Abstract
Background: Immune recovery is crucial for patients treated with allogeneic HSCT and in particular of those receiving a T-cell depleted haplo-HSCT. We recently developed a novel method of graft manipulation based on physical elimination of α/β T cells and B-lymphocytes for preventing graft-versus-host disease (GvHD) and EBV-related lymphoproliferative disorders, respectively. Thanks to this approach, we successfully conducted a prospective trial in children with malignant or non-malignant disorders (ClinicalTrial.gov identifier: NCT01810120). Although patients enrolled in this trial had faster immune recovery and lower incidence of infections than those given haplo-HSCT after infusion of positively selected CD34+ cells, reconstitution of adaptive T-cell immunity remains suboptimal. We therefore designed a phase I/II trial aimed at testing the effect on post-transplant immune recovery of adoptive infusion (within 14 + 4 days after transplantation) of BPX-501 cells in children given haplo-HSCT after depletion of α/β T and B cells (ClinicalTrials.gov identifier: NCT02065869).
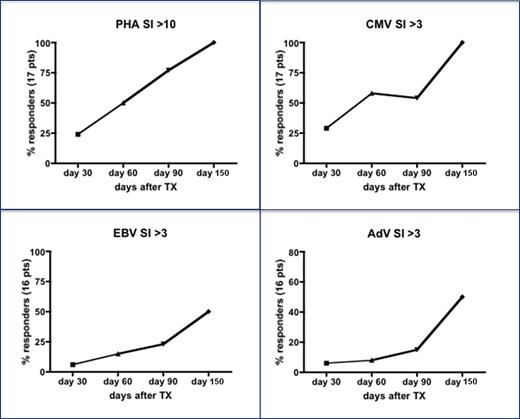
Patients and methods: As of July 25th 2015, 23 children have been infused with BPX-501 cells. The 9 children included in the phase I portion of the study were given 2.5x105, 5x105, and 1x106 BPX-501 cells/kg, respectively, while the 14 included in the phase II received 1x106 BPX-501 cells/kg. This analysis refers to the 16 patients with a minimum follow-up of 90 days; 7 children had acute leukemia and 9 non-malignant disorders. Basic phenotype of circulating lymphocytes was assessed by flow cytometry on fresh heparinized peripheral blood samples at 10, 20, 30, 60, 90, 120 and 150 days post haplo-HSCT, respectively. The following antibodies were used: anti-TCRαβ FITC/anti-TCRγδ PE/anti-CD3 PerCP-Cy™5.5 (WT31, 11F2, SK7), anti-CD4 APC Cy7 (RPA-T4), anti-CD19 BV 510 (SJ25C1), anti-CD3 BV 421 (UCHT1), anti-CD56 PeCy7 (B159), anti-CD16 APC (B73.1), anti-CD8 APC (RPA-T8) from BD Biosciences (San Diego, CA, USA). Antigen-driven activation of peripheral mononuclear cells was evaluated by standard lymphoproliferation assay (LPA) with 3H-thymidine pulsing on day 4 and harvesting 18 hours later. Antigens included PHA or CMV, EBV and AdV whole viral lysate. Results were scored positive with stimulation indexes (SI) >10 for PHA and >3 for viral antigens.
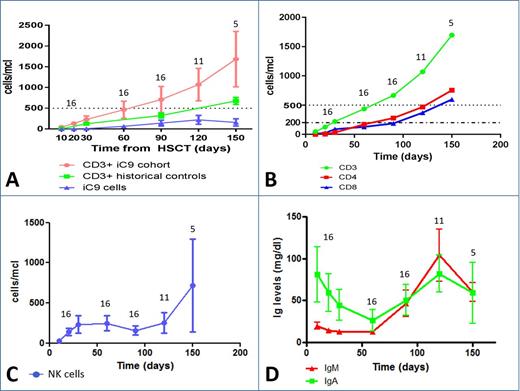
Results: None of the patients died from transplant-related complications. Chimerism analysis investigated through short tandem repeats showed that in all but 4 patients, cells were of donor origin before the infusion of BPX-501 cells. In the 4 patients, there was a reversion to complete donor chimerism after infusion of BPX-501 cells. At early time points after haplo-HSCT, gδ T cells predominated over αβ T lymphocytes; subsequently, this latter population became the more largely represented. The number of both CD3+ T lymphocytes and of BPX-501 cells is shown in Panel A of Figure 1, reconstitution of whole T cells in historical children given haplo-HSCT after depletion of α/β T cells is also shown. The number of CD3+ T lymphocytes reached greater than 0.5x109/L 2 months after infusion of BPX-501 cells. Remarkably, while usually immune recovery after transplantation is characterized by prevalence of CD8+ cells, in our patients the physiological predominance of CD4+ lymphocytes was maintained (Panel B of Figure 1. Reconstitution of natural killer cells (NK) is shown in Panel C of Figure 1. As compared to patients receiving CD34+ selected cell haplo-HSCT, children included in this study had a faster reconstitution of mature KIR+/NKG2A- NK cells. Serum levels of IgA and IgM over time are shown in Panel D of Figure 1: there was a recovery of newly synthetized Ig at 3 months. The analysis of the function of T cells showed that the proliferative response to a polyclonal mitogen or to CMV lysate was comparable to that of a healthy control in 50% of patients as early as day + 60 after haplo-HSCT and BPX-501; on day +150, all patients reached a normal SI. Response to both EBV and AdV antigens was slightly delayed, but progressively improved over time (see also Figure 2).
Conclusions: Overall, these data indicate that infusion of BPX-501 cells is able to accelerate the recovery of adaptive T-cell immunity since these cells, once infused, expand in vivo and persist over time, potentially contributing to protect patients from infections.
Moseley:Bellicum Pharmaceuticals: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal