Key Points
Fucosylated Tregs persist for a longer time in vivo.
Fucosylated Tregs are able to prevent GVHD at a lower cell dose compared with untreated Tregs.
Abstract
Adoptive therapy with regulatory T cells (Tregs) to prevent graft-versus-host disease (GVHD) would benefit from a strategy to improve homing to the sites of inflammation. We hypothesized that adding fucose to human Tregs, forming the Sialyl Lewis X moiety on P-selectin glycoprotein ligand-1, would improve their trafficking pattern. The selectin pathway recruiter, α-1,3-fucosyltransferase-VI enzyme, significantly increased Treg surface fucosylation (66% vs 8%). In a xenogenic GVHD mouse model, fucosylated Tregs showed prolonged periods of in vivo persistence. When given at a lower dose compared with the untreated Tregs, the murine recipients of fucosylated Tregs maintained weight, had ameliorated clinical GVHD, and improved survival (70% vs 30%; P < .0001). These preclinical data indicate that fucosylated human Tregs is an effective strategy for prevention of GVHD and, as such, warrants consideration for future clinical trials.
Introduction
The optimal way to translate the adoptive therapy with regulatory T cells (Tregs) in preventing graft versus host disease (GVHD) in the clinic remains unclear because of their small number, constituting only 1% to 5% of adult peripheral blood (PB) or umbilical cord blood (CB).1-9 In a phase 1/2 clinical trial, large donor-to-donor variability in Treg populations was responsible for their inability to reach a clinically meaningful dose in 20% of patients and suggests that current ex vivo expansion strategies are not sufficient for broad application of this approach.2 Novel approaches to increase Treg potency are needed
Tregs bind to endothelial E- and P-selectins for trafficking to the sites of inflammation.10 E-selectin levels have been shown to be increased in patients with extensive GVHD.11 Incubation of CB hematopoietic progenitor cells with the enzyme fucosyltransferase-VI results in the addition of fucose to their cell surface to form a tetrasaccharide, sialyl Lewis X (sLeX),12 the moiety found on P-selectin ligand.13 Such treatment is effective in increasing their homing and engraftment in nonobese-diabetic/severe combined immunodeficiency interleukin (IL)-2Rγnull (NSG) mice14 and is currently being evaluated in the clinical setting.15 We hypothesized that ex vivo fucosylation would also improve Treg homing and enhance their anti-GVHD potency.
Study design
CB Treg isolation and expansion
As shown previously,16 CB Treg expansion was performed with CD3/28 coexpressing Dynabeads (ClinExVivo CD3/CD28; Invitrogen Dynal AS, Oslo, Norway) in the presence of IL-2 (CHIRON Corporation, Emeryville, CA).
Fucosylation of expanded Tregs
Ex vivo expanded Tregs were harvested and incubated in fucosylation solution (1/25 dilution of FTVI in 1 mM GDP Fucose, phosphate-buffered saline 1% human serum albumin) (Targazyme Inc, Carlsbad, California) for 30 minutes at room temperature and resuspended in phosphate-buffered saline. Fucosylation was characterized by the presence of sLeX residues, as assessed by flow cytometry with antibody HECA-452 (BD Biosciences, San Jose, CA), raised against cutaneous lymphocyte antigen (CLA), shown to be sLeX30. A portion of the cells were removed pre- and postfucosylation for flow staining with CLA, CD4, CD127, and CD25 antibodies.
Mixed lymphocyte reaction
Allogeneic mixed lymphocyte reaction (alloMLR) was performed using T-effector cells (Teffs) from PB mononuclear cells (PBMCs) isolated from 2 unrelated donors.17 CB Tregs (fucosylated or untreated) were added in the following Teffs:Tregs ratios: 1:1, 4:1, 8:1, 10:1, 40:1, 80:1, and 100:1. Cellular proliferation was measured by incorporation of 3H-thymidine. Results were measured using cell harvester (PerkinElmer, Waltham, MA) and a liquid scintillation counter (Packard Meriden, Prospect, CT). Results are expressed in counts per minute.
Xenogenic GVHD mouse model
Sublethally irradiated (320 cGY) NSG mice (Jackson Laboratory, Bar Harbor, ME) received intravenous injection of 107 human PBMCs16 and were followed every other day for weight loss, GVHD score,18 and survival. Mice were sacrificed for moribund features, as per institutional policy. Survival was measured by Kaplan-Meier method. The GVHD score and weights were compared using the paired analysis of variance test. Protocols were approved by the Institutional Animal Care and Use Committee and the Institutional Review Board.
Selectin binding assay
Functional group analysis of E-, P-, L-selectin binding was made by incubating the Tregs with Fc Chimera of recombinant human E-, P-, or L-selectin (R&D Systems, Minneapolis, MN). The selectin-bound cells were washed with phosphate-buffered saline and then incubated with DyLight 649 anti-human immunoglobulin G, Fc-γ fragment (Jackson ImmunoResearch, West Grove, PA). The cell population interacted with E-, P-, or L-selectin was then analyzed by flow cytometry.
Noninvasive bioluminescence imaging
Ex vivo expanded Tregs were labeled with a retroviral vector that expressed Firefly luciferase and enhanced green fluorescent protein (eGFP-FFLuc). Imaging was performed by injection of luciferin (20 mg/mL, 100 μL subcutaneously), followed by noninvasive whole-animal bioluminescence on an IVIS Spectrum imager, as shown before.16
Results and discussion
Fucosylation of CB Tregs leads to increased binding to E-selectin
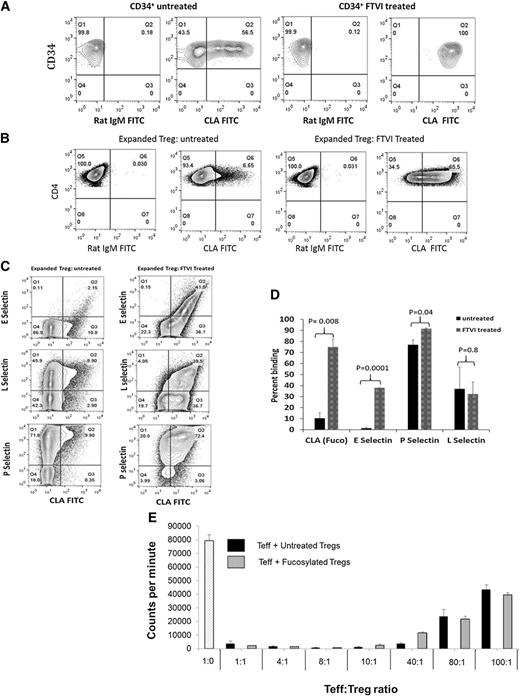
Ex vivo fucosylation of the expanded CB Tregs was performed on day 14. A portion of the cells was removed pre- and postfucosylation for flow staining with CLA, CD4, CD127, and CD25 antibodies. Successful fucosylation of CD34+ cells was performed as a positive control, where a relatively high level of baseline CLA positivity was observed in activated CB CD34+ cells (47% ± 7%) (Figure 1A). In contrast, the baseline CLA-positivity of expanded CB Tregs was low, with average levels of 8% ± 1.3%. On incubation with FTVI enzyme, the fucosylation levels of the expanded CB Tregs increased from 8% ± 1.3% to 66% ± 4.5% (n = 5; P < .00001) (Figure 1B). Varying degrees of fucosylation were seen on testing other cell types (supplemental Figure 1, available on the Blood Web site).
FTVI treatment increases Treg fucosylation. Fucosylation is characterized by the presence of sLeX residues, as assessed by flow cytometry, with antibody HECA-452 (BD Biosciences, San Jose, CA), raised against CLA, shown to be sLeX30. The left side of each contour plot shows the isotype control, and the right side the results of staining (percentage CLA-positive cells). (A) Positive control. CB CD34+ cells show an endogenous rate of 56.5% CLA+ cells that increases to 100% on treatment with FTVI enzyme. (B) Expanded Tregs. Untreated ex vivo-expanded Tregs show minimal endogenous fucosylation of 6.6% that increases to 65.5% on treatment with FTVI. (C) Fucosylation of Tregs leads to increased ability to bind E-selectin ligand. Expanded Tregs were treated with FTVI enzyme for 30 minutes, followed by biotin CLA–streptavidin phycoerythrin. Unlabeled selectins (E, P, L) with APC anti-Fc were used to study selectin binding ability in untreated Tregs (left) or fucosylated Tregs (right). Significant increase in the binding ability of fucosylated Tregs to E-selectin ligand was demonstrated (41% vs 2%) (upper). A high level of endogenous expression of P-selectin ligand was seen in untreated Tregs (81.7%) that increased on fucosylation (92.9%) (lower). No change was seen in the total L-selectin ligand expression (middle). (D) Increased E-selectin binding in fucosylated Tregs. Average values from 3 independent experiments show that the extent of fucosylation is related to the binding ability of E-, P-, or L- selectin. (E) Fucosylated Tregs suppress immune response in alloMLR similar to untreated Tregs. No differences in the degree of suppression of alloMLR was identified at the varying ratio of T-effector (Teff) and Treg (untreated or fucosylated). The x-axis denotes the varying ratio of Teff:Treg. Y-axis denotes counts per minute (mean ± SEM; n = 3).
FTVI treatment increases Treg fucosylation. Fucosylation is characterized by the presence of sLeX residues, as assessed by flow cytometry, with antibody HECA-452 (BD Biosciences, San Jose, CA), raised against CLA, shown to be sLeX30. The left side of each contour plot shows the isotype control, and the right side the results of staining (percentage CLA-positive cells). (A) Positive control. CB CD34+ cells show an endogenous rate of 56.5% CLA+ cells that increases to 100% on treatment with FTVI enzyme. (B) Expanded Tregs. Untreated ex vivo-expanded Tregs show minimal endogenous fucosylation of 6.6% that increases to 65.5% on treatment with FTVI. (C) Fucosylation of Tregs leads to increased ability to bind E-selectin ligand. Expanded Tregs were treated with FTVI enzyme for 30 minutes, followed by biotin CLA–streptavidin phycoerythrin. Unlabeled selectins (E, P, L) with APC anti-Fc were used to study selectin binding ability in untreated Tregs (left) or fucosylated Tregs (right). Significant increase in the binding ability of fucosylated Tregs to E-selectin ligand was demonstrated (41% vs 2%) (upper). A high level of endogenous expression of P-selectin ligand was seen in untreated Tregs (81.7%) that increased on fucosylation (92.9%) (lower). No change was seen in the total L-selectin ligand expression (middle). (D) Increased E-selectin binding in fucosylated Tregs. Average values from 3 independent experiments show that the extent of fucosylation is related to the binding ability of E-, P-, or L- selectin. (E) Fucosylated Tregs suppress immune response in alloMLR similar to untreated Tregs. No differences in the degree of suppression of alloMLR was identified at the varying ratio of T-effector (Teff) and Treg (untreated or fucosylated). The x-axis denotes the varying ratio of Teff:Treg. Y-axis denotes counts per minute (mean ± SEM; n = 3).
We next evaluated the effect of fucosylation on the selectin homing pathways used by T cells; namely, E-, P- and L-selectins. As compared with untreated Tregs, fucosylation of CB Tregs led to their increased binding ability to E-selectin compared with untreated controls (1.5% ± 0.5% vs 38% ± 0%; P = .0001) (Figure 1C-D). The binding ability of untreated vs fucosylated Tregs for P-selectin ligand was 77% ± 3% vs 91.5% ± 0.5% (P = .04). In contrast, no significant effects were observed for L-selectin ligand expression (37% ± 14% vs 32.5% ± 7.5%; P = .8), respectively. It is possible that the homing properties of Tregs permit superior survival as a result of local environmental effects as to where Tregs reside.
To quantify their suppressive function, we showed that in an alloMLR, both untreated and fucosylated Tregs exerted similar levels of suppression at different Treg ratios, demonstrating no significant advantage of fucosylation on their in vitro function (Figure 1E).
Fucosylated Tregs improve in vivo persistence, decrease GVHD, and increase overall survival in a xenogenic GVHD mouse model
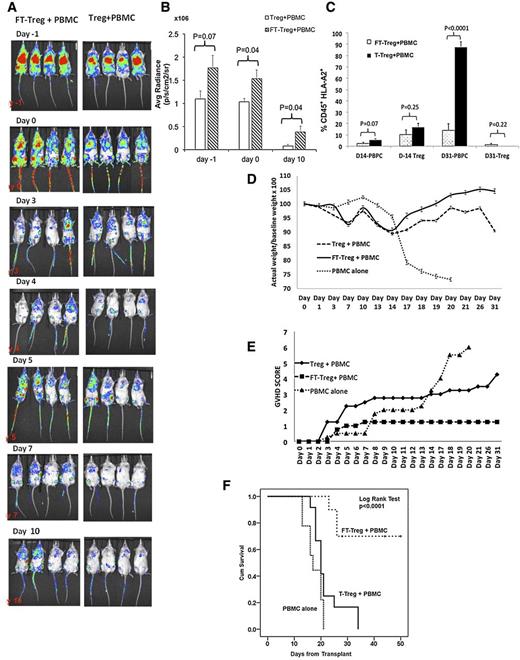
To study their in vivo persistence, ex vivo expanded Tregs were labeled with a retroviral vector that expressed Firefly luciferase and eGFP (eGFP-FFLuc), with a transduction efficiency of 20%, followed by division of the CB Tregs into 2 fractions, where 1 fraction was fucosylated and the other untreated. Both fractions were injected at 1 × 107 cells into sublethally irradiated NSG mice. The next day, mice were injected with PBMCs at 1 × 107 cells. Tregs were from an HLA-A2neg CB source and PBMCs were from an HLA-A2pos PB source. As shown in Figure 2A, the average bioluminescent radiance in the fucosylated Treg recipients was significantly higher than in the untreated Treg recipients on days 0 and 10 postinfusion (Figure 2B). The higher frequency of fucosylated vs untreated Tregs on day 10 was predictive of observed lower PB frequencies of GVHD-inducing CD45+HLA-A2+ PBMCs on day 31 posttransplant (14% vs 88%, respectively; P < .00001) (Figure 2C).
Fucosylated Tregs prevent xenogenic GVHD. (A) Longer in vivo persistence of fucosylated Tregs. Equal numbers (107 cells) of eGFP-FFLuc transduced fucosylated or untreated Tregs were injected in the NSG mice on day −1, followed by 107 PBMCs on day 0. Whole-animal luciferase imaging was performed on days −1, 0, 3, 4, 5, 7, and 1016. Continued persistence of fucosylated Tregs was demonstrated as compared with untreated Tregs. (B) On the quantification of the bioluminescence, significantly higher signal was seen on day 10 (P = .04). (C) A higher frequency of circulating PBMCs (CD45+HLA-A2pos) was seen on day 31 in the untreated Tregs cohort compared with the fucosylated Treg recipient. Fucosylated (FT) Treg cells prevent xenogeneic GVHD. Sublethally irradiated NSG mice received Treg cells or FT-Treg cells at a cell dose of 1 × 106 on day −1, followed by tail vein injection of PBMCs at a cell dose of 1 × 107 on day 0. Mice were monitored every other day for weight, GVHD score, and survival. Ten mice in each group from 3 different experiments were analyzed. (D). Weight. Fucosylated Treg recipients were able to maintain weight compared with Treg recipients and PBMC-only mice (P = .03). (E) Improved GVHD score was observed in the fucosylated Treg recipients (P = .009). (F) Significant improvement in overall survival was seen in fucosylated Treg recipients compared with untreated Tregs at 1 × 106 cell dose (P < .0001).
Fucosylated Tregs prevent xenogenic GVHD. (A) Longer in vivo persistence of fucosylated Tregs. Equal numbers (107 cells) of eGFP-FFLuc transduced fucosylated or untreated Tregs were injected in the NSG mice on day −1, followed by 107 PBMCs on day 0. Whole-animal luciferase imaging was performed on days −1, 0, 3, 4, 5, 7, and 1016. Continued persistence of fucosylated Tregs was demonstrated as compared with untreated Tregs. (B) On the quantification of the bioluminescence, significantly higher signal was seen on day 10 (P = .04). (C) A higher frequency of circulating PBMCs (CD45+HLA-A2pos) was seen on day 31 in the untreated Tregs cohort compared with the fucosylated Treg recipient. Fucosylated (FT) Treg cells prevent xenogeneic GVHD. Sublethally irradiated NSG mice received Treg cells or FT-Treg cells at a cell dose of 1 × 106 on day −1, followed by tail vein injection of PBMCs at a cell dose of 1 × 107 on day 0. Mice were monitored every other day for weight, GVHD score, and survival. Ten mice in each group from 3 different experiments were analyzed. (D). Weight. Fucosylated Treg recipients were able to maintain weight compared with Treg recipients and PBMC-only mice (P = .03). (E) Improved GVHD score was observed in the fucosylated Treg recipients (P = .009). (F) Significant improvement in overall survival was seen in fucosylated Treg recipients compared with untreated Tregs at 1 × 106 cell dose (P < .0001).
According to preclinical data, a 1:1 ratio of Treg:conventional T-cell is optimal for Tregs to effectively suppress an allogeneic reaction.3-5 We hypothesized that the greater persistence of fucosylated vs untreated Tregs would lead to superior GVHD protection by increasing the Treg:conventional T-cell ratio in vivo. We tested 10- to 1000-fold lower ratios of Tregs to PBMCs. Sublethally irradiated NSG mice were given fucosylated Tregs or untreated Tregs at a cell dose of 1 × 106 on day −1, followed by a 10-fold higher PBMC cell dose of 1 × 107 on day 0. Fucosylated Treg recipients regained their pretransplant body weight, in contrast to significant weight loss observed in the untreated Treg recipients (Figure 2D). Furthermore, an improved clinical GVHD score18 (5.7 vs 1.2; Figure 2E) was noted. Most important, at day 30, fucosylated Treg recipients had improved overall survival (70%) when compared with untreated Tregs (23%) and PBMCs alone (0%; P < .0001; Figure 2F). In preliminary experiments, improved survival was associated with fucosylated Tregs at 1 × 105 cell dose when comparing untreated Tregs and PBMCs alone (supplemental data; Figure 2). No significant survival difference was noted in the fucosylated Tregs recipients at a 1 × 104 cell dose (data not shown).
Therapeutic implications
If, as we demonstrate in this xenogeneic GVHD model, ex vivo fucosylation of expanded human CB Tregs can increase their persistence and anti-GVHD potency, this may allow more patients to benefit from the adoptive therapy with third-party CB-derived Tregs. Such benefits of ex vivo fucosylation may also be extended to Tregs derived from other donor products. Furthermore, by empowering the Tregs by fucosylation, we propose to potentially reduce GVHD as a result of improved homing and trafficking. Whether these benefits will be realized in patients receiving fucosylated Tregs will be addressed in a future phase 1 clinical trial.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work has been supported by the Anderson Cancer Center Start Up Funds and Lee Clark Award grants R01 HL11879, P01 CA065493, CA142106, and AI056299.
Authorship
Contribution: S.P., X.L., and A.N. designed research, conducted experiments, analyzed data, and participated in manuscript writing; and N.S., H.Y., E.Y., K.R., I.M., P.Z.-M., L.M., S.W., B.R.B., and E.J.S. designed research, analyzed data, and participated in manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simrit Parmar, University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: sparmar@mdanderson.org.