Key Points
Prophylactic transfusion in pregnant women with SCD may reduce maternal mortality, vaso-occlusive pain events, and pulmonary complications.
Prophylactic transfusion in pregnant women with SCD may similarly reduce perinatal mortality, neonatal death, and preterm birth.
Abstract
Pregnancy in women with sickle cell disease is associated with adverse maternal and neonatal outcomes. Studies assessing the effects of prophylactic red blood cell transfusions on these outcomes have drawn inconsistent conclusions. The objective of this systematic review was to assess the effect of prophylactic compared with on-demand red blood cell transfusions on maternal and neonatal outcomes in women with sickle cell disease. A systematic search of several medical literature databases was conducted. Twelve studies involving 1291 participants met inclusion criteria. The studies had moderate to high risk of bias. Meta-analysis demonstrated that prophylactic transfusion was associated with a reduction in maternal mortality (7 studies, 955 participants; odds ratio [OR], 0.23; 95% confidence interval [CI], 0.06-0.91), vaso-occlusive pain episodes (11 studies, 1219 participants; OR, 0.26; 95% CI, 0.09-0.76), pulmonary complications (9 studies, 1019 participants; OR, 0.25; 95% CI, 0.09-0.72), pulmonary embolism (3 studies, 237 participants; OR, 0.07; 95% CI, 0.01-0.41), pyelonephritis (6 studies, 455 participants; OR, 0.19; 95% CI, 0.07-0.51), perinatal mortality (8 studies, 1140 participants; OR, 0.43; 95% CI, 0.19-0.99), neonatal death (5 studies, 374 participants; OR, 0.26; 95% CI, 0.07-0.93), and preterm birth (9 studies, 1123 participants; OR, 0.59; 95% CI, 0.37-0.96). Event rates for most of the results were low. Prophylactic transfusions may positively impact several adverse maternal and neonatal outcomes in women with sickle cell disease; however, the evidence stems from a relatively small number of studies with methodologic limitations. A prospective, multicenter, randomized trial is needed to determine whether the potential benefits balance the risks of prophylactic transfusions.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 2437.
Disclosures
The authors, Associate Editor David Garcia, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe the effects of prophylactic transfusion in pregnant women with sickle cell disease (SCD) on maternal outcomes, based on a systematic review.
Discuss the effects of prophylactic transfusion in pregnant women with SCD on perinatal outcomes.
Determine potential mechanisms by which prophylactic transfusion may benefit pregnant women with SCD and their infants, and potential adverse effects.
Release date: November 19, 2015; Expiration date: November 19, 2016
Introduction
Sickle cell disease (SCD) is characterized by deformation of red blood cells (RBCs) into rigid, sickle shapes under conditions of hypoxic stress, leading to ischemia-reperfusion injury, hemolysis-mediated endotheliopathy, and multiorgan damage.1 Recent estimates in 2010 identified SCD to be responsible for ∼28 600 deaths annually worldwide.2 Disease course depends in part on SCD genotype, with greater severity encountered in HbSS and HbS/β0 thalassemia and a more benign course in HbSC and HbS/β+ thalassemia, although adverse events have been observed in all subtypes.3
With advancement in comprehensive care, most women now reach their reproductive years. Pregnancy in women with SCD is currently viewed more favorably4 than it was during the 1970s, when its avoidance was recommended.5 Nevertheless, maternal and fetal morbidity and mortality6-9 are still encountered, and further examination of interventions capable of mitigating these adverse pregnancy outcomes is needed.
Prophylactic RBC transfusion has been proposed to reduce complications by correcting severe anemia and the extent of sickling in both the maternal and placental circulation,10 thereby improving blood flow and oxygenation. The benefits of prophylactic transfusion in SCD have been established for stroke prophylaxis11 and as part of preoperative optimization.12 However, the use of prophylactic transfusion beyond these indications is tempered by concerns over acute and delayed transfusion reactions, alloimmunization, transfusion-related infections, and iron overload.13 Furthermore, reports assessing the effect of prophylactic RBC transfusions on pregnancy outcomes in SCD have been inconsistent.14-19 Thus, the objective of this study was to systematically review the impact of prophylactic transfusion compared with on-demand transfusion (defined as transfusion instituted to treat complications) on maternal and neonatal adverse pregnancy outcomes in pregnant women with SCD.
Methods
The systematic review protocol was registered on PROSPERO (CRD42014007277), and the review was conducted according to PRISMA guidelines20 and reported in accordance with the MOOSE guidelines.21 Studies were included if they (1) involved pregnant women with SCD (HbSS, HbSC, HbS/β0/+ thalassemia); (2) examined the role of simple transfusions (transfusion of ≥1 unit of RBCs) or partial or full exchange transfusions (either manually or involving automated erythrocytapheresis) given prophylactically or on-demand for obstetric or hematologic indications; and (3) were randomized controlled trials (RCTs), controlled trials, and noncontrolled comparative studies with 10 or more participants. Case reports of <10 participants, reviews, letters to the editor, animal studies, studies of laboratory investigations, and noncomparative studies were excluded.
Primary maternal outcomes were mortality; SCD-related morbidity, including vaso-occlusive pain episodes, acute chest syndrome, thromboembolic events (venous and arterial), systemic infections (sepsis/multiorgan failure), and local infections (renal [urinary tract infections/pyelonephritis], pulmonary [pneumonia], uterine [endometritis], and wound); and obstetric morbidity in the form of preeclampsia (as reported by authors). Primary neonatal outcomes were mortality (intrauterine fetal demise or neonatal death); small for gestational age/low birth weight infants (defined as weight below 10th percentile for gestational age [GA]/weight <2500 g); and preterm birth (defined as birth prior to 37 weeks GA). Secondary outcomes included transfusion-related alloimmunization (defined as newly formed alloantibodies following transfusion in pregnancy), need for induction of labor, and mode of delivery.
The literature search was conducted using the OvidSP search platform in the following databases: MEDLINE, EMBASE, and EBM Reviews containing the Cochrane Central Register of Controlled Trials, as well as the EBSCOHost search platform in CINAHL, to include articles indexed as of February 19, 2015 (supplemental Table 1 available on the Blood Web site). The search was limited to human data, without language restrictions. Additional articles were identified by scanning reference lists and searching the gray literature, for the last 5 years, for relevant abstracts from conference proceedings of the American Society of Hematology, the Society for Maternal Fetal Medicine, the Society of Obstetricians and Gynecologists of Canada, and the Royal College of Obstetricians and Gynecologists.
Title and abstract screening, data extraction for studies meeting inclusion criteria, and risk of bias assessment were independently carried out by 2 reviewers (A.K.M. and N.S.). Disagreements were resolved by discussion and consensus. When disagreement persisted, a third author (R.D.) adjudicated. Risk of bias was assessed at the individual study level according to the Cochrane collaboration’s risk of bias tool22 for RCTs and the Newcastle-Ottawa Scale23 for nonrandomized studies.
Data were analyzed according to the type of intervention. For dichotomous variables, relative effect measures, primarily consisting of odds ratios (ORs) and relative risk (RR) and their 95% confidence intervals (CIs), were calculated. A random effects model was chosen given the diversity of individual studies, particularly concerning study population, as well as protocols and targets of the intervention.24 For continuous variables, the mean difference was calculated. The degree of heterogeneity across the studies was examined using I2 values,25 classifying >50% as moderate heterogeneity, and >75% as high heterogeneity. Review Manager Software (version 5.3; the Cochrane Collaboration, Oxford, United Kingdom) was used to complete the meta-analysis. A subgroup analysis by type of SCD was conducted.
Results
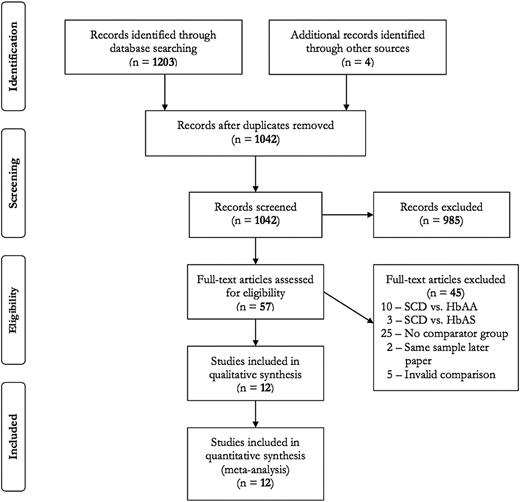
The flow diagram detailing selection of studies included in this review is provided in Figure 1. A total of 12 articles met inclusion criteria (11 cohort studies10,14,15,17-19,26-30 and 1 RCT16 ).
Search results for prophylactic transfusion in sickle cell disease in pregnancy.
Search results for prophylactic transfusion in sickle cell disease in pregnancy.
Description of study characteristics
Study characteristics are described in Tables 1 and 2. Only 2 of the cohort studies were multicenter,10,30 and all but 218,28 used a retrospective design with historical controls.19,26,27 Historical controls from the 1950s and 1960s were included in 2 studies.19,28 Seven studies specified outcomes based on β-globin genotype.10,15,18,19,26,28,30 Confounding variables were generally not addressed in the study design or analysis.
Characteristics and risk of bias assessment for cohort studies on transfusion therapy in pregnant women with sickle cell disease
| Author (year), country, setting . | Single vs multicenter . | Design . | Years of study . | Inclusion criteria . | Risk of bias assessment (NOS) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Selection ★★★★ . | Comparability ★★ . | Outcome ★★★ . | Total/9 . | Risk of bias (1-3: high; 4-6: moderate; 7-9: low) . | |||||
| Morrison (1976)28 * | Single | Prospective cohort, matched retrospectively with historical controls | Apr. 1970 – Mar. 1974 | SCD | ★★ | — | ★ | 3 | High |
| United States | Apr 1965 – Mar. 1970 - Historical controls | ||||||||
| Tertiary care | |||||||||
| Miller (1981)26 | Single | Retrospective cohort with historical controls | 1978-1980 (PT) | SCD | ★★ | — | ★ | 3 | High |
| United States | 1974-1979 (ODT) Historical controls | ||||||||
| Tertiary care | |||||||||
| Cunningham (1983)19 | Single | Retrospective cohort with historical controls | 1973-1982 (PT) | SCD | ★★ | — | ★ | 3 | High |
| United States | 1955-1972 (ODT) | ||||||||
| Tertiary care | |||||||||
| Tuck (1987)30 | Multi | Retrospective cohort | 1978-1984 (PT) | SCD | ★ | — | — | 1 | High |
| United Kingdom | 1975-1981 (Un-transfused) | ||||||||
| Tertiary care | |||||||||
| Koshy (1991)15 † | Single | Retrospective cohort | 1986-1990 | SCD | ★ | — | ★ | 2 | High |
| United States | |||||||||
| Tertiary care | |||||||||
| Morrison (1991)27 | Single | Retrospective cohort with historical controls | Jan 1981-Dec 1990 | HbSS, HbSC | ★★ | — | ★ | 3 | High |
| United States | |||||||||
| Tertiary care | |||||||||
| Howard (1995)10 | Multi | Retrospective cohort | 1991-1993 | SCD | ★★ | — | ★★ | 4 | Moderate |
| United Kingdom | |||||||||
| Hospitals England &Wales | |||||||||
| El-Shafei (1995)18 | Single | Mixed retrospective and prospective cohort | May 1988 – Oct. 1994 (prospective) vs Nov. 1986 – Oct. 1988 (retrospective) | SCD | ★★ | — | ★ | 3 | High |
| Bahrain | |||||||||
| MoH Hospital | |||||||||
| Moussaoui (2002)29 | Single | Retrospective cohort | Not stated | HbSS | ★★ | — | — | 2 | High |
| Libreville, Gabon | |||||||||
| Not stated | |||||||||
| Gilli (2007)17 | Single | Retrospective cohort | 1994-2004 | HbSS, HbSC | ★★ | ★ | ★ | 4 | Moderate |
| Brazil | |||||||||
| Tertiary care | |||||||||
| Asma (2015)14 | Single | Retrospective cohort | 2000-2013 | SCD | ★ | — | ★ | 2 | High |
| Turkey | |||||||||
| Tertiary care | |||||||||
| Author (year), country, setting . | Single vs multicenter . | Design . | Years of study . | Inclusion criteria . | Risk of bias assessment (NOS) . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Selection ★★★★ . | Comparability ★★ . | Outcome ★★★ . | Total/9 . | Risk of bias (1-3: high; 4-6: moderate; 7-9: low) . | |||||
| Morrison (1976)28 * | Single | Prospective cohort, matched retrospectively with historical controls | Apr. 1970 – Mar. 1974 | SCD | ★★ | — | ★ | 3 | High |
| United States | Apr 1965 – Mar. 1970 - Historical controls | ||||||||
| Tertiary care | |||||||||
| Miller (1981)26 | Single | Retrospective cohort with historical controls | 1978-1980 (PT) | SCD | ★★ | — | ★ | 3 | High |
| United States | 1974-1979 (ODT) Historical controls | ||||||||
| Tertiary care | |||||||||
| Cunningham (1983)19 | Single | Retrospective cohort with historical controls | 1973-1982 (PT) | SCD | ★★ | — | ★ | 3 | High |
| United States | 1955-1972 (ODT) | ||||||||
| Tertiary care | |||||||||
| Tuck (1987)30 | Multi | Retrospective cohort | 1978-1984 (PT) | SCD | ★ | — | — | 1 | High |
| United Kingdom | 1975-1981 (Un-transfused) | ||||||||
| Tertiary care | |||||||||
| Koshy (1991)15 † | Single | Retrospective cohort | 1986-1990 | SCD | ★ | — | ★ | 2 | High |
| United States | |||||||||
| Tertiary care | |||||||||
| Morrison (1991)27 | Single | Retrospective cohort with historical controls | Jan 1981-Dec 1990 | HbSS, HbSC | ★★ | — | ★ | 3 | High |
| United States | |||||||||
| Tertiary care | |||||||||
| Howard (1995)10 | Multi | Retrospective cohort | 1991-1993 | SCD | ★★ | — | ★★ | 4 | Moderate |
| United Kingdom | |||||||||
| Hospitals England &Wales | |||||||||
| El-Shafei (1995)18 | Single | Mixed retrospective and prospective cohort | May 1988 – Oct. 1994 (prospective) vs Nov. 1986 – Oct. 1988 (retrospective) | SCD | ★★ | — | ★ | 3 | High |
| Bahrain | |||||||||
| MoH Hospital | |||||||||
| Moussaoui (2002)29 | Single | Retrospective cohort | Not stated | HbSS | ★★ | — | — | 2 | High |
| Libreville, Gabon | |||||||||
| Not stated | |||||||||
| Gilli (2007)17 | Single | Retrospective cohort | 1994-2004 | HbSS, HbSC | ★★ | ★ | ★ | 4 | Moderate |
| Brazil | |||||||||
| Tertiary care | |||||||||
| Asma (2015)14 | Single | Retrospective cohort | 2000-2013 | SCD | ★ | — | ★ | 2 | High |
| Turkey | |||||||||
| Tertiary care | |||||||||
MoH, Ministry of Health; NOS, Newcastle-Ottawa Scale; ODT, on-demand transfusion; PNM, perinatal mortality; PT, prophylactic transfusion.
Data include Morrison (1976) J Pediatr.
Data for patients in the prophylactic transfusion arm also previously reported as part of RCT (Koshy 1988).
Characteristics and risk of bias assessment for RCT on transfusion therapy in pregnant women with sickle cell disease
| Author (year), country, setting . | Single vs multicenter . | Design . | Years of study . | Inclusion criteria . | Risk of bias assessment . |
|---|---|---|---|---|---|
| Koshy (1988)16 | Multi | RCT | Jan 1979 to Mar 1986 | HbSS <28 weeks GA | Selection bias: Unclear |
| United States | Only HbSS randomized | Excluded neurologic dysfunction, nephrotic syndrome, chronic renal failure, persistent liver disease, chronic lung disease, coagulopathy, or presence of multiple RBC antibodies | |||
| Tertiary care | 28 with HbSS not randomized; of those, 7 continued long-term PT initiated prior to study | Performance bias: High risk | |||
| Detection bias: Unclear | |||||
| Attrition bias: Low risk | |||||
| Reporting bias: Unclear risk | |||||
| Overall: High risk of bias |
| Author (year), country, setting . | Single vs multicenter . | Design . | Years of study . | Inclusion criteria . | Risk of bias assessment . |
|---|---|---|---|---|---|
| Koshy (1988)16 | Multi | RCT | Jan 1979 to Mar 1986 | HbSS <28 weeks GA | Selection bias: Unclear |
| United States | Only HbSS randomized | Excluded neurologic dysfunction, nephrotic syndrome, chronic renal failure, persistent liver disease, chronic lung disease, coagulopathy, or presence of multiple RBC antibodies | |||
| Tertiary care | 28 with HbSS not randomized; of those, 7 continued long-term PT initiated prior to study | Performance bias: High risk | |||
| Detection bias: Unclear | |||||
| Attrition bias: Low risk | |||||
| Reporting bias: Unclear risk | |||||
| Overall: High risk of bias |
The RCT16 was a multicenter study conducted in the United States of pregnancies with HbSS recruited before 28 weeks of gestation. Of note, participants with history of neurologic dysfunction, persistent liver disease, chronic lung disease, nephrotic syndrome, chronic renal failure, coagulopathy, or presence of multiple RBC antibodies were excluded, of whom 25% were on long-term transfusion therapy prior to pregnancy.
Risk of bias assessment
Nine cohort studies were deemed to be at high risk of bias and 2 at moderate risk (Tables 1 and 2). Most studies did not report whether the outcome of interest was present at the start of the study. Only 2 studies controlled for relevant confounders, but none controlled for the burden of disease prior to pregnancy. The duration of follow-up was not explicitly stated in most studies.
The only included RCT was deemed to be at high risk of bias due to high risk of selection bias (unclear randomization method), high risk of detection bias (unreported method of allocation concealment), low risk of attrition bias (all outcome data were obtained), and unclear risk of reporting bias (protocol outlining the prespecified outcomes was not available).
Summary of management and transfusion protocols
Transfusion protocols are detailed in Table 3. Protocols varied across reports and the type of transfusion depended on the degree of anemia in 5 studies.10,15,16,19,30 Timing of prophylactic transfusion differed, with only 2 studies instituting the intervention in early to mid-pregnancy.10,15 The proportion of patients receiving prophylactic transfusion and needing additional on-demand transfusions was not reported in the majority of studies and varied widely in the studies in which it was reported (5.6-60.7%).14,18,28,29 Initial transfusion targets and subsequent transfusion triggers in the prophylactic transfusion group differed between studies and involved a combination of values, including hemoglobin of 100 to 110 g/L,15,29,30 hematocrit (Hct) ≤35%,19,26,28 HbA >40% to >50%,19,27,28 and/or HbS <25% to <50%14,15,19,29,30 for the former and hemoglobin <70 g/L,14 hematocrit <25%,19,26,28 and/or HbA <20% to <40%19,26-28 for the latter. In the on-demand transfusion group, severe anemia, which was variably defined, was the indication for transfusion in 6 studies,14,15,18,26,27,29 acute SCD-related complications (including acute chest syndrome or nonresolving vaso-occlusive pain episodes) were the indications in 4 studies,15,17,18,27 and the indication was not defined in 4 studies.10,19,28,30 None of the cohort studies explicitly listed obstetric indications as reason for on-demand transfusion.10,14,15,17-19,26-30
Summary of management and transfusion protocols
| Author, year, journal . | Prophylactic transfusion . | ODT . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of transfusion . | Timing of initiation, weeks GA . | MUT . | Proportion requiring ODT, n (%) . | Initial targets subsequent triggers . | Reason for transfusion . | Timing of initiation, weeks GA . | MUT . | Proportion requiring ODT, % . | |
| Cohort studies | |||||||||
| Morrison 197627 Obstet Gynecol* | Partial exchange transfusion from 28 wks (routine admission) | 28 | 3-6 per transfusion | 2/36 (5.6%) | Target | Severe anemia (Hct <15%) with crisis &/or infection | NR | 1 | 38% |
| Hct 35% | |||||||||
| HbA 40% | |||||||||
| Trigger | |||||||||
| a) Hct <25% & HbA <20% | |||||||||
| b) 36-38 wks | |||||||||
| c) with crisis | |||||||||
| d) in labor | |||||||||
| Miller 198126 Am J Obstet Gynecol | Initially partial exchange transfusion, subsequently EA | 22.3 ± 8.4 | 13 | NR | Target | Hb <70 g/L or anemia-related symptoms | NR | 3.7 | NR |
| NR | |||||||||
| Trigger | |||||||||
| Hct or HbA <25% | |||||||||
| Cunningham 198319 Obstet Gynecol | Simple transfusion or partial exchange transfusion depending on degree of anemia | ∼20 | HbSS - 13 | NR | Target | Hb <70 g/L or Hct < 20% or infection, hypoxia, excessive blood loss | NR | NR | NR |
| HbSC - 15 | HbA 50% | ||||||||
| HbS/B-Thal – NR | HbS 50% | ||||||||
| Trigger | |||||||||
| Hct <25% HbA <40% | |||||||||
| Tuck 198730 BJOG | Simple or partial exchange transfusion depending on degree of anemia | <20 or | 12 (4-28) | NR | Target | NR | NR | NR | NR |
| Dulwich: PT for all SCD types | >20 in HbSS | Hb 110 g/L & | |||||||
| St. Thomas: PT for HbSS | depending on time of presentation | HbS 25% | |||||||
| Trigger | |||||||||
| NR | |||||||||
| Koshy 199115 J Clin Apher | Simple or partial exchange transfusion | “Early pregnancy” | 12 | NR | Target | Prior to C/S, ACS, PET, severe anemia | NR | 4 | 74% |
| Hb 100-110 g/L | |||||||||
| HbS <35% | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Morrison 199127 J Clin Apher | Partial exchange transfusion or EA if severe crisis or morbidity | 19.4 | NR | NR | Target | Severe and symptomatic anemia, or crisis un-responsive to treatment | NR | NR | 58% |
| HbA >50% | |||||||||
| Trigger | |||||||||
| HbA <20% or “significant crisis, severe morbidity” | |||||||||
| Howard 199510 BJOG | Simple or partial exchange transfusion depending on severity of anemia | “First or second trimester” | NR | NR | Target | Sickling complications | NR | NR | 26% |
| NR | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| El-Shafei 199518 Aust NZ J Obstet Gynaecol | PT for anemia (Hb <100 g/L) | NR | NR | 148 (60.7%) | Target | Hb <60 g/L, ongoing crisis, Hb <80 g/L prior to C/S, or PPH | NR | NR | 29.4% |
| NR | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Moussaoui 200229 Trop Med | Simple transfusion | 16 | 12 | 2/10 (for PPH) | Target | Severe anemia in pregnancy, severe anemia peripartum, or to treat PPH | NR | 7.83 | NR |
| Hb >100 g/L | |||||||||
| HbS <40% | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Gilli 200717 Int J Gyn Ob | Erythro-cytapheresis | 28 | 8.14 ± 4.31 | NR | Target | Acute SCD complications | NR | 3.76 ± 2.70 | NR |
| NR | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Asma 201514 Transfusion | Continuous flow apheresis; 60-70% RBCs exchanged | Variable | 6.2 ± 0.6 | 4/24 (16.7%) | Target | Simple transfusion - Hb <70g/L leukocytosis in absence of infection, and underlying pulmonary or cardiac disease | One in first & one in third trimester | NR | 15% |
| HbS <30% | |||||||||
| Trigger | |||||||||
| Clinical deterioration, Hb <70g/L, leukocytosis with no infection, and underlying pulmonary or cardiac disease - hyper-transfusion with target Hct of 27% | |||||||||
| RCT | |||||||||
| Koshy 198816 NEJM† | Simple or partial exchange transfusion | 8-14 wks | 12 | NR | Hb 100-110 g/L or | Hematologic or obstetric indications | NR | 6.5 | 44% |
| (78%) | Hct near 33% and | Hematologic | |||||||
| 20-26 wks | HbS <35% | Hb <60g/L | |||||||
| (22%) | Hct <18% | ||||||||
| Retics <3% | |||||||||
| Author, year, journal . | Prophylactic transfusion . | ODT . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type of transfusion . | Timing of initiation, weeks GA . | MUT . | Proportion requiring ODT, n (%) . | Initial targets subsequent triggers . | Reason for transfusion . | Timing of initiation, weeks GA . | MUT . | Proportion requiring ODT, % . | |
| Cohort studies | |||||||||
| Morrison 197627 Obstet Gynecol* | Partial exchange transfusion from 28 wks (routine admission) | 28 | 3-6 per transfusion | 2/36 (5.6%) | Target | Severe anemia (Hct <15%) with crisis &/or infection | NR | 1 | 38% |
| Hct 35% | |||||||||
| HbA 40% | |||||||||
| Trigger | |||||||||
| a) Hct <25% & HbA <20% | |||||||||
| b) 36-38 wks | |||||||||
| c) with crisis | |||||||||
| d) in labor | |||||||||
| Miller 198126 Am J Obstet Gynecol | Initially partial exchange transfusion, subsequently EA | 22.3 ± 8.4 | 13 | NR | Target | Hb <70 g/L or anemia-related symptoms | NR | 3.7 | NR |
| NR | |||||||||
| Trigger | |||||||||
| Hct or HbA <25% | |||||||||
| Cunningham 198319 Obstet Gynecol | Simple transfusion or partial exchange transfusion depending on degree of anemia | ∼20 | HbSS - 13 | NR | Target | Hb <70 g/L or Hct < 20% or infection, hypoxia, excessive blood loss | NR | NR | NR |
| HbSC - 15 | HbA 50% | ||||||||
| HbS/B-Thal – NR | HbS 50% | ||||||||
| Trigger | |||||||||
| Hct <25% HbA <40% | |||||||||
| Tuck 198730 BJOG | Simple or partial exchange transfusion depending on degree of anemia | <20 or | 12 (4-28) | NR | Target | NR | NR | NR | NR |
| Dulwich: PT for all SCD types | >20 in HbSS | Hb 110 g/L & | |||||||
| St. Thomas: PT for HbSS | depending on time of presentation | HbS 25% | |||||||
| Trigger | |||||||||
| NR | |||||||||
| Koshy 199115 J Clin Apher | Simple or partial exchange transfusion | “Early pregnancy” | 12 | NR | Target | Prior to C/S, ACS, PET, severe anemia | NR | 4 | 74% |
| Hb 100-110 g/L | |||||||||
| HbS <35% | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Morrison 199127 J Clin Apher | Partial exchange transfusion or EA if severe crisis or morbidity | 19.4 | NR | NR | Target | Severe and symptomatic anemia, or crisis un-responsive to treatment | NR | NR | 58% |
| HbA >50% | |||||||||
| Trigger | |||||||||
| HbA <20% or “significant crisis, severe morbidity” | |||||||||
| Howard 199510 BJOG | Simple or partial exchange transfusion depending on severity of anemia | “First or second trimester” | NR | NR | Target | Sickling complications | NR | NR | 26% |
| NR | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| El-Shafei 199518 Aust NZ J Obstet Gynaecol | PT for anemia (Hb <100 g/L) | NR | NR | 148 (60.7%) | Target | Hb <60 g/L, ongoing crisis, Hb <80 g/L prior to C/S, or PPH | NR | NR | 29.4% |
| NR | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Moussaoui 200229 Trop Med | Simple transfusion | 16 | 12 | 2/10 (for PPH) | Target | Severe anemia in pregnancy, severe anemia peripartum, or to treat PPH | NR | 7.83 | NR |
| Hb >100 g/L | |||||||||
| HbS <40% | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Gilli 200717 Int J Gyn Ob | Erythro-cytapheresis | 28 | 8.14 ± 4.31 | NR | Target | Acute SCD complications | NR | 3.76 ± 2.70 | NR |
| NR | |||||||||
| Trigger | |||||||||
| NR | |||||||||
| Asma 201514 Transfusion | Continuous flow apheresis; 60-70% RBCs exchanged | Variable | 6.2 ± 0.6 | 4/24 (16.7%) | Target | Simple transfusion - Hb <70g/L leukocytosis in absence of infection, and underlying pulmonary or cardiac disease | One in first & one in third trimester | NR | 15% |
| HbS <30% | |||||||||
| Trigger | |||||||||
| Clinical deterioration, Hb <70g/L, leukocytosis with no infection, and underlying pulmonary or cardiac disease - hyper-transfusion with target Hct of 27% | |||||||||
| RCT | |||||||||
| Koshy 198816 NEJM† | Simple or partial exchange transfusion | 8-14 wks | 12 | NR | Hb 100-110 g/L or | Hematologic or obstetric indications | NR | 6.5 | 44% |
| (78%) | Hct near 33% and | Hematologic | |||||||
| 20-26 wks | HbS <35% | Hb <60g/L | |||||||
| (22%) | Hct <18% | ||||||||
| Retics <3% | |||||||||
ACS, acute chest syndrome; C/S, Caesarean section; EA, erythrocytapheresis; Hb, hemoglobin, MUT, mean units transfused; NR, not reported; ODT, on-demand transfusion; PET, preeclampsia; PPH, postpartum hemorrhage; PT, prophylactic transfusion; Retics, reticulocyte count; RBCs, red blood cells.
Data include Morrison (1976) J Pediatr.
Data for patients in the prophylactic transfusion arm also previously reported as part of RCT (Koshy 1988).
In the single RCT conducted by Koshy et al,16 the prophylactic transfusion group received simple or partial exchange transfusions to achieve the following targets: hemoglobin of 100 to 110 g/L, Hct of 33%, and HbS <35%. Transfusions were initiated between 8 and 14 weeks of GA in three quarters and between 20 and 26 weeks in one quarter of participants. The proportion of women in the prophylactic transfusion group that required on-demand transfusion was not specified. Indications for transfusion in the on-demand transfusion group were listed as obstetric or hematologic and were not defined beyond the transfusion triggers that included hemoglobin <60 g/L, Hct <18%, and reticulocyte count <3%. Transfusion in the on-demand transfusion group was required by 44% of participants.
Meta-analysis
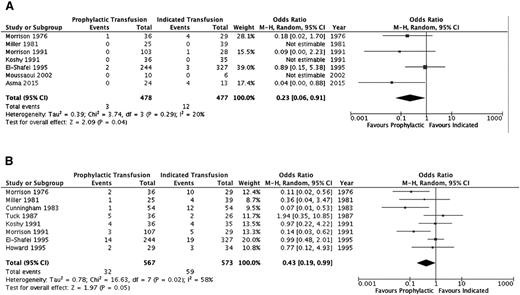
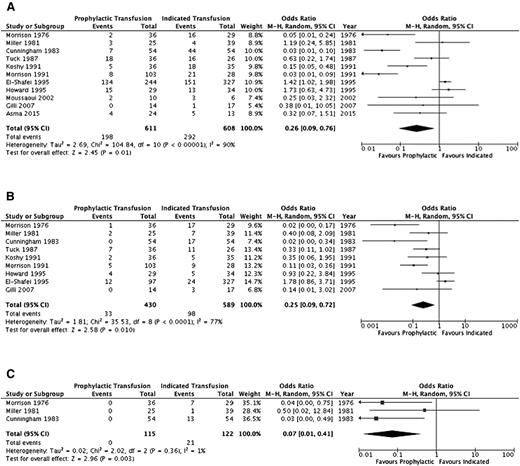
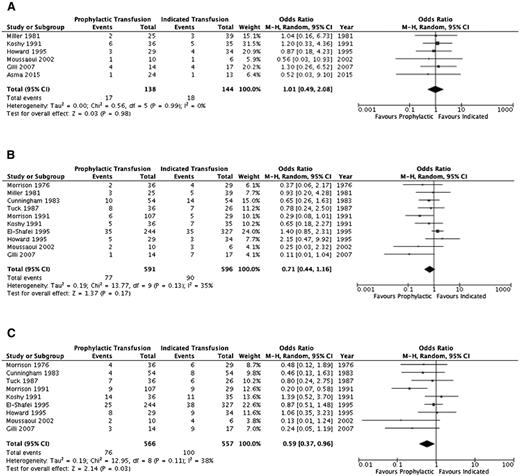
A summary of the ORs for the cohort studies is detailed in Table 4 and individual study results in supplemental Table 2. Thromboembolic events (aside from pulmonary emboli) and systemic infections (sepsis/multiorgan failure) could not be evaluated. Pulmonary infections, pulmonary embolism, and acute chest syndrome were assessed separately by some studies and in varying combinations by others; thus, the outcome of overall pulmonary complications was added to reflect this variability. Furthermore, an evaluation of alloimmunization, need for induction of labor, and mode of delivery was not completed secondary to lack of detail across studies. Figures 2–4 illustrate the effects of prophylactic transfusion on maternal and fetal mortality, maternal morbidities, and obstetric morbidities, respectively. Odds of maternal mortality, vaso-occlusive pain episodes, overall pulmonary complications, pulmonary embolism, pyelonephritis, perinatal mortality, neonatal death, and preterm birth were significantly lower in the prophylactic transfusion group compared with the on-demand transfusion group, whereas there was no difference between the groups in odds of pulmonary infection, acute chest syndrome, urinary tract infection, endometritis, preeclampsia, intrauterine fetal demise, and small for gestational age infants or low-birth-weight infants.
Outcomes in cohort studies of prophylactic transfusion compared with on-demand transfusion in pregnant women with SCD (cohort studies)
| Group . | Outcomes . | Studies, n . | Study subject, n . | OR (95% CI) . | Significance (heterogeneity), P (I2) . |
|---|---|---|---|---|---|
| Maternal | Mortality | 714,15,18,26-29 | 955 | 0.23 (0.06-0.91) | .04 (20%) |
| Vaso-occlusive pain episodes | 1110,15,17-19,26-30 | 1219 | 0.26 (0.09-0.76) | .01 (90%) | |
| Pulmonary complications* | 910,15,17-19,26-28,30 | 1019 | 0.25 (0.09-0.72) | .01 (77%) | |
| Pulmonary infection | 518,19,26-28 | 792 | 0.26 (0.05-1.27) | .10 (83%) | |
| Pulmonary embolism | 319,26,28 | 237 | 0.07 (0.01-0.41) | <.01 (1%) | |
| Acute chest syndrome | 215,17 | 102 | 0.28 (0.06-1.26) | .10 (0%) | |
| Urinary tract infection | 315,29,30 | 149 | 1.09 (0.22-5.42) | .92 (61%) | |
| Pyelonephritis | 615,19,26-29 | 455 | 0.19 (0.07-0.51) | <.01 (34%) | |
| Endometritis | 226,29 | 80 | 0.76 (0.17-3.44) | .72 (40%) | |
| Preeclampsia | 610,14,15,17,26,29 | 282 | 1.01 (0.49-2.08) | .98 (0%) | |
| Fetal | Perinatal mortality | 810,15,18,19,26-28,30 | 1140 | 0.43 (0.19-0.99) | <.05 (58%) |
| Intrauterine fetal demise | 814,15,17,19,26,28-30 | 458 | 0.47 (0.17-1.33) | .15 (32%) | |
| Neonatal death | 515,19,26,28,30 | 374 | 0.26 (0.07-0.93) | .04 (0%) | |
| Small for gestational age/low birth weight | 1010,15,17-19,26-30 | 1187 | 0.71 (0.44-1.16) | .17 (35%) | |
| Preterm delivery | 910,15,17-19,27-30 | 1123 | 0.59 (0.37-0.96) | .03 (38%) |
| Group . | Outcomes . | Studies, n . | Study subject, n . | OR (95% CI) . | Significance (heterogeneity), P (I2) . |
|---|---|---|---|---|---|
| Maternal | Mortality | 714,15,18,26-29 | 955 | 0.23 (0.06-0.91) | .04 (20%) |
| Vaso-occlusive pain episodes | 1110,15,17-19,26-30 | 1219 | 0.26 (0.09-0.76) | .01 (90%) | |
| Pulmonary complications* | 910,15,17-19,26-28,30 | 1019 | 0.25 (0.09-0.72) | .01 (77%) | |
| Pulmonary infection | 518,19,26-28 | 792 | 0.26 (0.05-1.27) | .10 (83%) | |
| Pulmonary embolism | 319,26,28 | 237 | 0.07 (0.01-0.41) | <.01 (1%) | |
| Acute chest syndrome | 215,17 | 102 | 0.28 (0.06-1.26) | .10 (0%) | |
| Urinary tract infection | 315,29,30 | 149 | 1.09 (0.22-5.42) | .92 (61%) | |
| Pyelonephritis | 615,19,26-29 | 455 | 0.19 (0.07-0.51) | <.01 (34%) | |
| Endometritis | 226,29 | 80 | 0.76 (0.17-3.44) | .72 (40%) | |
| Preeclampsia | 610,14,15,17,26,29 | 282 | 1.01 (0.49-2.08) | .98 (0%) | |
| Fetal | Perinatal mortality | 810,15,18,19,26-28,30 | 1140 | 0.43 (0.19-0.99) | <.05 (58%) |
| Intrauterine fetal demise | 814,15,17,19,26,28-30 | 458 | 0.47 (0.17-1.33) | .15 (32%) | |
| Neonatal death | 515,19,26,28,30 | 374 | 0.26 (0.07-0.93) | .04 (0%) | |
| Small for gestational age/low birth weight | 1010,15,17-19,26-30 | 1187 | 0.71 (0.44-1.16) | .17 (35%) | |
| Preterm delivery | 910,15,17-19,27-30 | 1123 | 0.59 (0.37-0.96) | .03 (38%) |
Pulmonary complications (infections, infarctions, and/or embolism).
Comparison of mortality between the prophylactic transfusion and on-demand transfusion groups. (A) Maternal mortality. (B) Perinatal mortality.
Comparison of mortality between the prophylactic transfusion and on-demand transfusion groups. (A) Maternal mortality. (B) Perinatal mortality.
Comparison of selected maternal morbidity indices between the prophylactic transfusion and on-demand transfusion groups. (A) Vaso-occlusive pain episodes. (B) Pulmonary complications. (C) Pulmonary embolism.
Comparison of selected maternal morbidity indices between the prophylactic transfusion and on-demand transfusion groups. (A) Vaso-occlusive pain episodes. (B) Pulmonary complications. (C) Pulmonary embolism.
Comparison of obstetric morbidity between the prophylactic transfusion and on-demand transfusion groups. (A) Pre-eclampsia. (B) Small for gestational age/low birth-weight infants. (C) Preterm birth.
Comparison of obstetric morbidity between the prophylactic transfusion and on-demand transfusion groups. (A) Pre-eclampsia. (B) Small for gestational age/low birth-weight infants. (C) Preterm birth.
The RCT16 did not include maternal mortality as an outcome, and there was no difference in the risk of perinatal mortality (RR, 3.0; 95% CI, 0.65-13.88), intrauterine fetal demise (RR, 2.0; 95% CI, 0.39-10.24), or neonatal death (RR, 5.0; 95% CI, 0.25-100.64) between the prophylactic transfusion and on-demand transfusion groups. With respect to maternal morbidity, the prophylactic transfusion group had a lower risk of vaso-occlusive pain events (RR, 0.28; 95% CI, 0.12-0.67) and acute chest syndrome (RR, 0.11; 95% CI, 0.03-0.44), whereas there were no pulmonary embolic events in either group. There was no difference between groups in the risk of urinary tract infection (RR, 0.33; 95% CI, 0.07-1.54), pyelonephritis (RR, 1.00; 95% CI, 0.07-15.38), endometritis (RR, 0.33; 95% CI, 0.01-7.92), or preeclampsia (RR, 0.75; 95% CI, 0.29-1.94). Similarly, there were no differences between groups in the risk of fetal morbidity, including small for gestational age and low-birth-weight infants (RR, 0.71; 95% CI, 0.25-2.04) or preterm birth (RR, 2.33; 95% CI, 1.0 1-5.39).
Subgroup analysis
Subgroup analysis of nonrandomized studies based on common β-globin genotypes, HbSS, HbSC, and HbS/β thalassemia (supplemental Table 3), demonstrated that among patients with HbSS in the prophylactic transfusion group, there was a significant reduction in odds of vaso-occlusive pain episodes, overall pulmonary complications, pulmonary embolism, and pulmonary infections compared with the on-demand transfusion group, but no significant difference in odds of maternal mortality, pyelonephritis, perinatal mortality, neonatal death, or preterm birth. Among patients with HbSC in the prophylactic transfusion group, there was a significant reduction in odds of vaso-occlusive pain events and pulmonary embolism compared with the on-demand transfusion group, but no significant difference in any of the remaining outcomes. Among patients with HbS/β thalassemia, no significant difference in odds was identified for any of the outcomes between the prophylactic transfusion and the on-demand transfusion groups; however, numbers were small.
Discussion
In contrast to recently drawn conclusions,31 this systematic review and meta-analysis invites further investigation into the merits of prophylactic transfusion in pregnant women with SCD. Although constrained by methodologic limitations of original study designs, the analysis suggested a reduction in vaso-occlusive pain episodes, maternal mortality, overall pulmonary complications, pulmonary embolism, neonatal mortality, and preterm birth, favoring institution of prophylactic transfusion.
The potential of prophylactic transfusion to reduce adverse pregnancy outcomes in women with SCD stems from its capacity to remedy SCD-driven physiologic derangements through (1) correction of anemia, enhancing RBC oxygen-carrying capacity; (2) reduction in the proportion of sickle hemoglobin carrying erythrocytes, diminishing vaso-occlusive episodes; (3) reduction in blood viscosity, if provided via RBC exchange transfusion; and to a lesser extent, (4) suppression of endogenous erythropoiesis.32,33 Although indiscriminate use of transfusion must be avoided, given its potential adverse effects of alloimmunization, acute and delayed transfusion reactions, transfusion-related infectious morbidity, and iron overload,13 transfusion in the appropriate context has merit and may even be lifesaving.
Studies to date have consistently supported the effectiveness of prophylactic transfusion in reducing the frequency of vaso-occlusive pain episodes,14,15,17,19,27-30 which tend to increase in pregnancy.3 As this systematic review demonstrates, maternal mortality in a majority of women with SCD occurred in the setting of vaso-occlusive pain episodes,14,18,28 with pulmonary embolism and acute chest syndrome accounting for 2 additional cases each.14,28 The positive impact of prophylactic transfusion on maternal morbidity may thus be the consequence of reducing the frequency of vaso-occlusive pain episodes. However, uncomplicated vaso-occlusive events in the nonpregnant state are typically self-limited and of themselves are not considered an indication for transfusion.34 To rationalize the use of prophylactic transfusion to address vaso-occlusive pain events in pregnant women, it may thus be useful to classify them as complicated when they occur in the setting of pregnancy.
The utility of prophylactic transfusion in stabilizing the pregnancy course in women with SCD may further be judged by the proportion of women who, given the institution of prophylactic transfusion, are able to avoid the need for urgent on-demand transfusion. Unfortunately, most studies did not address this variable, and those that did demonstrated significant variability in their estimates, ranging from 5.6% to 60.7%. Moreover, none of the cohort studies listed obstetric indications explicitly as a reason for on-demand transfusion, where their inclusion may have yielded higher rates of transfusion in the on-demand transfusion group. Precise estimates of these parameters are needed, as on-demand transfusion is often provided in urgent circumstances, whereas prophylactic transfusion allows for a nonurgent, planned approach, which may decrease the rate of transfusion-related complications. A recent study found that the proinflammatory state characteristic of SCD-related challenges, when present at the time of transfusion, was associated with an increased risk of alloimmunization, with acute chest syndrome and vaso-occlusive episodes demonstrating the strongest relationship with alloantibody formation.35 Although this finding needs replication in prospective studies, it may lend further credence to a preemptive approach aimed at preventing the need for transfusion in the acute setting.
Having considered possible mechanisms underlying the observed favorable impact of prophylactic transfusion on maternal mortality and morbidity, its seeming lack of effect on preeclampsia and fetal growth restriction is incongruous and deserves exploration. Although the etiology of fetal growth restriction can be heterogeneous,36 the most likely process in the context of SCD is placental insufficiency, which is supported by the recognition of a strong relationship between placental weight and birth weight for infants of mothers with homozygous SCD,37 and by reports of pathologic placental features consisting of calcifications, infarcts, intervillous hemorrhage, fibrin necrosis, and syncytial knots.38 Placental dysfunction in this setting is likely a consequence of both vaso-occlusion and impaired placental development,4,39 creating a condition of relative fetal hypoxia.40,41
The lack of impact of prophylactic transfusion on fetal growth restriction and preeclampsia demonstrated in this systematic review may potentially be explained by the circumstances under which prophylactic transfusion was instituted. The wide variation among studies in the timing of prophylactic transfusion commencement is of specific relevance, given that aberrant early placentation is typically the pathophysiologic origin of these conditions. In the first weeks of pregnancy, abnormal differentiation of the villous syncytiotrophoblast will lead to compromise in the placental barrier, inciting an inflammatory response and culminating in features of preeclampsia. However, abnormal extravillous trophoblast invasion will result in altered differentiation of spiral arteries, compromising uterine blood flow, consequently affecting umbilical blood flow, and culminating in growth restriction.42 The events of early trophoblast development are well underway by 3 weeks after conception.42,43 Given that none of the studies included in this review initiated the treatment early enough to have an effect on early placental development, the lack of effect on growth restriction and preeclampsia, both driven by abnormal placentation, becomes less surprising.
However, it must be considered that if prophylactic transfusion is to positively impact the downstream events of growth restriction and preeclampsia in sickle cell patients, it may need to be instituted as soon as pregnancy is diagnosed and perhaps even peri-conceptionally. Further studies are needed to advance these hypotheses.
The results of this study are largely contrary to results of the single RCT of prophylactic transfusion in pregnant women with SCD,16 which found that, aside from decreasing vaso-occlusive pain episodes, the rate of medical/obstetric complications did not differ between the prophylactic and on-demand transfusion groups. However, it is worth considering that the RCT’s sample size was very small, with 36 participants in each arm, and event rates were low; thus, the study was underpowered to assess the outcomes of interest. In addition, it excluded participants with considerable sickle cell-related comorbidities, who arguably comprise a group at higher risk of pregnancy-related complications. Similarly, a recent systematic review31 concluded that prophylactic transfusion carried no clinical benefit. Two trials, deemed to be at moderate risk of bias, were included.16,44 Further appraisal of these 2 trials reveals that participants for both were selected as part of the Co-operative Study and that the 26 pregnancies from the first trial were included in the analysis of the second trial, which reported a total of 36 pregnancies, thus potentially overestimating the sample size and rising questions with regard to the accuracy of analyses.
Strengths and limitations
Strengths of this review include the fact that it is a comprehensive objective appraisal of the available scientific literature. Additionally, the evidence from both RCT and non-RCT study designs was carefully evaluated. Limitations include the suboptimal methodologic quality of the included studies, the majority of which are at moderate to high risk of bias, and the absence and/or heterogeneity of definitions for exposure and the outcomes of interest. Furthermore, the subgroup analysis based on SCD genotypes was limited by very small sample sizes and low event rates. The lack of information from original studies pertaining to transfusion risks, particularly relating to alloimmunization, delayed hemolytic transfusion reactions, and iron overload precluded analysis of these important variables.
In conclusion, this systematic review advances the possibility that prophylactic transfusion might benefit pregnant women with SCD, leading to a decrease in maternal and neonatal morbidity and mortality. However, the review is limited by the quality and paucity of available research and the lack of studies addressing the impact of transfusion complications on maternal and fetal outcomes. This underscores the need to conduct a rigorously designed, multicenter RCT to conclusively settle this important concern.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Elizabeth Uleryk for invaluable assistance with the development and execution of the search strategy.
N.S. was supported by a Canadian Institute of Health Research/Canadian Blood Services New Investigator Award.
Authorship
Contribution: A.K.M. and N.S. contributed to study concept design, protocol development, independent review of all studies, data collection, data interpretation, drafting of manuscript, manuscript revision, and approval of final version; R.D. contributed to study concept design, adjudication, data interpretation, manuscript revision, and approval of final version; K.H.M.K. and K.M. contributed to study concept design, protocol development, data interpretation, manuscript revision, and approval of final version; R.W. contributed to study concept design, data interpretation, drafting of manuscript, manuscript revision, and approval of final version; and P.S.S. contributed to study concept design, data interpretation, manuscript revision, and approval of final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ann Kinga Malinowski, 700 University Ave, 3rd Floor, Suite 3-909,Toronto, ON, Canada M5G 1Z5; e-mail: amalinowski@mtsinai.on.ca.