Key Points
The emergence of 3q26.2 rearrangements in CML is associated with resistance to TKI treatment and poor prognosis.
3q26.2 rearrangements play a predominant role in determining prognosis, irrelevant to the presence or absence of other additional chromosomal abnormalities in CML.
Abstract
Chromosome 3q26.2 abnormalities in acute myeloid leukemia, including inv(3)/t(3;3) and t(3;21), have been studied and are associated with a poor prognosis. Their prevalence, response to tyrosine kinase inhibitor (TKI) treatment, and prognostic significance in chronic myelogenous leukemia (CML) are largely unknown. In this study, we explored these aspects using a cohort of 2013 patients with CML diagnosed in the era of TKI therapy. Chromosome 3 abnormalities were observed in 116 (5.8%) of 2013 cases. These cases were divided into 5 distinct groups: A, inv(3)(q21q26.2)/t(3;3)(q21;q26.2), 26%; B, t(3;21)(q26.2;q22), 17%; C, other 3q26.2 rearrangements, 7%; D, rearrangements involving chromosome 3 other than 3q26.2 locus, 32%; and E, gain or loss of partial or whole chromosome 3, 18%. In all, 3q26.2 rearrangements were the most common chromosome 3 abnormalities (50%, groups A-C). 3q26.2 rearrangements emerged at different leukemic phases. For cases with 3q26.2 rearrangements that initially emerged in chronic or accelerated phase, they had a high rate of transformation to blast phase. Patients with 3q26.2 abnormalities showed a marginal response to TKI treatment, and no patients achieved a long-term sustainable response at a cytogenetic or molecular level. Compared with other chromosomal abnormalities in CML, patients with 3q26.2 rearrangements had poorer overall survival. The presence or absence of other concurrent chromosomal abnormalities did not affect survival in these patients, reflecting the predominant role of 3q26.2 rearrangements in determining prognosis. Interestingly, although heterogeneous, chromosome 3 abnormalities involving non-3q26.2 loci (groups D, E) also conferred a worse prognosis compared with changes involving other chromosomes in this cohort.
Introduction
BCR-ABL1 is the primary driver in chronic myelogenous leukemia (CML)1,2 and is the sole chromosomal abnormality in 80% to 90% of cases in chronic phase (CP).3 As the disease progresses, clonal evolution with additional chromosomal abnormalities (ACAs) occurs.4,5 The emergence of ACAs can provide BCR-ABL1-independent survival pathways, especially in cases of ACAs involving oncogenes or tumor suppressors.6,7 The most common ACAs in CML include trisomy 8, an extra copy of Philadelphia chromosome (Ph), i(17)(q10), and trisomy 19.3,8 These are so-called “major route” changes, as described in literature.9,10 Other less common ACAs are “minor route” changes. Although ACAs are considered an indicator of disease progression, the clinical effect of ACAs in CML is heterogeneous and depends on the particular types of cytogenetic changes. Some chromosomal changes simply reflect the genetic instability of CML induced by BCR-ABL1, whereas others can be leukemogenic and cooperate with BCL-ABL1 to induce blastic transformation.4,6 Factors that are also important and affect prognosis include the disease phase of ACAs emergence, treatment regimens,11-13 the presence or absence of other concurrent chromosomal changes, and other features of accelerated phase (AP).13,14 Of note, in the most recent version of European LeukemiaNet recommendations for the management of CML, the expert panel has included the emergence of major route, not minor route, cytogenetic changes as a criterion for AP,10 mainly because these major route ACAs are associated with a poorer prognosis,3,10,13,15 and also because of the lack of consistent data on the roles of minor route ACAs.
3q26.2 abnormalities have been well studied in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Inv(3)/t(3;3) is observed in approximately 1% to 2% of AML cases,16 and affected patients have the worst survival among patients with AML with recurrent genetic abnormalities recognized in 2008 World Health Organization classification.16,17 In patients with MDS, inv(3)/t(3;3) is also associated with a poor prognosis.18 A recent study demonstrated that patients with MDS with inv(3)/t(3;3) have a prognosis similar to patients with AML with inv(3)/t(3;3), regardless of blast percentage.19 In the Revised International Prognostic Scoring system for MDS, inv(3)/t(3q)/del(3q) was categorized in poor prognostic subgroups, along with −7/del(7q) and complex cytogenetics (3 or more abnormalities).20 Another 3q26.2 rearrangement, t(3;21)(q26.2;q22.1), is considered a MDS-related cytogenetic abnormality and is more commonly observed in therapy-related AML. Similar to inv(3)/t(3;3), t(3;21) is also associated with a poor prognosis.21,22 Other forms of 3q26.2 abnormalities are also present, but with a much lower occurrence rate.
The 3q26.2 locus contains the EVI1 oncogene, and gene rearrangements involving 3q26.2 induce aberrant EVI1 overexpression. It was not until recently that mechanisms linking 3q26.2 rearrangements and EVI1 expression were clarified. Two studies demonstrated that in cases with inv(3)/t(3;3), the balanced rearrangements bring the GATA2 enhancer in proximity with EVI1gene and induce EVI1 expression.23,24 Aberrant EVI1 expression is also seen in other balanced translocations involving 3q26.2, such as t(3;21), probably via similar mechanisms.16 EVI1, a protooncogenic transcription factor, induces leukemogenesis by regulating various downstream pathways involving hematopoietic differentiation and proliferation.25 A recent study demonstrated that mutations activating RAS/receptor tyrosine kinase pathway are coupled with EVI1 overexpression in 98% of inv(3)/t(3;3) cases and contribute to leukemic transformation.26
Although studies have shown that 3q26.2 abnormalities in AML are associated with an unfavorable prognosis, the role of 3q26.2 in CML is largely unknown. Only occasional single-case reports and small case series composed of few cases are available in the literature.27,28 In the current study, we assessed a large cohort of patients with CML diagnosed in the era of tyrosine kinase inhibitor (TKI) therapy and analyzed the prevalence, treatment response, and prognosis of patients with CML with various chromosome 3 abnormalities, particularly focusing on the 3q26.2/EVI1 locus.
Patients and methods
Case selection and clinical information collection
Patients with CML admitted in our hospital in the era of TKI therapy were included in this study. Only cases with available conventional karyotyping results were included. Fluorescence in situ hybridization and real-time reverse transcription polymerase chain reaction analyses for BCR-ABL1 were routinely performed in these cases. Of note, chromosomal changes in 1 metaphase were not counted as ACAs unless these chromosomal abnormalities were clonal in the previous karyotyping analysis or they became clonal in the follow-up karyotyping study. Chromosomal changes in Ph-negative cells were not considered as ACAs in this study. The corresponding clinical data, including age, sex, phases when ACAs emerged, treatment regimens, and response and follow-up data, were obtained. The study was approved by the institutional review board and conducted according to the Declaration of Helsinki.
Criteria for AP and blast phase
We followed the criteria recommended by the European LeukemiaNet10 ; similar criteria have been used in many other studies.3,4,11,13,29,30 In detail, AP is defined as any of the following criteria: 15% to 29% blasts in peripheral blood or bone marrow; 20% or more basophils in peripheral blood or bone marrow; 30% or more blasts plus promyelocytes in peripheral blood or bone marrow, with blasts less than 30%; platelets less than 100 × 109/L unrelated to therapy; or clonal evolution with ACAs. Blast phase (BP) is defined as 30% or more blasts in peripheral blood or bone marrow or extramedullary blast proliferation.
For the purpose of this study, we designated the emergence phase of ACAs on the basis of whether there are other concurrent AP features. In detail, we defined that ACAs emerged in CP when no other AP features were present, and ACAs emerged in AP when ACAs were accompanied with other AP features.
Definition of cytogenetic and molecular response
Survival analysis
Survival curves were built using the Kaplan and Meier method, and differences in survival were evaluated by the log-rank test. Overall survival (OS) was calculated using 2 different starting points: one from the emerging date of ACAs (OS after ACAs emergence), and another from the date of CML diagnosis (OS after CML diagnosis). The end point is the date of last follow-up or death. Fisher’s exact test was performed to evaluate the difference of various clinical characteristics among groups in Table 1.
Clinical characteristics of patients with CML with chromosome 3 abnormalities
| Parameter . | Group A: inv(3)/t(3;3) (n=30) . | Group B: t(3;21) (n=20) . | Group C: other 3q26.2 (n=8) . | Group D: not 3q26.2 (n=37) . | Group E: del/add (n=21) . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 14 (47%) | 15 (75%) | 5 (62%) | 22 (59%) | 13 (62%) |
| Female | 16 (53%) | 5 (25%) | 3 (38%) | 15 (41%) | 8 (38%) |
| Age, y | |||||
| Median | 54 | 52 | 58 | 49 | 49 |
| Range | 31-80 | 22-67 | 32-77 | 15-78 | 14-64 |
| Emergence phase | |||||
| CP | 13 (43%) | 4 (20%) | 2 (25%) | 17 (46%) | 3 (14%) |
| AP | 2 (7%) | 4 (20%) | 4 (50%) | 6 (16%) | 3 (14%) |
| BP | 15 (50%) | 12 (60%) | 2 (25%) | 14 (38%) | 15 (72%) |
| ACAs | |||||
| Sole | 14 (47%) | 7 (35%) | 2 (25%) | 15 (41%) | 2 (10%) |
| With others | 16 (53%) | 13 (65%) | 6 (75%) | 22 (59%) | 19 (90%) |
| TKI response | 26 | 15 | 6 | 31 | 17 |
| CCyR | 0 (0%) | 1 (7%) | 2 (33%)* | 13 (42%) | 4 (24%) |
| MMR | 0 (0%) | 1 (7%) | 1 (17%)* | 9 (29%) | 0 (0%) |
| Transplant after ACAs | 10 (33%) | 8 (40%) | 0 (0%) | 7 (19%) | 7 (33%) |
| F/U (m) | |||||
| Median | 13.3 | 13.6 | 10.3 | 31 | 5.6 |
| Range | 1.8-88 | 2.9-131.9 | 0.9-21.2 | 0.9-193 | 0.03-49.1 |
| Status at last F/U | |||||
| Dead | 24 | 19 | 7 | 28 | 17 |
| Persistent | 4 | 1 | 1 | 1 | 1 |
| Remission | 2 | 0 | 0 | 8 | 3 |
| Parameter . | Group A: inv(3)/t(3;3) (n=30) . | Group B: t(3;21) (n=20) . | Group C: other 3q26.2 (n=8) . | Group D: not 3q26.2 (n=37) . | Group E: del/add (n=21) . |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 14 (47%) | 15 (75%) | 5 (62%) | 22 (59%) | 13 (62%) |
| Female | 16 (53%) | 5 (25%) | 3 (38%) | 15 (41%) | 8 (38%) |
| Age, y | |||||
| Median | 54 | 52 | 58 | 49 | 49 |
| Range | 31-80 | 22-67 | 32-77 | 15-78 | 14-64 |
| Emergence phase | |||||
| CP | 13 (43%) | 4 (20%) | 2 (25%) | 17 (46%) | 3 (14%) |
| AP | 2 (7%) | 4 (20%) | 4 (50%) | 6 (16%) | 3 (14%) |
| BP | 15 (50%) | 12 (60%) | 2 (25%) | 14 (38%) | 15 (72%) |
| ACAs | |||||
| Sole | 14 (47%) | 7 (35%) | 2 (25%) | 15 (41%) | 2 (10%) |
| With others | 16 (53%) | 13 (65%) | 6 (75%) | 22 (59%) | 19 (90%) |
| TKI response | 26 | 15 | 6 | 31 | 17 |
| CCyR | 0 (0%) | 1 (7%) | 2 (33%)* | 13 (42%) | 4 (24%) |
| MMR | 0 (0%) | 1 (7%) | 1 (17%)* | 9 (29%) | 0 (0%) |
| Transplant after ACAs | 10 (33%) | 8 (40%) | 0 (0%) | 7 (19%) | 7 (33%) |
| F/U (m) | |||||
| Median | 13.3 | 13.6 | 10.3 | 31 | 5.6 |
| Range | 1.8-88 | 2.9-131.9 | 0.9-21.2 | 0.9-193 | 0.03-49.1 |
| Status at last F/U | |||||
| Dead | 24 | 19 | 7 | 28 | 17 |
| Persistent | 4 | 1 | 1 | 1 | 1 |
| Remission | 2 | 0 | 0 | 8 | 3 |
Age, age at ACA emergence; F/U (m): time from the emergence of ACAs to death or the last follow-up; Persistent, cytogenetic level and/or hematologic level; Remission, at least cytogenetic remission.
Transient response (see “Results” for detailed information).
Results
Frequency and types of chromosome 3 abnormalities in CML
In total, 2013 CML cases with available karyotypes were included. There were 843 (42%) females and 1170 (58%) males (females:males = 1:1.4). The median age was 48 years at the diagnosis of CML (range, 1-88 years). Six hundred eight patients (30%) had ACAs in addition to t(9;22), including 223 (37%) females and 385 (63%) males (females:males = 1:1.7), with a median age of 49 years at the diagnosis of CML (range, 8-86). Among 608 cases with ACAs, 116 (5.8%) had chromosome 3 abnormalities (Figure 1A). We divided these 116 cases into 5 groups: A, inv(3)(q21q26.2)/t(3;3)(q21;q26.2), n = 30, 26%; B, t(3;21)(q26.2;q22), n = 20, 17%; C, other 3q26.2 rearrangements, n = 8, 7%; D, rearrangements involving chromosome 3 other than 3q26.2 locus, n = 37, 32%; and E, gain or loss of partial or whole chromosome 3, n = 21, 18%. As shown in Figure 1A, 3q26.2/EVI1 was the most commonly involved locus in CML cases with chromosome 3 abnormalities (groups A-C: 58/116, 50%).
The distribution of chromosome (Chr) 3 abnormalities in CML (116 cases) and their effects on survival. CML cases with chromosome 3 abnormalities were divided into 5 groups (A), and their corresponding survival after the emergence of ACAs was analyzed in B.
The distribution of chromosome (Chr) 3 abnormalities in CML (116 cases) and their effects on survival. CML cases with chromosome 3 abnormalities were divided into 5 groups (A), and their corresponding survival after the emergence of ACAs was analyzed in B.
In group A, 27 (90%) of 30 cases presented as inv(3)(q21q26.2), and 3 (10%) presented as t(3;3)(q21;q26.2). Excluding inv(3)/t(3;3) (group A) and t(3;21) (group B), 3q26.2 occurred infrequently in other forms, as demonstrated in group C. In this group, 7 of 8 patients had reciprocal translocations, and the translocation partners for 3q26.2 were diverse, with 1 case each of t(3;12)(q26.2;p12), t(3;8)(q26.2;q24.1), t(3;5)(q26.2;q22), t(2;3)(p16;q26.2), t(3;11)(q26.2;q25), t(3;12)(q26.2;p13), and t(3;17)(q26.2;q22); the remaining case carried inv(3)(q26q27). In group D with chromosome 3 abnormalities other than 3q26.2, 31 (84%) of 37 patients had various reciprocal translocations, including 8 cases involving the 3q21 locus. The remaining 6 (16%) patients had various forms of derivative chromosomes. Group E consisted of cases with deletion and addition of partial or whole chromosome 3. Fifteen (71%) of 21 cases had deletion, and 6 (29%) cases had addition. Most cases in this group (90%; 19/21) had other concurrent ACAs than chromosome 3 abnormalities (Table 1), and the presence of other concurrent ACAs is more frequent when compared with cases in group D (22/37, 59%; P = .02) and cases in groups A-C (35/58, 60%; P = .01). The karyotypes of all cases in these 5 groups are listed in supplemental Table 1 (available on the Blood Web site).
Emergence phases of chromosome 3 abnormalities in CML
The clinicopathological characteristics of patients with CML with chromosome 3 abnormalities are summarized in Table 1. The emergence of chromosome 3 abnormalities occurred in different CML phases. 3q26.2 rearrangements (groups A-C) emerged in CP in 33% of cases, in AP in 17%, and in BP in 50%. Non-3q26.2 chromosomal 3 rearrangements (group D) emerged in CP in 46% of cases, in AP in 16%, and in BP in 38%. There is no significant difference (P = .41) in terms of the pattern of emergence phase between cases with 3q26.2 (groups A-C) and cases with non-3q26.2 rearrangements (group D). In contrast, in group E, chromosomal abnormalities emerged more frequently in BP (72%) when compared with cases in group D (38%; P = .03).
Cases with 3q26.2 rearrangements (groups A-C) had a high rate of transformation to BP when they initially emerged during CP or AP. Nineteen (33%) of 58 cases developed 3q26.2 rearrangements in CP. Three underwent allogeneic stem cell transplant. In the remaining 16 patients, 13 had adequate clinical information to evaluate the status of blast transformation, and all of them (13/13) transformed to BP after a median time of 3.5 months (range, 1.2-18.6 months). In the same groups (A-C), 10 patients developed 3q26.2 abnormalities in AP. During the follow-up, all 10 transformed to BP after a median time of 3.1 months (range, 1.0-19.0 months).
Treatment response of patients with CML with chromosome 3 abnormalities
The majority of patients (95/116; 82%) received TKI treatment and had adequate clinical follow-up after the emergence of chromosome 3 abnormalities. The response to TKIs was variable. In cases with 3q26.2 (groups A-C), only 3 patients (3/47, 6%) achieved CCyR during the course of TKI treatment; 2 of them had transient CCyR with a duration of 2.6 and 3.1 months, respectively (case C6 and C8 in supplemental Table 1). The third patient had a relatively longer CCyR (12.1 months) and later relapsed with the same chromosomal abnormality. Of note, this patient initially presented with a small 3q26.2 clone (2 of 25 metaphases) (case B8 in supplemental Table 1). Among these 3 patients with CCyR, 2 also had MMR with a similar duration time as CCyR. In comparison with cases with 3q26.2, cases with chromosome 3 rearrangements other than 3q26.2 (group D) showed a much higher cytogenetic response rate (CCyR: 42% vs 6% in 3q26.2 cases; P = .0003) and MMR rate (29% vs 4% in 3q26.2 cases; P = .005). When compared with group D, group E patients had a CCyR of 24% (P = .34) and a MMR of 0% (P = .02).
In patients with 3q26.2 rearrangements (groups A-C, Table 1), 2 patients were in remission at the last follow-up, 26 and 88 months after 3q26.2 emergence (case A9 and A28 in supplemental Table 1); 1 had 3q26.2/inv(3) in CP and another in BP. Although neither patient showed CCyR to TKIs treatment, both patients achieved remission after allogeneic stem cell transplant and remained in remission at the last follow-up.
Survival comparison among patients with different chromosome 3 abnormalities
We compared the OS after the emergence of chromosome 3 abnormalities among groups A-E. As shown in Figure 1B, patients with chromosome 3 rearrangements involving 3q26.2 (groups A- C) had worse survival than patients with chromosome 3 rearrangements other than 3q26.2 (group D), with a P value of .02, .04, and .01, respectively. Deletion/addition of chromosome 3 (group E) also showed worse survival than group D (P = .004). Compared with group E, patients in group A, B, and C did not show significantly difference in survival (P = .15 for A vs E; P = .1 for B vs E; P = .69 for C vs E). The median survival time after the emergence of ACAs in groups A-E was 14.3, 13.6, 11.9, 31, and 7.5 months, respectively.
Survival comparison between patients with 3q26.2 rearrangements and patients with ACAs other than chromosome 3
We analyzed the effect of 3q26.2 rearrangements on survival and compared their prognosis with cases with chromosomal abnormalities involving other chromosomes. In total, 492 (24%) of 2013 cases were identified to have aberrations involving chromosomes other than chromosome 3. Among these, 437 cases had adequate clinical data. The clinical characteristics of these patients are listed in supplemental Table 2. We first calculated the OS after ACA emergence, using the date of ACA emergence as the starting point. Patients with 3q26.2 rearrangements had a worse survival than patients with ACAs involving other chromosomes (P < .0001), with a 2-year OS of 22% and 60%, respectively (Figure 2A). Next we analyzed the survival after CML diagnosis, using the date of initial CML diagnosis as the starting time. Similarly, patients with 3q26.2 rearrangements had a worse survival than patients with ACAs involving other chromosomes (P < .0001), with a 5-year OS of 30% and 62%, respectively (Figure 2B). In multivariate analysis, after adjusting age, sex, trisomy 8, extra Ph, and i(17)(q10), the emergence of 3q26.2 rearrangements remains an independent adverse prognostic factor (P = .0001).
Survival comparison between CML with 3q26.2 abnormalities and CML with ACAs involving chromosomes other than 3. (A) Date of ACA emergence (ACAs, 3q26.2, red; ACAs, not Chr 3, black) was used as the starting point to calculate OS. (B) Date of initial CML diagnosis was used as the starting point to calculate OS. CML without any ACAs was also included in B for survival comparison.
Survival comparison between CML with 3q26.2 abnormalities and CML with ACAs involving chromosomes other than 3. (A) Date of ACA emergence (ACAs, 3q26.2, red; ACAs, not Chr 3, black) was used as the starting point to calculate OS. (B) Date of initial CML diagnosis was used as the starting point to calculate OS. CML without any ACAs was also included in B for survival comparison.
We also included cases without any ACAs as a control. In our study, 1405 cases were negative for ACAs. Their basic clinical characteristics are listed in supplemental Table 2. As expected, patients with 3q26.2 had a worse OS than patients without ACAs (P < .0001; 5-year OS after CML diagnosis, 30% vs 90%, respectively) (Figure 2B).
Survival comparison between patients with 3q26.2 as the sole ACA and patients with other single ACAs
Clonal evolution with ACAs in CML is often involved more than 1 chromosomal change, especially during the course of treatment. This confounds the study of the prognostic significance of individual chromosomal abnormalities. To eliminate this confounding factor, we analyzed CML cases that presented with a single ACA at initial development of ACAs. Twenty-three cases had 3q26.2 rearrangements as the sole anomaly, and 248 cases had other single ACAs with adequate clinical follow-up. The same strategies as described in Figure 2 were used to build survival curves. Comparison between these 2 groups demonstrated that isolated 3q26.2 rearrangements conferred a worse prognosis than other single ACAs (P < .0001), with a 2-year OS of 32% for patients with 3q26.2 rearrangement vs 70% for patients with other single ACAs after the ACAs emergence (Figure 3A) and 5-year survival of 19% vs 66% after the CML diagnosis (Figure 3B).
Survival comparison between patients with CML with 3q26.2 abnormalities as the sole ACA and patients with CML with other single ACAs.
Survival comparison between patients with CML with 3q26.2 abnormalities as the sole ACA and patients with CML with other single ACAs.
Survival comparison between patients with 3q26.2 as the sole ACA and patients with trisomy 8 as the sole ACA
As mentioned earlier, major route changes are associated with poorer survival in CML and regarded as a criterion for disease acceleration.10 Trisomy 8 belongs to major route changes and is one of the most common ACAs in CML. In this study, we compared the effect of isolated 3q26.2 and isolated trisomy 8 abnormalities on survival. In our cohort, 41 cases presented with trisomy 8 as the sole ACA at the time of initial ACAs emergence, and 37 of them had adequate clinical follow-up for survival analysis. Survival analysis demonstrated that 3q26.2 abnormality was associated with a much worse survival than trisomy 8 (Figure 4A-B).
Survival comparison between patients with CML with 3q26.2 abnormalities as the sole ACA and patients with CML with trisomy 8 as the sole ACA.
Survival comparison between patients with CML with 3q26.2 abnormalities as the sole ACA and patients with CML with trisomy 8 as the sole ACA.
Survival comparison between patients with 3q26.2 as the sole abnormality and patients with 3q26.2 plus other ACAs
To examine whether other concurrent chromosomal abnormalities contribute to the poor survival of cases with 3q26.2 aberrations, we divided cases with 3q26.2 rearrangements into 2 groups: 1 group with 3q26.2 rearrangements as the sole aberration (n = 23) and a second group with 3q26.2 rearrangements plus additional chromosomal aberrations (n = 35). As shown in Figure 5A-B, there is no survival difference between these 2 groups, indicating that the poor prognosis of patients with 3q26.2 rearrangements is not dependent on other concurrent chromosomal abnormalities. In contrast, for cases with ACAs involving chromosomes other than 3, cases with multiple ACAs (more than 1 abnormality) had a worse survival than cases with single ACAs (P < .0001 in Figure 5C; P = .0003 in Figure 5D).
The predominant role of 3q26 rearrangements in determining survival. (A-B) Survival comparison between patients with 3q26.2 alone and patients with 3q26.2 plus other ACAs. (C-D) In cases with ACAs involving chromosomes other than 3, survival was compared between cases with single ACAs and cases with multiple ACAs.
The predominant role of 3q26 rearrangements in determining survival. (A-B) Survival comparison between patients with 3q26.2 alone and patients with 3q26.2 plus other ACAs. (C-D) In cases with ACAs involving chromosomes other than 3, survival was compared between cases with single ACAs and cases with multiple ACAs.
Chromosome 7 deletion has been shown to be associated with 3q26.2 rearrangements, and a recent study showed that 37.3% of patients with MDS and AML with inv(3)/t(3;3) also had concurrent −7/del(7q).19 In this study, we analyzed the effect of concurrent chromosome 7 deletion on survival. For patients with inv(3)/t(3;3) (group A), 30% (9/30) had −7/del(7q). The occurrence rate was lower in other cases with 3q26.2 rearrangements: 10% (2/20) in patients with t(3;21) (group B) and 12.5% (1/8) in group C. The emergence of −7/del(7q) occurred before, concurrent with, or after the emergence of 3q26.2 rearrangements. Survival analysis showed there was no significance difference in survival in patients with or without concurrent −7/del(7q) (supplemental Figure 1).
The effect of chromosome 3 abnormalities other than 3q26.2 on CML survival
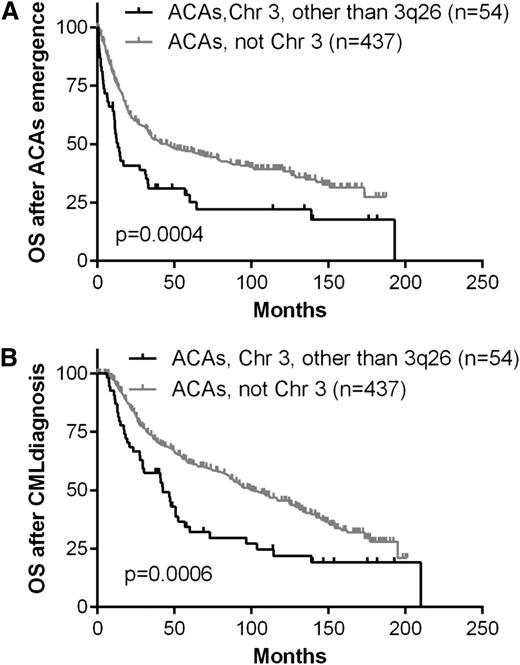
Patients with CML with chromosome 3 abnormalities other than 3q26.2 (groups D and E) are heterogeneous in terms of chromosomal aberrations, including various rearrangements, deletions, and additions. When compared with cases with ACAs other than chromosome 3, these patients also had a poor survival. The 2-year OS after emergence of ACAs was 39% compared with 60% in cases with ACAs other than chromosome 3 (P = .0004; Figure 6A). Their 5-year OS after CML diagnosis was 32% vs 62%, respectively (P = .0006; Figure 6B).
The effect of chromosome 3 abnormalities other than 3q26.2 on patients’ survival.
The effect of chromosome 3 abnormalities other than 3q26.2 on patients’ survival.
Discussion
In this study, we analyzed the frequency and clinical behavior of CML cases with chromosome 3 abnormalities, with a focus on 3q26.2 rearrangements. We demonstrated that 3q26.2 was the most common aberration in this cohort, frequently presented as inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and t(3;21)(q26.2;q22). These abnormalities arose at different phases, and for cases initially developing 3q26.2 rearrangements in CP and AP, they had a very high rate of transformation to BP. The emergence of 3q26.2 was associated with a poor response to TKI therapy and a poor clinical outcome. Rare patients (3%, 2/58; Table 1) achieved a relatively long-term remission (26 and 79 months at the last follow-up) after allogeneic stem cell transplant. Patients with 3q26.2 rearrangements (groups A-C; Table 1), as a group, had a poorer prognosis when compared with patients with chromosome 3 rearrangements other than 3q26.2 (group D; Figure 1B). Similarly, when compared with cases with ACAs involving other chromosomes, cases with 3q26.2 also had a poorer survival (Figures 2–4). Although commonly associated with other ACAs, 3q26.2 aberration plays a predominant role in determining prognosis, irrelevant to the presence or absence of other ACAs (Figure 5). When compared with group D, patients in group E with deletion or addition of chromosome 3 also showed a poorer prognosis (Figure 1B). This is likely a result of the higher frequency of the presence of other concurrent ACAs or complex karyotypes (90% in group E vs 59% in group D; P = .02; Table 1).
The poor prognosis of CML cases with 3q26.2 rearrangements is believed to attribute to EVI1 overexpression. The overexpression of EVI1 by 3q26.2 rearrangements in clonal evolution in CML offers a BCR-ABL1-independent oncogenic pathway. Studies had shown that the cooperation of BCR-ABL1 and EVI1 signaling can block myeloid differentiation and induce blast crisis.32 The presence of BCR-ABL1 independent signaling by EVI1 overexpression also explains the resistance of CML to TKI therapy, which is targeted to BCR-ABL1.
By conventional karyotyping, our current study demonstrated that 3q26.2/EVI1 abnormalities are present in about 3% (58/2013) of CML cases. The occurrence rate of EVI1 aberrant overexpression in CML, however, seems much higher. In a study by Ogawa et al, 10 (71%) of 14 cases of CML in BP had EVI1 mRNA overexpression.33 In CP, increased expression is also not uncommon. In a study of patients with CP CML who failed imatinib treatment, 8 (11%) of 75 patients had EVI1 overexpression.34 Thus, the rate of EVI1 overexpression at the mRNA level seems higher than 3q26.2/EVI1 abnormalities detected by conventional karyotyping. The mechanisms underlying this discordance may be diverse. In AML, Lugthart et al showed that 3q26.2 rearrangement can be cryptic and missed by conventional karyotyping, while being captured by fluorescence in situ hybridization studies in a subset of cases.35 Whether a similar scenario occurs in CML needs further investigation. Other mechanisms, such as gene amplification and transcriptional and translational induction, may also play roles in EVI1 aberrant expression. Arai et al demonstrated that EVI1 is transcriptionally regulated by mixed-lineage leukemia/lysine (K)-specific methyltransferase 2A (MLL/KMT2A) oncoproteins in hematopoietic stem cells.36 In a following study, Bindels et al showed that approximately 43% of all cases with MLL/KMT2A rearrangement are positive for EVI1 by real-time reverse transcription polymerase chain reaction, and EVI1 expression plays a critical role for the pathogenesis of a subset of AMLs with MLL-AF9 rearrangement.37 Similar to patients with EVI1 gene rearrangements, EVI1 overexpression without 3q26.2/EVI1 translocation is also associated with poor prognosis in patients with AML.38 In CML, Daghistani et al have shown that EVI1 overexpression is associated with imatinib resistance.34 The prognostic role of EVI1 overexpression in CML, however, is not systematically studied and needs more investigation.
The lack of response to TKI treatment in patients with CML with 3q26.2 rearrangements raises the issue of how to manage these patients. TKIs themselves are not sufficient to control the disease with 3q26.2 abnormalities. Intensive therapy, stem cell transplantation, or investigational therapy targeted to EVI1 should be considered. In this study, 18 (31%) of 58 cases with 3q26.2 rearrangements underwent stem cell transplant, and 2 patients achieved a long-term remission. All patients without transplant died at the last follow-up. Thus, it appears that a small subset of patients may benefit from stem cell transplant if they qualify clinically. EVI1 targeted therapy is a promising direction39 and needs further exploration.
In addition, in this study we showed that cases with chromosome 3 abnormalities other than 3q26.2 (groups D and E; Table 1) also had a worse survival than cases with ACAs involving other chromosomes (Figure 6). The mechanisms that mediate the poorer survival in these patients remain unknown and likely vary from case to case, as the chromosomal changes in these 2 groups are very heterogeneous (supplemental Table 1).
Studies have indicated that minor route cytogenetic changes, as a group, have no negative effect on TKI treatment response and prognosis of patients with CML.3 This may be oversimplified, as the group of minor route cytogenetic changes is very heterogeneous and composed of a variety of different chromosomal changes involving different chromosomes, and either balanced or unbalanced changes. As each individual chromosomal change in minor route is not common, it is difficult to analyze their prognosis individually, which may mask specific minor route changes that are associated with a poorer prognosis. Indeed, we recently showed that 11q23/MLL rearrangement, a rare minor route cytogenetic change in CML, is associated with poor prognosis.40 In the current study, we found that 3q26.2 aberration, although regarded as a minor route change, confers a worse prognosis than other ACAs, including trisomy 8, 1 of major route changes. In addition, the concurrent presence of other chromosomal changes in cases with 3q26.2 rearrangements did not affect the survival (Figure 5), indicating the predominant role of 3q26.2 in determining prognosis in these cases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: W.W. wrote the manuscript; W.W. and S.H. designed the study; W.W., D.A., and S.H. collected the data; and authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shimin Hu, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030; e-mail: shu1@mdanderson.org.