Key Points
The THBD c.1611C>A mutation (p.Cys537Stop) causes extremely high soluble thrombomodulin levels resulting in trauma-related bleeding.
Soluble thrombomodulin acts by enhancing activated protein C generation and by impairing factor Va, factor VIIIa, and thrombin generation.
Abstract
We describe a family with an autosomal dominant disorder characterized by severe trauma- and surgery-related bleeding. The proband, who experienced life-threatening bleeding during a routine operation, had normal clotting times, but markedly reduced prothrombin consumption. Plasma levels of all coagulation factors and of the main coagulation inhibitors were normal. Thrombin generation at low triggers was severely impaired and mixing experiments suggested the presence of a coagulation inhibitor. Using whole exome sequencing, the underlying genetic defect was identified as the THBD c.1611C>A mutation (p.Cys537Stop), which predicts a truncated form of thrombomodulin that is shed from the vascular endothelium. The patient had decreased expression of endothelium-bound thrombomodulin, but extremely elevated levels of soluble thrombomodulin in plasma, impairing the propagation phase of coagulation via rapid activation of protein C and consequent inactivation of factors Va and VIIIa. The same thrombomodulin mutation has been recently described in an unrelated British family with strikingly similar features.
Introduction
Thrombomodulin (TM)1-3 is an endothelial transmembrane glycoprotein, encoded by the THBD gene and mainly expressed in the microvasculature. The mature protein (557 amino acids) comprises a lectin-like domain, 6 epidermal growth factor–like modules, a serine/threonine-rich region, a transmembrane domain, and a short cytoplasmic tail.4 TM binds thrombin and switches its substrate specificity from fibrinogen, factor V (FV), factor VIII (FVIII), and platelets to protein C and thrombin-activatable fibrinolysis inhibitor, enhancing the activation of these substrates ∼1000-fold.5 Activated protein C (APC) proteolytically inactivates FVa and FVIIIa, the essential cofactors of the prothrombinase and intrinsic tenase complexes, respectively.6,7
TM also circulates at a low concentration (<10 ng/mL) in plasma.8,9 Soluble TM (sTM) derives from proteolytic cleavage of membrane-bound TM10-12 and is elevated in several pathologic conditions associated with endothelial dysfunction.13,14
Here we describe an inherited bleeding disorder caused by extremely high levels of sTM in plasma.
Materials and methods
Blood collection
Investigations were conducted with the participants’ informed consent in agreement with the Helsinki Declaration. Blood was collected from the proband and 8 family members in 0.106 M trisodium citrate (1:9, v/v) and 18.3 µg/mL corn trypsin inhibitor (Haematologic Technologies, Essex Junction, VT). Platelet-poor plasma was prepared by double centrifugation at 2500g for 15 minutes. Normal pooled plasma was used as control.
Thrombin generation
Plasma was activated with tissue factor (TF), factor IXa (FIXa) or factor Xa (FXa), phospholipids (4 μM), and CaCl2 (16.6 mM). Thrombin generation was measured by calibrated automated thrombography15,16 and quantified as the area under the curve (endogenous thrombin potential, ETP). In some experiments, plasma was preincubated with antibodies against TM (Acris Antibodies, Herford, Germany) or protein C (Affinity Biologicals, Ancaster, ON, Canada) for 15 minutes at 37°C.
APC and FVa generation
Plasma was activated with TF (1 pM), phospholipids (4 μM), and CaCl2 (16.6 mM). For APC generation, timed subsamples were transferred to EDTA buffer containing hirudin (300 nM) and assayed for APC activity using chromogenic substrate S2366 (0.2 mM). For FVa generation, timed subsamples were assayed for FVa activity in a prothrombinase-based assay as described.17
Intrinsic FXa generation
Plasma was activated with FIXa (2 nM), phospholipids (4 μM), and CaCl2 (16.6 mM). Timed subsamples were assayed for FXa activity in a mixture containing prothrombin (400 nM), FVa (0.1 nM), phospholipids (5 μM), CaCl2 (5 mM), and the thrombin substrate S2238 (0.25 mM). The rate of substrate conversion is proportional to the FXa concentration.
TM enzyme-linked immunosorbent assay
Plasma sTM was quantified using the IMUBIND Thrombomodulin ELISA kit (Sekisui Diagnostics, Stamford, CT).
