In this issue of Blood, Liang and colleagues1 demonstrate that cartilage oligomeric matrix protein (COMP) acts as a major endogenous plasma- and platelet-derived inhibitor of thrombin activity in vitro and in vivo.
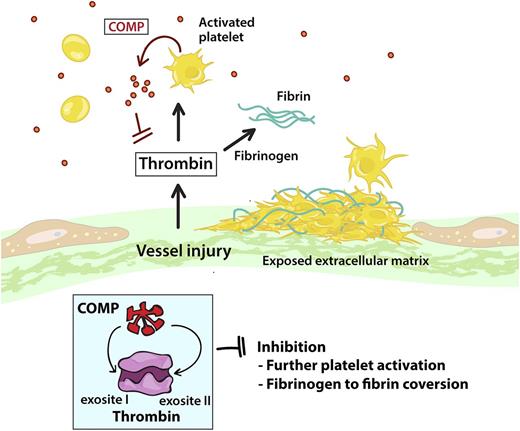
In response to vascular injury, platelets adhere to the exposed vascular extracellular matrix, become activated, and form a thrombus. The activated platelet surface is procoagulant and promotes the generation of thrombin. COMP circulates in plasma and is locally released from, and synthesized by, activated platelets, where it functions as an endogenous inhibitor of thrombin, inhibiting the conversion of fibrinogen to fibrin and limiting the degree of thrombin-induced platelet activation through PAR receptors. COMP binding to thrombin occurs through thrombin exosites I and II.
In response to vascular injury, platelets adhere to the exposed vascular extracellular matrix, become activated, and form a thrombus. The activated platelet surface is procoagulant and promotes the generation of thrombin. COMP circulates in plasma and is locally released from, and synthesized by, activated platelets, where it functions as an endogenous inhibitor of thrombin, inhibiting the conversion of fibrinogen to fibrin and limiting the degree of thrombin-induced platelet activation through PAR receptors. COMP binding to thrombin occurs through thrombin exosites I and II.
The production of thrombin through activation of the intrinsic and/or extrinsic coagulation pathways is a fundamental event in thrombosis and hemostasis. Among its many roles, thrombin is critical to blood clotting by converting fibrinogen to fibrin.2 It also functions in thrombosis and hemostasis as a potent platelet agonist through protease activated receptor (PAR)1 and PAR4 thrombin receptors on human platelets and the PAR4 receptor on mouse platelets.3 A number of inhibitors of thrombin have been identified that limit the vascular consequences of inadvertent or pathophysiological thrombin generation, including antithrombin III, heparin cofactor II, protein C inhibitor, and nexin I, all of which belong to the serpin family of protease inhibitors.4 In contrast, COMP is a member of the thrombospondin (TSP) protein family.
COMP, also known as TSP5, is an abundant extracellular matrix protein expressed in tissues of the musculoskeletal system including cartilage and tendon.5 COMP circulates in human plasma at a concentration of ∼1000 ng/mL.6 In the current issue of Blood, Liang and colleagues demonstrate that COMP is also stored in platelet α-granules and is released and further synthesized following platelet activation by platelet agonists including thrombin, collagen, and adenosine 5′-diphosphate (ADP).1 Human COMP exists as a disulfide-linked pentamer composed of 737-amino acid–length subunits. It has an N-terminal coiled-coil region containing the interchain disulfide bonds responsible for pentamer assembly, 4 type 2 epidermal growth factor-like repeats, 8 type 3 TSP repeats, and a COOH-terminal globular domain. COMP contains up to 30 calcium divalent cation binding sites that affect its structure and protein binding properties.5 In vivo, it is degraded by a subset of matrix metalloproteinase and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) metalloproteinases.5 COMP is known to bind a large number of proteins, including extracellular matrix constituents such as collagens, aggrecan, fibronectin, and matrilins; cell-surface receptors, including the integrins α5β1 and αvβ3 as well as CD47; and complement proteins and growth factors such as bone morphogenetic protein 2 (BMP-2), BMP-4, BMP-7, and transforming growth factor β.5,7 Using surface plasmon resonance, Liang and colleagues demonstrate that thrombin also binds immobilized COMP with a dissociation constant (KD) of 1.4 μM. This binding involves both thrombin exosites I and II and the COMP epidermal growth factor motifs, but not the thrombin catalytic active site, as COMP does not inhibit the cleavage of small synthetic thrombin peptide substrates (see figure).1
Liang and colleagues initially established a potential role for COMP in regulating thrombosis and hemostasis by analysis of COMP−/− mice.1 Relative to wild-type (WT) mice, COMP−/− mice had shortened tail bleeding times, reduced clotting times, accelerated thrombin-induced clot retraction in platelet-rich plasma (PRP), and more rapid vessel occlusion times in an FeCl3-induced in vivo thrombosis assay. The accelerated thrombin-induced clot retraction in COMP−/− mouse PRP was reversed by the addition of exogenously purified COMP (300 ng/mL). The thrombin time (TT) was significantly reduced for COMP−/− mice relative to WT, although the activated partial thromboplastin time (APTT) and prothrombin time (PT) were similar to WT. Addition of exogenous COMP (250-1000 ng/mL) to WT plasma progressively prolonged the TT, but the APTT and PT were only prolonged at the highest additional concentration of 1000 ng/mL, consistent with COMP inhibiting the thrombin-dependent conversion of fibrinogen to fibrin.
Exogenously purified COMP (100-300 ng/mL) dose-dependently inhibited mouse platelet aggregation induced by thrombin (0.1 U/mL), but not by PAR4 agonist peptide, collagen, and ADP. Similar results were obtained with human platelets. Importantly, COMP released by platelets was shown to inhibit further thrombin-dependent platelet activation and to be directly complexed with thrombin by coimmunoprecipitation analysis. The overall findings suggest that COMP binds thrombin, preventing the activation of platelet PAR receptors and subsequent downstream events.
Bone marrow transfer experiments with WT and COMP−/− donor mice and irradiated WT and COMP−/− recipient mice indicate that the cellular origin of COMP is bone marrow derived.
In additional experiments, washed WT and COMP−/− platelets were resuspended in COMP−/− platelet-poor plasma and infused into COMP−/− recipient mice. Relative to infusion of COMP−/− platelets, the infusion of WT platelets into COMP−/− recipient mice significantly prolonged tail bleeding times, clotting times, and occlusion times in the FeCl3-induced in vivo thrombosis assay, consistent with platelet-derived COMP as a critical and sufficient component in regulating thrombosis and hemostasis. That platelet-derived COMP prolongs the occlusion time in the FeCl3-induced in vivo thrombosis assay is consistent with previous evidence for an important role for thrombin in this thrombosis model.8
Although the present findings indicate an important role for plasma- and platelet-derived COMP as an endogenous thrombin inhibitor, there remain several important unanswered questions. Firstly, the measured KD for thrombin binding to immobilized COMP, 1.4 μM, is inconsistent with nanomolar concentrations of COMP inhibiting nanomolar concentrations of thrombin. This suggests either that the interaction is much stronger in the fluid phase, perhaps by the thrombin exosites I and II interacting with different subunits in the COMP pentamer, or that another protein or glycosaminoglycan may be involved in stabilizing the interaction. Secondly, because the authors examined platelet secretion of COMP in the presence of EDTA, it remains unclear whether COMP would be surface expressed on platelets at physiological Ca2+ concentrations. Platelets express the integrins α5β1 and αvβ3, as well as CD47, all of which are known to bind COMP.5,7 Expression of COMP on the surface of activated platelets inhibiting thrombin in the vicinity of activated platelets would provide an explanation for the significant prolongation of the tail bleeding times, clotting times, and occlusion times in the FeCl3-induced in vivo thrombosis assay when WT platelets were infused into COMP−/− recipient mice. Finally, it remains to be established whether there is a potential therapeutic benefit in utilizing or targeting COMP to affect the hemostatic balance in patients.
Conflict-of-interest disclosure: The authors declare no competing financial interests.