In this issue of Blood, Price et al report the results of a phase 3 prospective randomized trial comparing the clinical outcome of infected, severely neutropenic patients after receiving or not receiving granulocyte transfusions.1
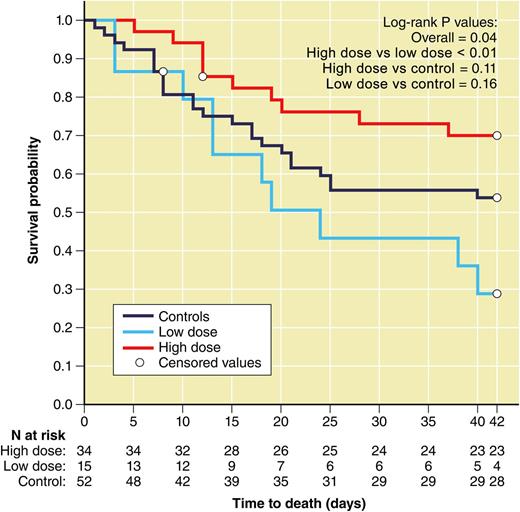
Survival to 42 days by dose group of patients enrolled in the RING trial. The high-dose group (n = 29) included all subjects in the transfusion arm who received a mean dose of ≥0.6 × 109 granulocytes/kg and transfusion and had data available on the primary outcome (red line); the low-dose group (n = 13) included all subjects in the transfusion arm who received a mean dose of <0.6 × 109 granulocytes/kg and transfusion and had data on the primary outcome (blue line); the control group for this comparison (n = 43) included subjects in the control arm who received no transfusions and had data on the primary outcome (black line). The figure is a modification of Figure 6 in the article by Price et al that begins on page 2153. Figure prepared by Patrick Lane, ScEYEnce Studios.
Survival to 42 days by dose group of patients enrolled in the RING trial. The high-dose group (n = 29) included all subjects in the transfusion arm who received a mean dose of ≥0.6 × 109 granulocytes/kg and transfusion and had data available on the primary outcome (red line); the low-dose group (n = 13) included all subjects in the transfusion arm who received a mean dose of <0.6 × 109 granulocytes/kg and transfusion and had data on the primary outcome (blue line); the control group for this comparison (n = 43) included subjects in the control arm who received no transfusions and had data on the primary outcome (black line). The figure is a modification of Figure 6 in the article by Price et al that begins on page 2153. Figure prepared by Patrick Lane, ScEYEnce Studios.
Functioning granulocytes are a vital component of the defense system against bacterial and fungal infections in humans. For patients undergoing stem cell transplantation or induction for acute leukemia, modern intensive chemotherapy often results in prolonged periods of neutropenia, a major risk factor for severe bacterial and fungal infections.2,3 Despite the administration of granulocyte colony-stimulating factor and appropriate antimicrobials, systemic infections in patients with neutropenia are associated with extended hospital admission, organ damage, and a significant mortality, which in some series reaches >20%.3 Clinical experience and data from animal studies suggest that control of infection in these patients requires the recovery of bone marrow neutrophil production.4
The first documented attempt to reverse neutropenia using granulocyte transfusions was documented in 1934.5 In the 1950s, Brecher et al gave granulocyte transfusions to neutropenic dogs and showed that the transfused cells migrated to the areas of infection,6 indicating that the content of the transfused product rapidly migrated into tissues and cleared from circulation. Although a significant enthusiasm developed for the use of granulocyte transfusions in the 1970s, the evidence for the clinical efficacy of high-dose granulocyte transfusion therapy has remained elusive despite significant advances in the granulocyte mobilization methods that optimized the quantity and viability of granulocytes collected.7,8
Several retrospective and early prospective studies have suggested that the efficacy of granulocyte transfusions in neutropenic patients may be proportional to the dose of granulocytes transfused. Doses of ≥1 × 109 granulocytes/kg body weight in daily transfusions seem to be required to treat or prevent infection.9 This dose requirement is difficult to achieve when heavier patients are treated because the usual level of granulocytes collected ranges from 10 to 70 × 109 granulocytes per transfusion.
Price et al report the results of the Resolving Infection in Neutropenia with Granulocytes (RING) trial. This was a 5-year, 14-center, randomized controlled clinical trial conducted as part of the National Heart, Lung, and Blood Institute's Transfusion Medicine/Hemostasis Clinical Trials Network, undertaken to evaluate the efficacy of granulocytes to treat infection in neutropenic patients. This trial represents a comprehensive attempt to test the hypothesis that granulocyte transfusions as typically performed in the clinical setting are beneficial.
The primary end point of this study was a composite of survival plus microbial response at 42 days, which enrolled adult and pediatric patients with severe neutropenia. Initially, only patients with chemotherapy-related neutropenia and a documented infection were enrolled. The eligibility criteria were changed (due to poor recruitment) after 31 months to include patients with presumed infection where the identification of the organism was not necessary and patients with neutropenia due to underlying marrow disease. Response for bloodstream infections was defined as a negative blood culture. For invasive bacterial or fungal infections, response was defined as resolution or evidence demonstrating clinical improvement. A stable infection was considered to be a failure. Microbial response was determined by a blinded adjudication panel. The study was carefully designed and excluded patients for whom a primary outcome could not be determined (modified intention-to-treat principle [MITT]), and in a secondary analysis, the study excluded patients who did not adhere to the assigned treatment (per-protocol [PP]). The study was designed to include 236 subjects to provide 80% power if the true success rates in the control and transfused groups were 50% and 70%, respectively. A total of only 114 subjects were enrolled (56 assigned to the granulocyte transfusion group and 58 to the control group). Transfused subjects received a median of 5 transfusions, with a mean transfusion dose of 54.9 × 109 granulocytes. Overall success rates for the granulocyte and control groups were 42% and 43% and 49% and 41%, respectively. Neither of these differences was statistically significant. Granulocyte transfusion therapy had no effect on the primary outcome, whether analyzed by the MITT or PP. There was no significant difference between treatment groups in a model of the primary outcome adjusted for prognostic factors such as ventilator use or Zubrod score. Neither were there any significant differences between the granulocyte and control arms in the primary end point success rates for any infection type or patient risk category, whether analyzed by MITT or PP. In addition, the 90-day survival was not affected by the assigned randomization group.
The interpretation of this study is firstly hampered by the low accrual rates associated with the intrinsic difficulties to recruit centers who believed that a randomized study was needed and secondly due to a lower than expected recruitment per participating center. Due to poor enrollment, the study only reached ∼47% power to detect the proposed difference, which may have resulted in missing a clinically positive effect by chance. The granulocyte dose was also lower than anticipated. The target of ≥40 × 109 granulocytes/kg was only achieved in 70% of subjects. In a post hoc analysis, subjects who received an average dose per transfusion of >0.6 × 109 granulocytes/kg tended to have better outcomes than those receiving a lower dose (see figure). Caution in this interpretation is warranted, however, because whether or not subjects received high-dose or low-dose transfusions was not a random occurrence and was largely site specific. Additionally, success in the control group was intermediate between that of the low-dose group and that of the high-dose group, although these differences were not statistically significant.
In summary, this study failed to provide a definitive answer on the efficacy of high-dose granulocyte transfusion therapy. Two questions arise. First, what should be the therapeutic attitude of clinicians? At this time, the only advice that can be provided is that if a decision is made to provide granulocyte transfusion therapy, it is important that clinicians determine whether procedures at the collection facilities are adequate to ensure that doses >0.6 (or ideally 1) × 109 granulocytes/kg are actually delivered. Second, what alternative methods can ensure that consistent, large doses of granulocytes can be delivered? The fact that we cannot generate enough viable granulocytes per dose or several doses a day per patient is a major handicap. The paradigm of mature blood cell transfusion that has helped so much in oncological therapy for transfusion of red cells and platelets may not be directly applicable to the transfusion of granulocytes. New products are in clinical and preclinical stages of development. These products include hematopoietic stem cell-derived ex vivo-expanded myeloid progenitors, which were shown to be efficacious in animal models of congenic transfusion.10 Whether these myeloid progenitors are efficacious in humans as off-the-shelf products remains to be seen in ongoing multicenter phase 2 trials for allogeneic transfusion. Alternatively, phenotypically typed functional granulocytes from pluripotent stem cells, which are theoretically expandable to much larger amounts than those obtained from primary hematopoietic stem cells, are in the preclinical phase. Stay tuned for future developments in this area!
Conflict-of-interest disclosure: The authors declare no competing financial interests.