Genetic analysis
Genomic DNA was isolated from peripheral blood leukocytes. Whole exome sequencing was outsourced to the Department of Clinical Genetics of Maastricht University, Maastricht, the Netherlands. Single nucleotide polymorphism (SNP) genotyping for THBD haplotype analysis was performed using 5′ nuclease assays (rs1042580, rs3176123, rs1692) and direct sequencing (rs2007363).
Immunohistochemistry
Immunohistochemical staining was performed essentially as described previously,18 using mouse monoclonal antibodies against TM (Abcam, Cambridge, MA) or CD31 (DAKO, Glostrup, Denmark) as primary antibodies and an horseradish peroxidase–labeled goat anti-mouse immunoglobulin G as a secondary antibody.
Results and discussion
A 37-year-old French Caribbean woman developed acute intraabdominal bleeding during surgery for a hemorrhagic ovarian cyst, despite a negative personal history of bleeding and normal preoperative coagulation screening tests. Neither red blood cell transfusions, fresh frozen plasma, desmopressin, nor tranexamic acid could effectively stop the hemorrhage. Two days later, a bilateral ovariectomy was undertaken to control the bleeding, but the patient developed a life-threatening intraperitoneal hemorrhage and a large abdominal wall hematoma. Laparotomy was rapidly performed for surgical control of the bleeding and clot removal. Two liters of blood were drained from the hematoma and the patient was admitted to the intensive care unit with hemorrhagic shock. Bleeding was eventually stopped through the concomitant transfusion of platelet concentrates and fresh frozen plasma. The family anamnesis revealed that several of the patient’s relatives had experienced trauma- and/or surgery-related bleeding, suggesting an autosomal dominant genetic disorder (Figure 1A). However, evaluation of the patient’s hemostatic system failed to detect any abnormality of coagulation, platelet function, or fibrinolysis, except for markedly reduced prothrombin consumption (residual prothrombin 74% to 91%, normal value <10%).
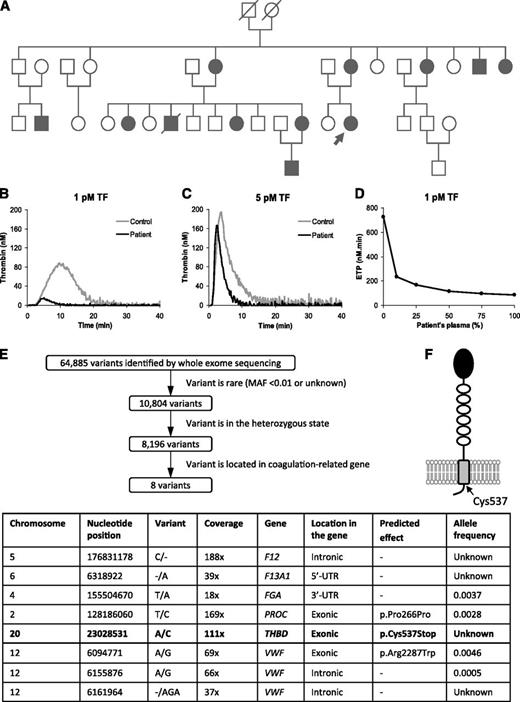
Characterization of the bleeding disorder. (A) Pedigree of the affected family. The proband is indicated by an arrow. Family members with a history of trauma- and/or surgery-related bleeding are marked by closed symbols. (B-D) Thrombin generation experiments. Plasma was activated with the indicated concentrations of TF, 4 μM phospholipids, and 16.6 mM CaCl2. Thrombin generation was measured using calibrated automated thrombography in the patient’s plasma (black lines) or control plasma (gray lines). (D) Mixtures of the patient’s and control plasma in different proportions were activated with 1 pM TF, and the ETP was plotted as a function of the percentage of patient’s plasma in the mixtures. (E) Analysis of the whole exome sequencing data. The 64 885 variants identified in the patient’s DNA were filtered for minor allele frequency (MAF) <0.01 (based on the assumption that the mutation responsible for this bleeding disorder would be rare), for heterozygosity (because of the dominant inheritance pattern of the disorder), and for location in coagulation-related genes. These criteria yielded 8 candidate variants (table), which were further analyzed individually. (F) Schematic representation of the TM molecule anchored to the endothelial membrane. The position of the Cys537 residue is indicated.
Characterization of the bleeding disorder. (A) Pedigree of the affected family. The proband is indicated by an arrow. Family members with a history of trauma- and/or surgery-related bleeding are marked by closed symbols. (B-D) Thrombin generation experiments. Plasma was activated with the indicated concentrations of TF, 4 μM phospholipids, and 16.6 mM CaCl2. Thrombin generation was measured using calibrated automated thrombography in the patient’s plasma (black lines) or control plasma (gray lines). (D) Mixtures of the patient’s and control plasma in different proportions were activated with 1 pM TF, and the ETP was plotted as a function of the percentage of patient’s plasma in the mixtures. (E) Analysis of the whole exome sequencing data. The 64 885 variants identified in the patient’s DNA were filtered for minor allele frequency (MAF) <0.01 (based on the assumption that the mutation responsible for this bleeding disorder would be rare), for heterozygosity (because of the dominant inheritance pattern of the disorder), and for location in coagulation-related genes. These criteria yielded 8 candidate variants (table), which were further analyzed individually. (F) Schematic representation of the TM molecule anchored to the endothelial membrane. The position of the Cys537 residue is indicated.
Thrombin generation was markedly decreased in the patient’s plasma (Figure 1B-C), especially at low TF (ETP 61 nM⋅min vs 835 nM⋅min in normal plasma), but also at higher TF (ETP 486 nM⋅min vs 1062 nM⋅min). Moreover, in mixing experiments, the patient’s plasma potently inhibited thrombin generation in normal plasma (Figure 1D), suggesting the presence of a coagulation inhibitor. Because thrombin generation triggered with FIXa (1 nM) or FXa (0.1 nM) was also severely impaired (not shown), we hypothesized a genetically determined excess or hyperactivity of 1 of the natural anticoagulants acting on FXa, FVa, and/or thrombin. However, the levels of antithrombin, protein C, protein S, and tissue factor pathway inhibitor were all normal in the patient’s plasma, prompting us to search for a different inhibitor.
Whole exome sequencing identified 64 885 variants in the patient’s DNA. Data analysis (Figure 1E) pointed at THBD c.1611C>A, introducing a premature stop codon in TM (p.Cys537Stop, Figure 1F) as the most promising candidate mutation. This was confirmed by Sanger sequencing, which showed complete cosegregation between this mutation and the bleeding phenotype in 8 family members. The same mutation has been recently described in a British family with strikingly similar features.19 However, THBD haplotype analysis indicated that the mutation resides on different haplotypes in the 2 families (Figure 2A), suggesting 2 independent mutational events.
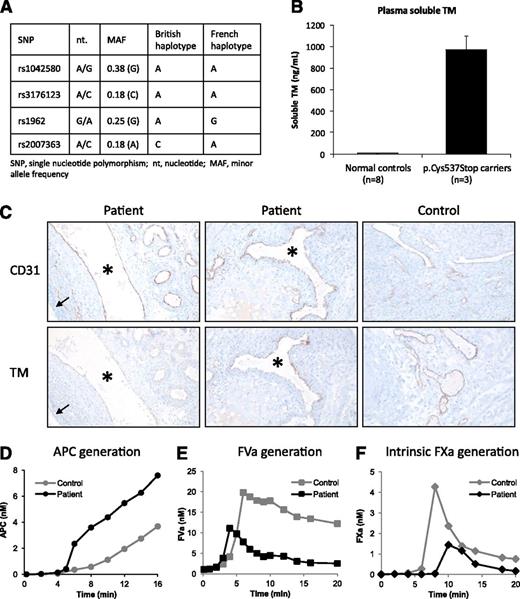
Functional consequences of the THBD c.1611C>A (p.Cys537Stop) mutation. (A) THBD haplotype analysis. Four haplotype-tagging SNPs (MAF ≥ 0.05, r2 threshold 0.8) capturing most of the common genetic variation in the THBD gene in the Caucasian population were identified using the Genome Variation Server of SeattleSNPs (http://gvs.gs.washington.edu/GVS138/). All available members of the British (n = 3) and French (n = 9) families with the c.1611C>A mutation were genotyped for these SNPs and the haplotype underlying the mutation in each family was reconstructed by allele segregation analysis. (B) Plasma concentrations of soluble TM in normal controls (n = 8) and in carriers of the p.Cys537Stop mutation (n = 3, proband and 2 family members). (C) Expression of membrane-bound TM in ovarian tissue. Histological sections of the patient’s ovarian tissue (left and middle panels) prepared at the time of her ovariectomy (1995) were stained for the endothelial marker CD31 and for TM, as indicated. Archival ovarian tissue obtained from a woman undergoing surgical resection at the same time as the patient, processed in the same way and stored in the same room for a comparable time, served as a normal control (right panels). (Left panels) A large venous vessel (*), associated with numerous smaller arterial and venous vessels, is visible in the ovarian stroma near a follicular ovarian cyst (arrow). CD31 is readily discernible along the endothelial lining of all visible vessels, whereas TM is barely detectable. (Middle panels) A large vessel (*) in the ovarian stroma is cross-sectioned. Although CD31 is detectable all along the endothelial lining, TM shows heterogeneous expression and is not detectable in the smaller adjacent vessels. (Right panels) CD31 and TM show strong and comparable apparent expression levels and their distribution is homogeneous along the endothelial linings. Immunoperoxidase technique followed by nuclear counterstaining with Mayer’s hematoxylin. Original magnifications: left and middle panels, ×250; right panels, ×350. (D) APC generation. Control plasma (gray circles) and the patient’s plasma (black circles) were activated with 1 pM TF, 4 μM phospholipids, and 16.6 mM CaCl2. At timed intervals, aliquots were removed and assayed for APC as described in “Materials and methods”. (E) FVa generation. Control plasma (gray circles) and the patient’s plasma (black circles) were activated with 1 pM TF, 4 μM phospholipids, and 16.6 mM CaCl2. At timed intervals, aliquots were removed and assayed for FVa as described in “Materials and methods”. Addition of a neutralizing anti-protein C antibody (not shown) abolished the difference in FVa generation between the patient’s and control plasma. (F) Intrinsic FXa generation. Control plasma (gray diamonds) and patient’s plasma (black diamonds) were activated with 2 nM FIXa, 4 μM phospholipids, and 16.6 mM CaCl2. At timed intervals, aliquots were removed and assayed for FXa as described in “Materials and methods”. Because the experiment was conducted at limiting FVIIIa concentrations, the generated FXa is proportional to the FVIIIa concentration.
Functional consequences of the THBD c.1611C>A (p.Cys537Stop) mutation. (A) THBD haplotype analysis. Four haplotype-tagging SNPs (MAF ≥ 0.05, r2 threshold 0.8) capturing most of the common genetic variation in the THBD gene in the Caucasian population were identified using the Genome Variation Server of SeattleSNPs (http://gvs.gs.washington.edu/GVS138/). All available members of the British (n = 3) and French (n = 9) families with the c.1611C>A mutation were genotyped for these SNPs and the haplotype underlying the mutation in each family was reconstructed by allele segregation analysis. (B) Plasma concentrations of soluble TM in normal controls (n = 8) and in carriers of the p.Cys537Stop mutation (n = 3, proband and 2 family members). (C) Expression of membrane-bound TM in ovarian tissue. Histological sections of the patient’s ovarian tissue (left and middle panels) prepared at the time of her ovariectomy (1995) were stained for the endothelial marker CD31 and for TM, as indicated. Archival ovarian tissue obtained from a woman undergoing surgical resection at the same time as the patient, processed in the same way and stored in the same room for a comparable time, served as a normal control (right panels). (Left panels) A large venous vessel (*), associated with numerous smaller arterial and venous vessels, is visible in the ovarian stroma near a follicular ovarian cyst (arrow). CD31 is readily discernible along the endothelial lining of all visible vessels, whereas TM is barely detectable. (Middle panels) A large vessel (*) in the ovarian stroma is cross-sectioned. Although CD31 is detectable all along the endothelial lining, TM shows heterogeneous expression and is not detectable in the smaller adjacent vessels. (Right panels) CD31 and TM show strong and comparable apparent expression levels and their distribution is homogeneous along the endothelial linings. Immunoperoxidase technique followed by nuclear counterstaining with Mayer’s hematoxylin. Original magnifications: left and middle panels, ×250; right panels, ×350. (D) APC generation. Control plasma (gray circles) and the patient’s plasma (black circles) were activated with 1 pM TF, 4 μM phospholipids, and 16.6 mM CaCl2. At timed intervals, aliquots were removed and assayed for APC as described in “Materials and methods”. (E) FVa generation. Control plasma (gray circles) and the patient’s plasma (black circles) were activated with 1 pM TF, 4 μM phospholipids, and 16.6 mM CaCl2. At timed intervals, aliquots were removed and assayed for FVa as described in “Materials and methods”. Addition of a neutralizing anti-protein C antibody (not shown) abolished the difference in FVa generation between the patient’s and control plasma. (F) Intrinsic FXa generation. Control plasma (gray diamonds) and patient’s plasma (black diamonds) were activated with 2 nM FIXa, 4 μM phospholipids, and 16.6 mM CaCl2. At timed intervals, aliquots were removed and assayed for FXa as described in “Materials and methods”. Because the experiment was conducted at limiting FVIIIa concentrations, the generated FXa is proportional to the FVIIIa concentration.
Cys537 is located at the end of the transmembrane domain of TM. Truncation at this position disturbs the transmembrane domain conformation and eliminates the cytoplasmic domain, causing shedding of the protein from the vascular endothelium. Accordingly, the concentration of sTM in the patient’s plasma (1120 ng/mL) was ∼180 times higher than in plasma from normal controls (6.3 ± 1.1 ng/mL) (Figure 2B), whereas expression of membrane-bound TM, as assessed by immunohistochemical staining of the patient’s ovarian tissue, was reduced (Figure 2C).
Thrombin generation in the patient’s plasma could be largely restored using neutralizing antibodies against TM or protein C (not shown), indicating that sTM is responsible for the patient’s hypocoagulable state and that its anticoagulant effects are (mainly) mediated by APC. Accordingly, detailed biochemical analysis showed increased APC generation (Figure 2D) and markedly reduced FVa and FVIIIa generation (Figures 2E-F) in the patient’s plasma. These findings account for the observed impairment of thrombin generation, especially at low triggers where FVIIIa is crucial to generate enough FXa (and thrombin) for the propagation phase. Moreover, they may explain why the patient’s hemorrhage could only be stopped by the concomitant administration of platelets (which contain partially activated20 and intrinsically APC-resistant21 FV) and fresh frozen plasma (a source of FVIII). Combining platelets and an FVIII concentrate might be clinically even more effective.
The absence of spontaneous bleeding in the patient is probably because sTM must form a complex with thrombin to activate protein C. Although the patient may have constitutively elevated circulating APC, trauma would trigger a sudden burst of APC generation via the thrombin formed at the site of injury, causing bleeding.
Why an increase in sTM at the expenses of the membrane-bound pool results in a bleeding tendency remains unclear, but might be explained by the possibility of sTM reaching high local concentrations at the site of injury, thereby hampering the transition from the initiation to the propagation phase. Whatever the mechanism, this represents a rare example of a bleeding disorder caused by a gain-of-function mutation in an anticoagulant factor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr M. Alhenc Gelas for her valuable help in collecting blood samples from the patient’s relatives living in Paris, Dr J. Vanoevelen and R. Kamps for running the whole exome sequencing, Professor T. Baglin for providing DNA samples from the British family with the same THBD mutation for haplotype comparison, and Dr G. Tans for helpful discussions.
This research was supported by the Association pour le Developpement et la Recherche en Hemostase Clinique, Lyon, France.
Authorship
Contribution: Y.D. was in charge of the clinical management of the patient, designed the study, performed research, and wrote the manuscript; J.Y.S. performed pathology and immunohistochemistry analyses; S.J.H.W. performed research; C.T. was in charge of the patient in 1997 and performed the routine hemostasis tests; T.M.H. designed part of the study and reviewed the manuscript; C.N. reviewed the manuscript; H.C.H. designed the study and reviewed the manuscript; T.L. designed the study, performed research, and reviewed the manuscript; E.C. analyzed the whole exome sequencing data, performed the haplotype analysis, and contributed to study design, data interpretation, and manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yesim Dargaud, Unite d’Hemostase Clinique, Hopital Cardiologique Louis Pradel, 28 Bd du Doyen Jean Lepine, F-69500 Bron, France; e-mail: ydargaud@univ-lyon1.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal