µM aprotinin; reactions were stopped with aprotinin (50 µM). Subsequently, recalcified plasma pretreated with 20 µg/mL 1A6 was added to HUVECs, and the time for fibrin generation to reach half-maximal (Thalf-max) was measured. P < .05 with respect to vehicle in the presence of FXIa. Mann-Whitney U test was used for statistical comparisons.
Previous studies have shown that Zn2+ facilitates FXIa–endothelial cell interactions.21 Our data show that the addition of Zn2+ decreased the rate of fibrin generation (Figure 4C) and increased FXa generation (supplemental Figure 6C) in the presence of FXIa. The procoagulant activity of FXIa was completely reversed by the addition of aprotinin, anti-TFPI antibodies, or 10C9. In contrast, the addition of 1A6 did not block the effect of FXIa. These results suggest that the inhibition of TFPI-β by FXIa promotes the procoagulant activity of ECs.
FXIa promotes the shedding of the K1 domain of TFPI-β expressed on HUVECs
On the basis of our observation that FXIa can cleave TFPI at Lys86, we next investigated whether FXIa was able to promote the release of the TFPI K1 domain from the EC surface. TFPI was detected in HUVEC lysates as a broad band at approximately 48 kDa on a western blot under nonreducing conditions (Figure 5A). After the pretreatment of HUVECs with FXIa, the 48-kDa band decreased and the appearance of a 38-kDa species was detected in the cell lysates in a FXIa concentration-dependent manner, suggesting that FXIa could release TFPI K1 domain from the endothelial cell surface. The effect of FXIa was abrogated by the addition of the serine protease inhibitor, PPACK. We next used a cell surface immunoassay to determine whether FXIa caused the loss of the TFPI K1 domain. The detection of the TFPI K1 domain on HUVECs with an anti-TFPI K1 domain antibody was reduced after incubation of the cells with increasing concentrations of FXIa (Figure 5B). Treatment of HUVECs with FXIa did not affect the binding of the polyclonal anti-TFPI antibody. These results suggest that FXIa cleavage of TFPI at Lys86 promotes the shedding of the K1 domain of TFPI on HUVECs.
FXIa cleaves the K1 domain of TFPI on endothelial cells. (A) HUVECs were pretreated with increasing concentrations of FXIa for 2 hours. Cell lysates were used for western blotting with a polyclonal anti-TFPI antibody under nonreducing conditions. (B) HUVECs were pretreated with increasing concentrations of FXIa for 2 hours, followed by cell surface detection of TFPI, using an anti-TFPI K1 antibody (●) or a polyclonal anti-TFPI antibody (○). (C) HUVECs were pretreated with 30 nM FXIa (black bars) or vehicle (white bars) for 2 hours in the presence or absence of 25 µM Zn2+, 50 nM HK, or 50 µM aprotinin, followed by cell surface detection of TFPI, using an anti-TFPI K1 antibody. P < .05 with respect to FXIa in the presence of Zn2+. (D) HUVECs were pretreated with increasing concentrations (0-50 nM) of FXIa (●), elastase (▪), plasmin (▲), kallikrein (◱), or FXIIa (○) for 2 hours, followed by cell surface detection of TFPI, using an anti-TFPI K1 antibody. Data are mean ± standard error (n = 3). Mann-Whitney U test was used for statistical comparisons.
FXIa cleaves the K1 domain of TFPI on endothelial cells. (A) HUVECs were pretreated with increasing concentrations of FXIa for 2 hours. Cell lysates were used for western blotting with a polyclonal anti-TFPI antibody under nonreducing conditions. (B) HUVECs were pretreated with increasing concentrations of FXIa for 2 hours, followed by cell surface detection of TFPI, using an anti-TFPI K1 antibody (●) or a polyclonal anti-TFPI antibody (○). (C) HUVECs were pretreated with 30 nM FXIa (black bars) or vehicle (white bars) for 2 hours in the presence or absence of 25 µM Zn2+, 50 nM HK, or 50 µM aprotinin, followed by cell surface detection of TFPI, using an anti-TFPI K1 antibody. P < .05 with respect to FXIa in the presence of Zn2+. (D) HUVECs were pretreated with increasing concentrations (0-50 nM) of FXIa (●), elastase (▪), plasmin (▲), kallikrein (◱), or FXIIa (○) for 2 hours, followed by cell surface detection of TFPI, using an anti-TFPI K1 antibody. Data are mean ± standard error (n = 3). Mann-Whitney U test was used for statistical comparisons.
Previous studies have shown that HK facilitates FXIa–endothelial cell interactions.21 After treatment of HUVECs with 30 nM FXIa for 2 hours, the addition of 25µM Zn2+, but not 50 nM HK, potentiated the cleavage of the K1 domain of TFPI, as evidenced by the reduced binding of the anti-TFPI K1 domain antibody to HUVECs (Figure 5C). Addition of the proteolytic enzyme inhibitor, aprotinin, abrogated the ability of FXIa to cleave the K1 domain of TFPI.
TFPI has been shown to be cleaved by serine proteases, such as elastase and plasmin.22,23 When compared with several other serine proteases at equivalent concentrations, the ability of FXIa to cleave TFPI K1 was found to be similar to the ability of elastase or plasmin to cleave the K1 domain of TFPI (Figure 5D). Neither kallikrein nor FXIIa appeared to cleave the K1 domain of TFPI on HUVECs.
Endogenous FXIa generated in plasma cleaves the K1 domain of TFPI-β and increases fibrin generation on HUVEC
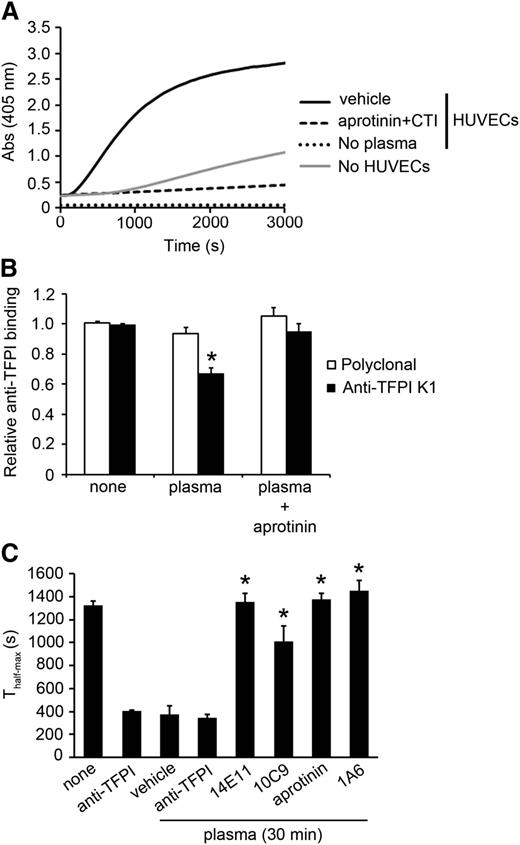
We studied the effects of endogenous generation of FXI by FXIIa in plasma on the ability of TFPI-β to inhibit fibrin formation on HUVECs. We analyzed whether HUVECs or the culture plate could act as a surface for induction of contact activation using a FXIIa/kallikrein chromogenic substrate (S-2302). As shown in Figure 6A, addition of citrated plasma to HUVECs resulted in hydrolysis of S-2302; this reaction was blocked by aprotinin and CTI, indicating that the contact pathway was activated by HUVECs. We then studied whether FXIa generated by FXIIa inhibited TFPI on HUVECs. Treatment of HUVECs with citrated plasma promoted the cleavage of the K1 domain from TFPI-β; this effect was reversed by the addition of aprotinin (Figure 6B). To determine whether endogenous FXIa generated in plasma by HUVECs promoted fibrin formation, HUVECs were preincubated with tumor necrosis factor α to induce TF expression, followed by incubation with citrated plasma for 30 minutes; reactions were then stopped with aprotinin and CTI. In selected experiments, citrated plasma was incubated with 14E11, 1A6, 10C9, or aprotinin. Cells were then washed, and fibrin generation of recalcified plasma was measured. 1A6 was included in the recalcified plasma to block the intrinsic pathway. As shown in Figure 6C, incubation of HUVECs with citrated plasma increased the rate of fibrin generation on recalcification. Pretreatment of citrated plasma with 14E11, 1A6, or 10C9 dramatically reduced the rate of fibrin generation. The presence of a blocking anti-TF mAb abrogated fibrin generation (data not shown), suggesting the endogenous FXIa generated by HUVECs in plasma reduced the anticoagulant activity of cultured ECs, likely via inhibition of TFPI-β.
Endogenous FXIa generation cleaves the K1 domain of TFPI and increases fibrin generation on HUVEC. (A) FXIIa and kallikrein generation in citrated plasma pretreated with vehicle or aprotinin (50 µM) and CTI (50 µg/mL) in the presence or absence of HUVECs was determined using S-2302 hydrolysis. (B) HUVECs were pretreated with citrated plasma for 30 minutes; reactions were stopped with aprotinin (50 µM) and CTI (50 µg/mL). Cell surface detection of TFPI was measured by an anti-TFPI K1 antibody or a polyclonal anti-TFPI antibody. P < .05 with respect to vehicle in the presence of the anti-K1 antibody. (C) HUVECs were pretreated with citrated plasma for 30 minutes in the presence or absence of anti-TFPI K1 and K2 antibodies (10 µg/mL), 14E11 (20 µg/mL), 1A6 (20 µg/mL), 10C9 (50 µg/mL), or aprotinin (50 µM); reactions were stopped with aprotinin (50 µM) and CTI (50 µg/mL). Subsequently, recalcified plasma pretreated with 20 µg/mL 1A6 was added to HUVECs, and the time for fibrin generation to reach half-maximal (Thalf-max) was measured. Data are mean ± SE (n = 3). P < .05 with respect to vehicle in the presence of plasma. Mann-Whitney U test was used for statistical comparisons.
Endogenous FXIa generation cleaves the K1 domain of TFPI and increases fibrin generation on HUVEC. (A) FXIIa and kallikrein generation in citrated plasma pretreated with vehicle or aprotinin (50 µM) and CTI (50 µg/mL) in the presence or absence of HUVECs was determined using S-2302 hydrolysis. (B) HUVECs were pretreated with citrated plasma for 30 minutes; reactions were stopped with aprotinin (50 µM) and CTI (50 µg/mL). Cell surface detection of TFPI was measured by an anti-TFPI K1 antibody or a polyclonal anti-TFPI antibody. P < .05 with respect to vehicle in the presence of the anti-K1 antibody. (C) HUVECs were pretreated with citrated plasma for 30 minutes in the presence or absence of anti-TFPI K1 and K2 antibodies (10 µg/mL), 14E11 (20 µg/mL), 1A6 (20 µg/mL), 10C9 (50 µg/mL), or aprotinin (50 µM); reactions were stopped with aprotinin (50 µM) and CTI (50 µg/mL). Subsequently, recalcified plasma pretreated with 20 µg/mL 1A6 was added to HUVECs, and the time for fibrin generation to reach half-maximal (Thalf-max) was measured. Data are mean ± SE (n = 3). P < .05 with respect to vehicle in the presence of plasma. Mann-Whitney U test was used for statistical comparisons.
Discussion
Mice lacking both FIX and FXI are more resistant to chemical injury-induced arterial thrombosis than are mice deficient in FIX alone.3 In vitro, FXIa is able to activate FX and shorten the aPTT clotting time of FIX-depleted plasma.2,3 Moreover, FXIa has been shown to promote thrombin generation by direct activation of FV and FVIII.3,6 These observations suggest that the role of FXIa in hemostasis and thrombus formation may include activities that bypass the FIX-mediated intrinsic pathway of thrombin generation. Here we show that FXIa binds to TFPI and inhibits its anticoagulant activity.
TFPI inhibits the early stages of the blood coagulation cascade through inhibition of the TF-FVIIa complex and FXa. TFPI is an alternatively spliced protein, and the major isoforms are TFPI-α and TFPI-β. TFPI-α, which has K1, K2, K3 domain and a basic C-terminal region, is stored within quiescent platelets and released after platelet activation. TFPI-α reversibly inhibits FXa through the K2 domain and, in a FXa-dependent manner, the TF-FVIIa complex through the K1 domain.12,13 Although the K3 domain lacks protease inhibitor activity, it is required for the binding of protein S, which acts as a cofactor for the inhibition of FXa by TFPI-α.24 We observed that FXIa cleaves TFPI-α from platelet releasate and inhibits its capacity to block the TF-FVIIa complex and FXa. We also observed that the anticoagulant effect of rTFPI-α in plasma was blocked by FXIa that was either generated endogenously in plasma by ellagic acid or directly added to plasma. We demonstrated that this effect of FXIa was independent of the generation of FXa, FVa, or FIXa by FXIa. On the basis of our data, the mechanisms by which FXIa inhibits TFPI-α may be explained by the fact that FXIa cleaves TFPI-α between the K1 and K2 domains (Lys86/Thr87) and at the active site of the K2 domain (Arg107/Gly108), which would block the capacity of TFPI-α to inhibit FXa and the TF-FVIIa complex. The interaction of protein S and TFPI requires K3 residues Arg199 and Glu226.25 The fact that FXIa also cleaves at the active site of the K3 (Arg199/Ala200) domain suggests that FXIa may block the access of TFPI-α to protein S and inhibit the capacity of protein S to enhance the inhibition of FXa by TFPI-α. In addition to proteolysis of TFPI, based on the efficiency of FXIa to block TFPI-α in plasma, our data suggest that the rapid binding of FXIa to TFPI may also directly block the access of TFPI-α to FXa. As the experiments performed for N-terminal sequencing required high enzyme/substrate ratios, future structure–function studies will be required to verify the physiological relevance of the specific FXIa-mediated cleavages.
The activation of endogenous FXI in plasma by the negatively charged substance ellagic acid blocked the anticoagulant effect of rTFPI-α, suggesting that activation of the contact pathway may promote thrombin generation in plasma through inhibition of TFPI. Physiological activators of the contact pathway, such as polyphosphate polymers (polyP) released from platelet-dense granules, have been shown to bind to FXI and enhance FXI autoactivation and FXI activation by thrombin in plasma.26 As polyP has been shown to inhibit the inhibitory effect of rTFPI on TF-induced clotting time in plasma,27 perhaps the inhibition of TFPI by polyP is at least partly a result of the ability of polyP to enhance FXIa generation. Because TFPI-α also binds to polyanions such as cell surface glycosaminoglycan heparan through its basic C-terminal region and neutrophil extracellular traps, perhaps TFPI-α also binds to polyP through its basic C-terminal region and acts as a cofactor for the inhibition of TFPI by FXIa.
TFPI-β is expressed on endothelial cells and comprises the K1 and K2 domains attached to a glycosylphosphatidyl inositol-anchored C terminus. We observed that the inhibition of HUVEC-derived TFPI-β by FXIa generated endogenously in plasma resulted in the loss of the K1 domain of TFPI-β and promotion of TF-dependent fibrin formation. The cleavage of TFPI between the K1 and K2 domain at Lys86 explains the appearance of the lower-molecular-weight bands under nonreducing conditions and the loss of the K1 domain of TFPI-β on HUVECs. We also observed that the inhibition of TFPI-β by FXIa was enhanced by Zn2+. Previous studies have shown that Zn2+ facilitates FXIa–endothelial cell or FXIa–platelet interactions.21,28 In addition, it has been shown that FXI binds to platelets through the A3 domain heparin-binding site (residues Lys252 and Lys253) in a Zn2+-dependent manner29 and that Zn2+ enhances FXI autoactivation and FXI activation by thrombin in the presence of glycosaminoglycans.30 Taken together, these data suggest that the binding of FXIa to glycosaminoglycans could enhance the capacity of FXIa to inhibit TFPI-β.
Complete TFPI deficiency induces a severe prothrombotic state that is incompatible with extrauterine life.31 Whether TFPI inhibits normal hemostasis is not known. However, TFPI appears to assume a pathologic role during hemostasis in patients with hemophilia. TFPI-blocking antibodies shorten the clotting time of FVIII-deficient plasma, and administration of TFPI-blocking antibodies improves hemostasis in hemophilic mice.32 It has been difficult to predict bleeding tendencies in FXI-deficient patients, as bleeding manifestations are poorly correlated with plasma FXI activity, and bleeding can be highly variable among patients with similar FXI levels. It has been reported that lower fibrin network density and lower clot stability distinguish bleeding risk in patients with severe FXI deficiency. Interestingly, in the same study, patients with severe FXI deficiency with a history of bleeding had higher levels of TFPI than asymptomatic patients.33 Perhaps FXI-deficient patients who have relatively mild bleeding problems have lower than normal TFPI levels that compensate for the absence of FXI.
During hemostasis, initiation of coagulation via the extrinsic pathway results in the activation of FX and FIX, leading to thrombin generation. FXI activation contributes to the amplification of thrombin generation through the intrinsic pathway. The contributions of both the extrinsic and intrinsic pathways can be downregulated in part by plasma or platelet-derived TFPI through direct inhibition of FXa. Here, we show that TFPI is a substrate of FXIa and that FXIa is capable of reversing the inhibitory function of platelet-derived TFPI-α, suggesting an additional mechanism by which FXIa may enhance FXa and thrombin generation during hemostasis. As the observed events in the clotting time and fibrin generation assays are specific to the conditions used, further studies would be required to define the effect of FXIa on the rate constants of thrombin generation through inhibition of TFPI function, as well as to determine whether these in vitro events occur in the wound environment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr George J. Broze, Dr Alan Mast, and Dr Jiaqing Pang for insightful comments and technical assistance.
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute grants (R01HL101972, R44HL106919) and an Oregon Clinical and Translational Research Institute grant UL1TR000128. O.J.T.M. is an American Heart Association Established Investigator (13EIA12630000). C.P. is an AHA Fellow (14POST18180011).
National Institutes of Health
Authorship
Contribution: C.P., E.I.T., D.G., A.G., and O.J.T.M. designed research and wrote the manuscript; C.P., A.M., Q.C., and K.D.Z. performed research; and C.P., E.I.T., D.G., A.G., and O.J.T.M. analyzed and interpreted data.
Conflict-of-interest disclosure: A.G., E.I.T., and Oregon Health & Science University have a significant financial interest in Aronora Inc., a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by the Oregon Health & Science University Conflict of Interest in Research Committee. The remaining authors declare no competing financial interests.
Correspondence: Cristina Puy, Department of Biomedical Engineering, Oregon Health & Science University, CHH-13B, 3303 SW Bond Ave, Portland, OR 97239; e-mail: puygarci@ohsu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal