Key Points
Ibrutinib combined with ofatumumab in relapsed CLL had had an ORR of 83% with median time to response of <3 months in all groups.
All 3 sequences of administration were acceptably tolerated and active; responses were durable, and median PFS was not yet reached.
Abstract
Ibrutinib represents a therapeutic advance in chronic lymphocytic leukemia (CLL) but as monotherapy produces few complete remissions in previously treated patients. Anti-CD20 antibodies have improved response and progression-free survival (PFS) when combined with chemotherapy. We evaluated the safety and activity of adding ofatumumab to ibrutinib in 3 different administration sequences. Patients with CLL/small lymphocytic lymphoma (SLL), prolymphocytic leukemia, or Richter’s transformation who failed ≥2 prior therapies were enrolled. Patients received ibrutinib 420 mg daily and 12 doses of ofatumumab 300/2000 mg in 3 schedules: ibrutinib lead-in (group 1; n = 27), concurrent start (group 2; n = 20), or ofatumumab lead-in (group 3; n = 24). Seventy-one patients were treated; most had high-risk disease including del(17)(p13.1) (44%) or del(11)(q22.3) (31%). The most frequent adverse events (any grade) were diarrhea (70%), infusion-related reaction (45%), and peripheral sensory neuropathy (44%). Overall response rates in CLL/SLL patients (n = 66) were 100%, 79%, and 71% in groups 1, 2, and 3, respectively. Estimated 12-month PFSs for all patients were 89%, 85%, and 75%, respectively. Four patients in group 3 progressed prior to receiving ibrutinib. This study demonstrates the tolerability and clinical activity of this combination with quicker time to best response than single-agent ibrutinib and with durable responses. This trial was registered at www.clinicaltrials.gov as #NCT01217749.
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent form of leukemia among adults in Western countries, with increasing incidence in older individuals; median age of diagnosis is 72 years.1,2 Although chemoimmunotherapy has become the standard front-line treatment for fit patients,2,3 CLL remains incurable. Moreover, the presence of high-risk features such as unmutated immunoglobulin heavy chain variable region (IGHV), del(17)(p13.1), or transformation to high-grade lymphoma is associated with poor outcomes.4-10 Thus, new and effective regimens are needed for patients with pretreated CLL.
During the last decades, the B-cell receptor (BCR) pathway has emerged as a new therapeutic target in B-cell malignancies. Proximal within this pathway, Bruton tyrosine kinase (BTK), a member of the Tec kinase family, plays a central role in activation of downstream signaling required for survival and proliferation of malignant B cells.11-15 BTK is also critical for B-cell development and function in relation to the homing, migration, and adhesion of B cells to bone marrow or lymphoid tissues.16,17
Ibrutinib is a first-in-class, orally administered, once-daily covalent inhibitor of BTK. In preclinical models, ibrutinib induced apoptosis and decreased survival of CLL cells and inhibited their homing, migration, and adhesion to the tumor microenvironment.18-20 Recently, in the phase 3 RESONATE trial (PCYC-1112-CA) in relapsed/refractory CLL, ibrutinib demonstrated a statistically significant 78% reduction in the risk of progression or death and a 56% reduction in the risk of death compared with ofatumumab.21 Ibrutinib was US Food and Drug Administration-approved for treatment of patients with CLL who received ≥1 prior therapy and for patients with CLL with del(17)(p13.1).22
Ofatumumab is an anti-CD20 monoclonal antibody that binds to an epitope distinct from that for rituximab.23 It exhibits more potent complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) compared with rituximab in B-cell lines including CLL cells.24-26 Ofatumumab is approved in the United States for treatment of CLL refractory to fludarabine and alemtuzumab27 and in combination with chlorambucil for previously untreated CLL where fludarabine-based treatment is inappropriate.28,29
Studies with single-agent ibrutinib showed early lymphocytosis in patients with CLL,30-32 which is considered a pharmacodynamic effect of ibrutinib resulting in mobilization of lymphocytes into the peripheral blood from tissue compartments.18,20 Combining ibrutinib with an anti-CD20 monoclonal antibody to clear blood lymphocytes was thought to reduce the duration and incidence of lymphocytosis, thereby potentially shortening the time to response. Given its improved single-agent activity in CLL relative to rituximab and its availability in 2011,27 ofatumumab was chosen for this study. Although recent preclinical studies have reported potential antagonistic effects of ibrutinib when combined with anti-CD20 monoclonal antibodies,33-35 these data were unknown at the time of study conception and are unconfirmed in recent clinical studies.36
On the basis of studies showing the feasibility and activity of ofatumumab combined with chemotherapy,28,37,38 we hypothesized that the addition of ofatumumab may improve the already impressive single-agent activity of ibrutinib. The rationale for this combination is based on proven single-agent activity in relapsed/refractory CLL, nonoverlapping toxicities, and different mechanisms of antileukemic activity. The present study evaluates safety, tolerability, and efficacy of 3 different fixed-dose regimens of ibrutinib combined with ofatumumab in patients with relapsed/refractory CLL and related diseases. Because it was unknown whether the initial lymphocytosis commonly observed with ibrutinib would predispose to development of ofatumumab infusion-related reactions or tumor lysis syndrome, 3 different administration sequences were evaluated: ibrutinib was started either 4 weeks before (group 1), 1 day before (group 2), or 8 weeks after ofatumumab (group 3).
Patients and methods
Patients
Patients were enrolled between January 2011 and June 2012 and treated at The Ohio State University James Comprehensive Cancer Center after providing written informed consent. Key eligibility criteria included histologically confirmed CLL, small lymphocytic lymphoma (SLL), B-cell prolymphocytic leukemia (PLL) per the World Health Organization classification, or Richter’s transformation; indication for treatment based on the 2008 International Working Group for CLL (IWCLL) guidelines39 or requiring cytoreduction prior to stem cell transplant; failure of ≥2 prior therapies including a nucleoside analog (unless contraindicated); ≥10% expression of CD20 on CLL/SLL cells by flow cytometry; Eastern Cooperative Oncology Group performance status ≤2; and adequate end-organ function.
Study design and treatment plan
This phase 1b/2, single-center, open-label, sequential group study was institutional review board approved and conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization (ICH) Guidelines for good clinical practice (GCP) and is registered at clinicaltrials.gov (#NCT01217749).
At screening, patients underwent a complete history, physical examination, and laboratory and prognostic factor testing. Pretreatment assessments included flow cytometry, bone marrow evaluation, and computed tomography (CT) scans of the chest, abdomen, and pelvis. A baseline positron emission tomography/CT scan was performed for patients with SLL and Richter’s transformation.
Study treatment was administered in 28-day cycles. Ibrutinib was administered orally at a dose of 420 mg once daily and continued until disease progression or unacceptable toxicity. Ofatumumab was administered intravenously per prescribing information (300 mg for dose 1/2000 mg for doses 2-12). Patients could then continue daily ibrutinib in extension study PCYC-1103-CA until progression or intolerability. Patients were enrolled sequentially to receive 3 different administration sequences: group 1 with ibrutinib lead-in, group 2 with concomitant administration (ofatumumab on day 1/ibrutinib on day 2), and group 3 with ofatumumab lead-in (Figure 1). After the first 6 patients in group 1 demonstrated no DLT (within 56 days) as defined in the supplemental Materials (available on the Blood Web site), enrollment was expanded to 27 patients. Expansion of enrollment in group 2 was similarly guided by safety (≤1 DLT among the first 6 patients observed for 28 days) and antitumor activity. Based on the absence of DLTs in the first 2 cohorts, the third cohort was designed without DLT assessment. After completion of enrollment in group 1, enrollment in group 2 began, and after group 2 was completed, enrollment in group 3 began.
Treatment schema by group. Oral ibrutinib 420 mg was given once daily until disease progression or unacceptable toxicity. Intravenous ofatumumab was given as 8 weekly infusions followed by 4 monthly infusions for a total of 12 doses (dose 1, 300 mg; doses 2-12, 2000 mg). Group 1, ibrutinib monotherapy during cycle 1 and then ofatumumab added starting cycle 2; group 2, ofatumumab and ibrutinib starting on days 1 and 2 of cycle 1, respectively; group 3, ofatumumab monotherapy for the first 2 cycles and then ibrutinib added starting at cycle 3.
Treatment schema by group. Oral ibrutinib 420 mg was given once daily until disease progression or unacceptable toxicity. Intravenous ofatumumab was given as 8 weekly infusions followed by 4 monthly infusions for a total of 12 doses (dose 1, 300 mg; doses 2-12, 2000 mg). Group 1, ibrutinib monotherapy during cycle 1 and then ofatumumab added starting cycle 2; group 2, ofatumumab and ibrutinib starting on days 1 and 2 of cycle 1, respectively; group 3, ofatumumab monotherapy for the first 2 cycles and then ibrutinib added starting at cycle 3.
Treatment was withheld for any grade 4 toxicity, or the individual drug was withheld for ibrutinib-related or ofatumumab-related, clinically significant, unmanageable grade 3 adverse events (AEs). Treatment was resumed after the AE returned to baseline or resolved.
Patients received premedication with acetaminophen 650 mg, cetirizine 10 mg or equivalent, and dexamethasone 20 mg intravenously prior to each dose of ofatumumab. For doses 3 to 12 of ofatumumab, the dexamethasone dose could be gradually reduced or discontinued if no grade 3 or higher infusion reactions occurred during prior doses. Patients considered at risk for tumor lysis syndrome were to be hydrated and pretreated with antihyperuricemics as per standard ofatumumab guidance.29 Use of standard supportive care treatments was permitted.
Assessments
Responses were assessed and reported by the investigators for patients with CLL and PLL according to IWCLL guidelines39 and for SLL and Richter’s transformation according to the revised International Working Group criteria.40 Bone marrow evaluation was required to confirm a complete response (CR). Lymphocytosis (an increase in absolute lymphocyte count [ALC] of ≥50% from baseline and to >5 × 109/L) alone was not considered a treatment failure or progressive disease in the absence of other indications of disease progression.41,42 Response evaluations were performed following cycles 1 and 3 and then every 3 cycles, including CT scans of the chest, abdomen, and pelvis. Safety assessments included laboratory evaluation and physical examination. Severity of AEs was defined using the Common Terminology Criteria for Adverse Events (version 4.0) for nonhematologic AEs, and per IWCLL criteria for hematologic AEs.
Statistical considerations
Safety was evaluated in all patients who received ≥1 dose of study drug. Efficacy was determined among evaluable patients who received ≥1 dose of study drug and had baseline and ≥1 postbaseline response assessment. The minimax Simon 2-stage design was chosen to provide 85% power to reject the null hypothesis of an overall response rate (ORR) of 25% (at an ORR of 50%) when using a 1-sided 10% α-level test based on early single-agent data, which demonstrated only a modest partial response (PR) rate with short follow-up due to persistent lymphocytosis. An interim analysis for groups 1 and 2 was conducted for the first 10 evaluable patients, and the groups were expanded only if ≥3 patients achieved objective responses during the first 3 cycles. Efficacy was evaluated in each group separately, with no formal statistical analysis planned to compare outcomes among sequentially enrolled groups.
Primary end points were the number of DLTs observed among the first 6 patients enrolled in groups 1 and 2 and ORR in all groups. Secondary end points included incidence of AEs (including AEs leading to ibrutinib discontinuation), grade 3 or higher AEs, serious AEs (SAEs), time to response, duration of response (DOR), progression-free survival (PFS), and hematologic improvement (evaluated among patients with baseline cytopenias). Kaplan-Meier methodology was used to estimate DOR and PFS; descriptive statistics were used for analysis of all other end points.
Results
Patients
A total of 71 patients were enrolled. Baseline characteristics varied among the 3 sequentially enrolled groups, with a higher proportion of older patients and patients with Richter’s transformation, but fewer with del(17)(p13.1), in group 1 (Table 1). Sixty-five patients (92%) had CLL, 1 (1%) had SLL, 2 (3%) had PLL, and 3 (4%) had Richter’s transformation. Median age was 64 years (range, 48-85 years). The majority (61%) had high-risk disease stage (Rai stage III or IV); 75% had bulky lymph nodes (≥5 cm), 44% had del(17)(p13.1), and 31% had del(11)(q22.3) (Table 1). Baseline cytopenias were present in 70% of patients. Patients had received a median of 3 prior therapies (range, 2-13) that typically included an alkylating agent, purine analog, and rituximab (Table 2).
Baseline demographic and clinical characteristics
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . | All patients (N = 71) . |
|---|---|---|---|---|
| Median age (range), years | 66 (51-85) | 63 (48-75) | 63 (50-71) | 64 (48-85) |
| ≥65 years | 14 (52) | 8 (40) | 9 (38) | 31 (44) |
| ≥70 years | 12 (44) | 4 (20) | 1 (4) | 17 (24) |
| Diagnosis, n (%) | ||||
| CLL | 22 (82) | 19 (95) | 24 (100) | 65 (92) |
| SLL | 1 (4) | 0 (0) | 0 (0) | 1 (1) |
| PLL | 1 (4) | 1 (5) | 0 (0) | 2 (3) |
| Richter’s transformation | 3 (11) | 0 (0) | 0 (0) | 3 (4) |
| ECOG performance status, n (%) | ||||
| 0 | 10 (37) | 8 (40) | 5 (21) | 23 (32) |
| 1 | 16 (59) | 11 (55) | 15 (63) | 42 (59) |
| 2 | 1 (4) | 1 (5) | 4 (17) | 6 (9) |
| Bulky disease, n (%) | ||||
| Lymph nodes ≥5 cm | 19 (70) | 13 (65) | 21 (88) | 53 (75) |
| Lymph nodes ≥10 cm | 3 (11) | 3 (15) | 5 (21) | 11 (16) |
| Rai risk classification,*n (%) | ||||
| Low risk | 1 (4) | 0 (0) | 1 (4) | 2 (3) |
| Intermediate risk | 11 (41) | 5 (25) | 8 (33) | 24 (34) |
| High risk | 14 (52) | 14 (70) | 15 (63) | 43 (61) |
| Not reported | 1 (4) | 1 (5) | (0) | 2 (3) |
| Cytopenia at baseline, n (%) | ||||
| ANC ≤1500/µL | 5 (19) | 6 (30) | 9 (38) | 20 (28) |
| Hemoglobin ≤11 g/dL | 11 (41) | 12 (60) | 10 (42) | 33 (47) |
| Platelets ≤100 000/µL | 13 (48) | 12 (60) | 11 (46) | 36 (51) |
| Prognostic factors, n (%) | ||||
| Unmutated IGHV | 20 (74) | 13 (65) | 17 (71) | 50 (70) |
| Del17(17)(p13.1) | 10 (37) | 9 (45) | 12 (50) | 31 (44) |
| Del(11)(q22.3) | 9 (33) | 6 (30) | 7 (29) | 22 (31) |
| β2-Microglobulin >3 mg/L | 15 (56) | 12 (60) | 13 (54) | 40 (56) |
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . | All patients (N = 71) . |
|---|---|---|---|---|
| Median age (range), years | 66 (51-85) | 63 (48-75) | 63 (50-71) | 64 (48-85) |
| ≥65 years | 14 (52) | 8 (40) | 9 (38) | 31 (44) |
| ≥70 years | 12 (44) | 4 (20) | 1 (4) | 17 (24) |
| Diagnosis, n (%) | ||||
| CLL | 22 (82) | 19 (95) | 24 (100) | 65 (92) |
| SLL | 1 (4) | 0 (0) | 0 (0) | 1 (1) |
| PLL | 1 (4) | 1 (5) | 0 (0) | 2 (3) |
| Richter’s transformation | 3 (11) | 0 (0) | 0 (0) | 3 (4) |
| ECOG performance status, n (%) | ||||
| 0 | 10 (37) | 8 (40) | 5 (21) | 23 (32) |
| 1 | 16 (59) | 11 (55) | 15 (63) | 42 (59) |
| 2 | 1 (4) | 1 (5) | 4 (17) | 6 (9) |
| Bulky disease, n (%) | ||||
| Lymph nodes ≥5 cm | 19 (70) | 13 (65) | 21 (88) | 53 (75) |
| Lymph nodes ≥10 cm | 3 (11) | 3 (15) | 5 (21) | 11 (16) |
| Rai risk classification,*n (%) | ||||
| Low risk | 1 (4) | 0 (0) | 1 (4) | 2 (3) |
| Intermediate risk | 11 (41) | 5 (25) | 8 (33) | 24 (34) |
| High risk | 14 (52) | 14 (70) | 15 (63) | 43 (61) |
| Not reported | 1 (4) | 1 (5) | (0) | 2 (3) |
| Cytopenia at baseline, n (%) | ||||
| ANC ≤1500/µL | 5 (19) | 6 (30) | 9 (38) | 20 (28) |
| Hemoglobin ≤11 g/dL | 11 (41) | 12 (60) | 10 (42) | 33 (47) |
| Platelets ≤100 000/µL | 13 (48) | 12 (60) | 11 (46) | 36 (51) |
| Prognostic factors, n (%) | ||||
| Unmutated IGHV | 20 (74) | 13 (65) | 17 (71) | 50 (70) |
| Del17(17)(p13.1) | 10 (37) | 9 (45) | 12 (50) | 31 (44) |
| Del(11)(q22.3) | 9 (33) | 6 (30) | 7 (29) | 22 (31) |
| β2-Microglobulin >3 mg/L | 15 (56) | 12 (60) | 13 (54) | 40 (56) |
ECOG, Eastern Cooperative Oncology Group.
Low risk, stage 0; intermediate risk, stage I or II; high risk, stage III or IV.
Prior systemic therapy
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . | All patients (N = 71) . |
|---|---|---|---|---|
| Median number (range) of prior systemic therapies | 3 (2-10) | 3 (2-13) | 4 (2-12) | 3 (2-13) |
| Types of prior systemic therapy, n (%) | ||||
| Immunotherapy | ||||
| Antibody therapy | 27 (100) | 20 (100) | 24 (100) | 71 (100) |
| Rituximab | 26 (96) | 20 (100) | 24 (100) | 70 (99) |
| Alemtuzumab | 4 (15) | 1 (5) | 4 (17) | 9 (13) |
| Corticosteroids | 15 (56) | 10 (50) | 14 (58) | 39 (55) |
| Chemotherapy | ||||
| Purine analogs | 24 (89) | 20 (100) | 21 (88) | 65 (92) |
| Alkylating agents | 20 (74) | 9 (45) | 14 (58) | 43 (61) |
| Anthracyclines | 6 (22) | 2 (10) | 2 (8) | 10 (14) |
| Other therapy | ||||
| Small molecule | 9 (33) | 5 (25) | 6 (25) | 20 (28) |
| Immunomodulating agent | 8 (30) | 4 (20) | 5 (21) | 17 (24) |
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . | All patients (N = 71) . |
|---|---|---|---|---|
| Median number (range) of prior systemic therapies | 3 (2-10) | 3 (2-13) | 4 (2-12) | 3 (2-13) |
| Types of prior systemic therapy, n (%) | ||||
| Immunotherapy | ||||
| Antibody therapy | 27 (100) | 20 (100) | 24 (100) | 71 (100) |
| Rituximab | 26 (96) | 20 (100) | 24 (100) | 70 (99) |
| Alemtuzumab | 4 (15) | 1 (5) | 4 (17) | 9 (13) |
| Corticosteroids | 15 (56) | 10 (50) | 14 (58) | 39 (55) |
| Chemotherapy | ||||
| Purine analogs | 24 (89) | 20 (100) | 21 (88) | 65 (92) |
| Alkylating agents | 20 (74) | 9 (45) | 14 (58) | 43 (61) |
| Anthracyclines | 6 (22) | 2 (10) | 2 (8) | 10 (14) |
| Other therapy | ||||
| Small molecule | 9 (33) | 5 (25) | 6 (25) | 20 (28) |
| Immunomodulating agent | 8 (30) | 4 (20) | 5 (21) | 17 (24) |
All 71 patients received study treatment, 68 (96%) were evaluable for response, and 66 (93%) had CLL/SLL. Reasons for treatment discontinuation are shown in Table 3. Nine patients (13%) discontinued treatment due to progressive disease including 4 patients in group 3 who had progressive disease during ofatumumab monotherapy; 7 (10%) discontinued due to AEs. Fifty-four patients (76%) continued ibrutinib treatment in a long-term extension study, and 2 (3%) received an allogeneic stem cell transplant. Median duration of ibrutinib therapy in the primary study was 15.8 months in group 1, 11.3 months in group 2, and 9.2 months in group 3 (Table 3). The duration of ofatumumab treatment was similar for all 3 groups (median, 5.6 months).
Patient disposition and study treatment exposure
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . |
|---|---|---|---|
| Received study treatment, n (%) | 27 (100) | 20 (100) | 24 (100) |
| Enrolled into extension study, n (%)* | 19 (70) | 16 (80) | 19 (79)* |
| Discontinued treatment, n (%) | |||
| Adverse event | 2 (7) | 3 (15) | 2 (8) |
| Progressive disease | 4 (15) | 0 (0) | 5 (21) |
| Underwent stem cell transplant | 1 (4) | 1 (5) | 0 (0) |
| Noncompliance with study drug | 1 (4) | 0 (0) | 0 (0) |
| Median duration of treatment with ibrutinib (range), months | 15.8 (4.5-19.5) | 11.3 (0.4-14.9) | 9.2 (0.7-11.6) |
| Median duration of treatment with ofatumumab (range), months | 5.6 (1.4-9.6) | 5.6 (0-7.4) | 5.6 (0-8.8) |
| Median number of infusions with ofatumumab (range) | 12 (7-12) | 10.5 (1-12) | 12 (1-12) |
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . |
|---|---|---|---|
| Received study treatment, n (%) | 27 (100) | 20 (100) | 24 (100) |
| Enrolled into extension study, n (%)* | 19 (70) | 16 (80) | 19 (79)* |
| Discontinued treatment, n (%) | |||
| Adverse event | 2 (7) | 3 (15) | 2 (8) |
| Progressive disease | 4 (15) | 0 (0) | 5 (21) |
| Underwent stem cell transplant | 1 (4) | 1 (5) | 0 (0) |
| Noncompliance with study drug | 1 (4) | 0 (0) | 0 (0) |
| Median duration of treatment with ibrutinib (range), months | 15.8 (4.5-19.5) | 11.3 (0.4-14.9) | 9.2 (0.7-11.6) |
| Median duration of treatment with ofatumumab (range), months | 5.6 (1.4-9.6) | 5.6 (0-7.4) | 5.6 (0-8.8) |
| Median number of infusions with ofatumumab (range) | 12 (7-12) | 10.5 (1-12) | 12 (1-12) |
Includes 2 patients in group 3 who discontinued ofatumumab during the ofatumumab lead-in period due to disease progression, then started on ibrutinib monotherapy, and later enrolled into the extension study.
Safety
No DLTs occurred. The most common treatment-emergent AEs were diarrhea (70%), infusion-related reaction (45%), peripheral sensory neuropathy (44%), and stomatitis (38%) (Table 4). These AEs were mostly grade 1 or 2. A total of 37 events of peripheral sensory neuropathy occurred among 31 patients; 13 of these patients (42%) had a prior history of sensory neuropathy, and 13 (42%) had a history of diabetes mellitus or hyperglycemia prior to study start. The median time to first onset of peripheral sensory neuropathy was 57 days (range, 8-451 days); 25 of the 37 events (68%) resolved with a median time to resolution of 205 days (95% confidence interval [CI], 114-365). Atrial fibrillation of any grade occurred in 6 patients (8.5%), of which 2 (3%) were grade 3 or higher events. Overall, 45 patients (63%) had grade 3 or higher AEs, the most common events being neutropenia (24%), pneumonia (17%), and diarrhea (7%). During the study, 8 patients (11%) received treatment with neutrophil growth factor (filgrastim). Only 1 grade 3 infusion reaction (group 2) was reported in all 71 patients. Eight patients (11%) had AEs that led to ibrutinib discontinuation, 3 patients each in groups 1 and 2 and 2 patients in group 3.
Summary of AEs
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . | All patients (N = 71) . | ||||
|---|---|---|---|---|---|---|---|---|
| Any . | Grade ≥3 . | Any . | Grade ≥3 . | Any . | Grade ≥3 . | Any . | Grade ≥3 . | |
| Patients with AEs leading to treatment discontinuation, n (%) | 3 (11) | 3 (11) | 3 (15) | 3 (15) | 2 (8) | 2 (8) | 8 (11) | 8 (11) |
| Most common treatment-emergent AEs* | ||||||||
| Diarrhea | 18 (67) | 2 (7) | 16 (80) | 3 (15) | 16 (67) | 0 (0) | 50 (70) | 5 (7) |
| Infusion-related reaction | 9 (33) | 0 (0) | 14 (70) | 1 (5) | 9 (38) | 0 (0) | 32 (45) | 1 (1) |
| Peripheral sensory neuropathy | 11 (41) | 1 (4) | 8 (40) | 0 (0) | 12 (50) | 1 (4) | 31 (44) | 2 (3) |
| Stomatitis | 11 (41) | 2 (7) | 9 (45) | 0 (0) | 7 (29) | 0 (0) | 27 (38) | 2 (3) |
| Contusion | 9 (33) | 0 (0) | 8 (40) | 0 (0) | 3 (13) | 0 (0) | 20 (28) | 0 (0) |
| Upper respiratory tract infection | 7 (26) | 0 (0) | 8 (40) | 0 (0) | 4 (17) | 0 (0) | 19 (27) | 0 (0) |
| Nausea | 5 (19) | 0 (0) | 6 (30) | 0 (0) | 6 (25) | 0 (0) | 17 (24) | 0 (0) |
| Increased tendency to bruise | 10 (37) | 0 (0) | 5 (25) | 0 (0) | 2 (8) | 0 (0) | 17 (24) | 0 (0) |
| Petechiae | 6 (22) | 0 (0) | 5 (25) | 0 (0) | 6 (25) | 0 (0) | 17 (24) | 0 (0) |
| Neutropenia† | 5 (19) | 5 (19) | 7 (35) | 7 (35) | 5 (21) | 5 (21) | 17 (24) | 17 (24) |
| Muscle spasms | 10 (37) | 0 (0) | 4 (20) | 0 (0) | 2 (8) | 0 (0) | 16 (23) | 0 (0) |
| Fatigue | 13 (48) | 2 (7) | 1 (5) | 0 (0) | 1 (4) | 0 (0) | 15 (21) | 2 (3) |
| Pneumonia | 7 (26) | 5 (19) | 3 (15) | 3 (15) | 4 (17) | 4 (17) | 14 (20) | 12 (17) |
| Peripheral edema | 7 (26) | 0 (0) | 2 (10) | 0 (0) | 4 (17) | 0 (0) | 13 (18) | 0 (0) |
| Pain in extremity | 6 (22) | 0 (0) | 3 (15) | 1 (5) | 3 (13) | 0 (0) | 12 (17) | 1 (1) |
| Arthralgia | 5 (19) | 0 (0) | 2 (10) | 0 (0) | 5 (21) | 0 (0) | 12 (17) | 0 (0) |
| Dyspepsia | 5 (19) | 0 (0) | 4 (20) | 0 (0) | 2 (8) | 0 (0) | 11 (16) | 0 (0) |
| Sinusitis | 6 (22) | 0 (0) | 2 (10) | 0 (0) | 3 (13) | 0 (0) | 11 (16) | 0 (0) |
| Insomnia | 5 (19) | 0 (0) | 6 (30) | 0 (0) | 0 (0) | 0 (0) | 11 (16) | 0 (0) |
| Anemia | 7 (26) | 0 (0) | 2 (10) | 0 (0) | 2 (8) | 0 (0) | 11 (16) | 0 (0) |
| . | Group 1: ibrutinib → ofatumumab (N = 27) . | Group 2: ibrutinib/ofatumumab (N = 20) . | Group 3: ofatumumab → ibrutinib (N = 24) . | All patients (N = 71) . | ||||
|---|---|---|---|---|---|---|---|---|
| Any . | Grade ≥3 . | Any . | Grade ≥3 . | Any . | Grade ≥3 . | Any . | Grade ≥3 . | |
| Patients with AEs leading to treatment discontinuation, n (%) | 3 (11) | 3 (11) | 3 (15) | 3 (15) | 2 (8) | 2 (8) | 8 (11) | 8 (11) |
| Most common treatment-emergent AEs* | ||||||||
| Diarrhea | 18 (67) | 2 (7) | 16 (80) | 3 (15) | 16 (67) | 0 (0) | 50 (70) | 5 (7) |
| Infusion-related reaction | 9 (33) | 0 (0) | 14 (70) | 1 (5) | 9 (38) | 0 (0) | 32 (45) | 1 (1) |
| Peripheral sensory neuropathy | 11 (41) | 1 (4) | 8 (40) | 0 (0) | 12 (50) | 1 (4) | 31 (44) | 2 (3) |
| Stomatitis | 11 (41) | 2 (7) | 9 (45) | 0 (0) | 7 (29) | 0 (0) | 27 (38) | 2 (3) |
| Contusion | 9 (33) | 0 (0) | 8 (40) | 0 (0) | 3 (13) | 0 (0) | 20 (28) | 0 (0) |
| Upper respiratory tract infection | 7 (26) | 0 (0) | 8 (40) | 0 (0) | 4 (17) | 0 (0) | 19 (27) | 0 (0) |
| Nausea | 5 (19) | 0 (0) | 6 (30) | 0 (0) | 6 (25) | 0 (0) | 17 (24) | 0 (0) |
| Increased tendency to bruise | 10 (37) | 0 (0) | 5 (25) | 0 (0) | 2 (8) | 0 (0) | 17 (24) | 0 (0) |
| Petechiae | 6 (22) | 0 (0) | 5 (25) | 0 (0) | 6 (25) | 0 (0) | 17 (24) | 0 (0) |
| Neutropenia† | 5 (19) | 5 (19) | 7 (35) | 7 (35) | 5 (21) | 5 (21) | 17 (24) | 17 (24) |
| Muscle spasms | 10 (37) | 0 (0) | 4 (20) | 0 (0) | 2 (8) | 0 (0) | 16 (23) | 0 (0) |
| Fatigue | 13 (48) | 2 (7) | 1 (5) | 0 (0) | 1 (4) | 0 (0) | 15 (21) | 2 (3) |
| Pneumonia | 7 (26) | 5 (19) | 3 (15) | 3 (15) | 4 (17) | 4 (17) | 14 (20) | 12 (17) |
| Peripheral edema | 7 (26) | 0 (0) | 2 (10) | 0 (0) | 4 (17) | 0 (0) | 13 (18) | 0 (0) |
| Pain in extremity | 6 (22) | 0 (0) | 3 (15) | 1 (5) | 3 (13) | 0 (0) | 12 (17) | 1 (1) |
| Arthralgia | 5 (19) | 0 (0) | 2 (10) | 0 (0) | 5 (21) | 0 (0) | 12 (17) | 0 (0) |
| Dyspepsia | 5 (19) | 0 (0) | 4 (20) | 0 (0) | 2 (8) | 0 (0) | 11 (16) | 0 (0) |
| Sinusitis | 6 (22) | 0 (0) | 2 (10) | 0 (0) | 3 (13) | 0 (0) | 11 (16) | 0 (0) |
| Insomnia | 5 (19) | 0 (0) | 6 (30) | 0 (0) | 0 (0) | 0 (0) | 11 (16) | 0 (0) |
| Anemia | 7 (26) | 0 (0) | 2 (10) | 0 (0) | 2 (8) | 0 (0) | 11 (16) | 0 (0) |
Occurring in >15% of the study population.
Includes preferred terms of neutropenia and decreased neutrophil counts.
Overall, 31 patients (44%) had SAEs, including 12 (44%) in group 1, 9 (45%) in group 2, and 10 (42%) in group 3. The most common SAEs included pneumonia (16%) and atrial fibrillation (6%). Seven patients (10%) had protocol-defined major bleeding events. Seven patients experienced a clinically significant hemorrhagic event, 1 of which (subdural hematoma) had a fatal outcome. Four of the 7 major hemorrhages were postprocedural; 2 of the 4 procedures occurred without a prior ibrutinib dose hold, which was implemented after additional ibrutinib dosing experience and is currently recommended. The 7 events were grade 3 hemothorax (after thoracentesis for symptomatic, recurrent pleural effusion, n = 1), grade 3 postprocedural hemorrhage (sinus surgery, n = 1; bone marrow biopsy, n = 1), grade 3 hematoma (in the knee [Baker’s cyst], postsurgical, n = 1), grade 2 gastrointestinal hemorrhage (due to gastric ulcer, n = 1), grade 3 gastrointestinal hemorrhage (due to esophageal varices hemorrhage in a patient with ongoing medical history of esophageal varices, n = 1), and subdural hematoma (n = 1). The patient who experienced a subdural hematoma was taking warfarin and enoxaparin as thromboprophylaxis during the study due to a recent history of deep vein thrombosis. Other fatal non–progressive disease events within 30 days of the last dose of study treatment occurred in 6 patients including pneumonia (n = 2), organizing pneumonia (n = 1), cardiorespiratory arrest (n = 1), ischemic stroke (n = 1), and sepsis (n = 1).
Efficacy
The ORR among patients with CLL/SLL was 100% (95% CI, 85.2-100%) in group 1, 78.9% (95% CI, 54.4-93.9%) in group 2, and 70.8% (95% CI, 48.9-87.4%) in group 3. Among all CLL/SLL patients, the ORR was 83.3% (95% CI, 72.1-91.4%). One patient (1.5%) achieved a CR and 54 patients (81.8%) achieved a PR. Two additional patients (3%) had a PR with lymphocytosis (PR-L). The best response among patients with CLL/SLL is shown in Figure 2A. Both PLL patients responded to treatment with best responses of CR and PR, with DORs of 9.2+ and 11.3+ months, respectively, continued on ibrutinib in the long-term extension study, and were in response at the time of this report. Minimal residual disease was detected by flow cytometry in the 2 patients (CLL, PLL) with a CR, although with short follow-up. Two of the patients with Richter’s transformation had stable disease for 471 and 137 days before developing disease progression; 1 other patient with Richter’s transformation had a best response of PR (DOR, 4.6 months) before developing disease progression on day 168. Four patients (17%) in group 3 progressed on ofatumumab monotherapy before initiating ibrutinib. The presence of high-risk features did not appear to mitigate efficacy with short follow-up (Figure 2B).
Outcomes with study treatment. (A) Best response among CLL/SLL patients by group. PD, progressive disease; SD, stable disease. *Four patients (17%) in group 3 developed PD while receiving ofatumumab monotherapy. (B) Forest plot of response rates by patient subgroups. (C) Median percent change in ALC from baseline by group. (D) Median percent change in the sum of the products of lymph node diameters (SPD) and ALC by group.
Outcomes with study treatment. (A) Best response among CLL/SLL patients by group. PD, progressive disease; SD, stable disease. *Four patients (17%) in group 3 developed PD while receiving ofatumumab monotherapy. (B) Forest plot of response rates by patient subgroups. (C) Median percent change in ALC from baseline by group. (D) Median percent change in the sum of the products of lymph node diameters (SPD) and ALC by group.
Overall, 37 of 70 patients (53%) developed lymphocytosis, including 17 (63%) in group 1, 11 (55%) in group 2, and 9 (39%) in group 3. The median time to peak ALC was 3.1 weeks in group 1, 1.1 weeks in group 2, and 13.1 weeks in group 3, reflecting the dosing schedule (Figure 2C). At the time of analysis, lymphocytosis resolved in all patients in group 1 with a median time to resolution of 12.1 weeks; in 9 of 11 patients in group 2 (median time to resolution, 7.6 weeks); and in 6 of 9 patients in group 3 (median time to resolution, 21.1 weeks). A reduction in lymph node size was noted for all groups (Figure 2D). As expected, concomitant lymphocytosis was more pronounced for patients in group 1 than groups 2 and 3 due to the later start of ofatumumab treatment; in all groups, ALC decreased over time.
Overall, 39 of 50 patients (78%) who had baseline cytopenias showed improvement in ≥1 hematologic parameter. Sustained improvement (defined as ≥50% improvement above baseline values or ANC >1500/µL or hemoglobin >11 g/dL or platelets >100 000/µL lasting ≥56 days without transfusion or use of growth factors) was seen in 12 of 20 patients (60%) with baseline neutropenia, 18 of 33 patients (55%) with anemia, and 25 of 36 patients (69%) with thrombocytopenia.
Among the 58 patients who responded to study treatment across diseases, the median time to initial response was 2.8 months (range, 1.0-6.0) in group 1, 1 month (range, 1.0-3.1) in group 2, and 2.8 months (range, 2.7-7.4) in group 3. Both patients who achieved a CR had a confirmatory bone marrow biopsy at 12.2 months, which is the time point CR was noted. The median time to best response was 2.8 months (range, 1.0-12.2) for all responders; in groups 1, 2, and 3, the time to best response was 3.8, 2.8, and 4.6 months, respectively. The median DOR has not yet been reached for the overall study population or individual groups. At 12 months, the estimated rate of continued response was 88.9% (95% CI, 74.3-95.4%). At the end of the study, 52 responding patients (89.7%) remained alive and progression free.
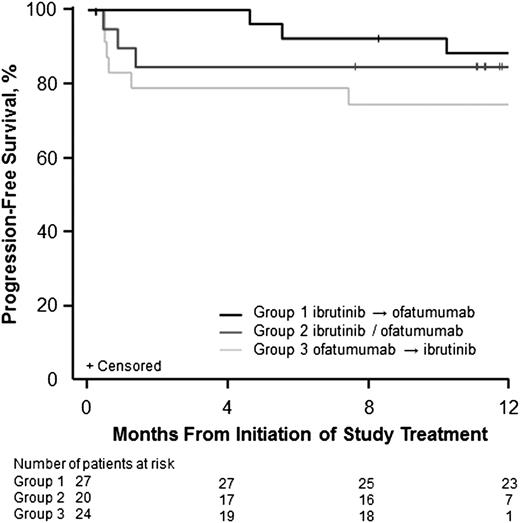
At a median time on study of 12.5 months, median PFS had not yet been reached; median follow-up was 16.4, 11.8, and 11.1 months for group 1, 2, and 3, respectively. The majority (76%) of patients continued on ibrutinib in a long-term extension study. Two patients (2.8%) had a stem cell transplant. The estimated 12-month PFS rate was 83.1% (95% CI, 72.1-90%) for the entire study population, 88.7% (95% CI, 69.0-96.2%) in group 1, 85% (95% CI, 60.4-94.9%) in group 2, and 75% (95% CI, 52.6-87.9%) in group 3 (Figure 3). Estimated 12-month overall survival was 88.6% (95% CI, 78.6-94.2%) for the entire study population, 92.3% (95% CI, 72.5-98%) in group 1, 85% (95% CI, 60.4-94.9%) in group 2, and 87.5% (95% CI, 66.1-95.8%) in group 3.
Discussion
This study demonstrates that ibrutinib and ofatumumab showed high clinical activity in patients with relapsed/refractory CLL/SLL in all 3 dose administration sequences investigated. These patients were heavily pretreated, and the majority had high-risk disease features. The response rates in patients with CLL/SLL in all 3 groups appeared to occur quicker than expected based on previous experience with single-agent ibrutinib with this relatively short follow-up time.27,31 Response to therapy was highest in the group that received ibrutinib for 1 month prior to ofatumumab and lowest among patients receiving the lead-in with ofatumumab, possibly reflective of the diminished efficacy of ofatumumab compared with ibrutinib as recently demonstrated in the phase 3 RESONATE trial in previously treated patients with CLL.21 The high ORR of the combination was consistent across patient subgroups, even among patients with high-risk features such as del(17)(p13.1), unmutated IGHV, and elevated β2-microglobulin levels. Further, the present study, which enrolled patients with PLL arising from CLL and also diffuse large B-cell lymphoma (Richter’s transformation), suggests the possibility of disease control with this combination regimen in patients with aggressive disease, which is confirmed by recent data demonstrating activity with single-agent ibrutinib in a subset of patients with large-cell transformation.43 Toxicity was similar among groups but did include neuropathy (mostly less than grade 2), which did not limit completion of therapy in the majority of patients.
Relative to single-agent ibrutinib, the CR rate was not appreciably enhanced with the combination regimen; this may be explained by the relatively short median follow-up time (12.5 months). In patients with CLL receiving ibrutinib, the quality of response has been shown to improve over time.31 The median time to best response in the 2 patients who achieved a CR was 12.2 months in both patients (due to the timing of the confirmatory bone marrow biopsy). Thus, longer follow-up may be needed to show a CR benefit.
By standard response criteria, investigator-determined ORR for the CLL/SLL cohort (83.3%) appeared favorable relative to ORR with single-agent ibrutinib in PCYC-1102-CA (71% with a median of 20.9 months of follow-up, with an additional 15% of patients achieving PR-L) when that study was initially reported,31 with a median time to best response of 2.8 months with the combination. Notably, the rate of PR-L with the combination (3%; 95% CI, 0.4-10.5%) was appreciably lower than with single-agent ibrutinib.31 It is well established at this point that response improves with time with ibrutinib, with a 90% ORR reported in the 3-year follow-up of patients on PCYC-1102-CA, where 94% of patients with a PR-L ultimately converted to a deeper response.44 Further follow-up is needed to evaluate the longer-term PFS outcomes with the combination relative to single-agent ibrutinib therapy. Additionally, randomized studies of ibrutinib with ofatumumab or other CD20 antibody study will be required to determine the added benefit of combination treatment.
Concurrent administration of ibrutinib and rituximab has been reported with similar efficacy as assessed by response in a recent phase 2 trial in patients with high-risk CLL (N = 40).36 The ORR with the combination was 95% (CR in 8% including 1 minimal residual disease–negative CR); the 18-month PFS was 78%, and the median PFS was not reached.36 Similar to the present study, responses with lymphocytosis (PR-L) were uncommon (8% after 6 cycles; 0% after 12 months) given the early resolution of lymphocytosis with the combination.
Lymphocytosis is a well-described pharmacodynamic class effect of BCR-inhibiting agents; with ibrutinib, this occurs by inhibition of BTK-mediated B-cell homing and adhesion to the tumor microenvironment, resulting in mobilization of leukemic cells from the lymph node compartment to the peripheral blood.18,20 Lymphocytosis developed in 53% of the total population of the present study, with higher rates observed when ibrutinib was started 1 month before ofatumumab (group 1: 63%) and lower rates when ofatumumab was started as monotherapy for 2 months (group 3: 39%). In previous studies with single-agent ibrutinib in CLL, lymphocytosis developed in a higher proportion of patients (more than two-thirds), suggesting that the addition of an anti-CD20 monoclonal antibody may decrease the rate of lymphocytosis observed with ibrutinib.21,31 Temporal differences in lymphocytosis patterns across the 3 groups were expected given the difference in the dosing sequence of ibrutinib relative to ofatumumab. A recent analysis from the phase 2 trial of single-agent ibrutinib in patients with relapsed/refractory CLL showed that PR with prolonged lymphocytosis was not associated with inferior PFS outcomes compared with a traditional clinical response.32 This finding with single-agent ibrutinib32 along with data from the present study with the combination of ibrutinib and ofatumumab and reported attenuation of lymphocytosis with the combination of ibrutinib and rituximab36 raises an important question on whether additional targeting of lymphocytosis with anti-CD20 monoclonal antibodies improves long-term PFS/overall survival outcomes over single-agent ibrutinib therapy.
The most common AEs (eg, diarrhea, infusion-related reactions, contusion/bruising, upper respiratory tract infection) observed with the combination were consistent with the safety profile of the single agents in previous trials.21,27,31 Major bleeding events ≥ grade 3 occurred in 6 patients (8%), which is consistent with the rate reported for single-agent ibrutinib in PCYC-1102-CA, albeit slightly higher than in the recent randomized RESONATE study of single-agent ibrutinib (1%) vs ofatumumab (1.6%).21,31 This may be due to implementation of an ibrutinib dose hold around procedures in the RESONATE study. Although 13 patients did have a prior history of neuropathy, peripheral sensory neuropathy was reported at a relatively high rate (42%) in the present study. However, the observed neurotoxicity was mostly grade 1 or 2 severity, was not treatment limiting in any case, and generally resolved with time following completion of ofatumumab. Notably, peripheral sensory neuropathy was not a frequent AE in earlier single-agent studies with ibrutinib.21,31 However, in the randomized RESONATE study, peripheral sensory neuropathy (grade 1 and 2) was noted at a higher rate (13%) with ofatumumab compared with ibrutinib (4%).21 The time course of neuropathy in the current study suggests overlap with the ofatumumab administration. These results suggest that, although peripheral neuropathy may be associated with ofatumumab therapy,21 it likely is exacerbated in combination with ibrutinib. Peripheral neuropathy was not commonly noted in another trial investigating rituximab and ibrutinib (9%).36 Generally, this complication did not limit the ability to administer either therapy as part of the current trial. Moving forward, given that ofatumumab has this unique toxicity different from observed with rituximab, superiority to this later agent would have to be demonstrated to actively encourage use of this regimen.
Recently, preclinical studies have reported that ibrutinib may inhibit ADCC observed with anti-CD20 monoclonal antibodies.33-35 However, these findings have not been substantiated clinically, suggesting that preclinical findings may not directly translate to the clinical setting. Moreover, other recent preclinical studies showed conflicting results on the effect of ibrutinib on ADCC.45,46 In 1 report, although ibrutinib was found to initially abrogate B-cell depletion with rituximab, over time, a significant recovery of ADCC activity and restoration of anti-CD20 antibody–mediated B-cell depletion was noted in CLL cells from ibrutinib-treated patients.46 In another report, under physiologically relevant experimental conditions, ibrutinib pretreatment had a minimal effect on ADCC of anti-CD20 antibodies in lymphoma cell lines.45 Clinical data from the current study, as well as the phase 2 study of ibrutinib combined with rituximab in high-risk CLL,36 consistently show additive activity with the combination regimen compared with either ibrutinib or anti-CD20 monoclonal antibody administered as a single agent. In the current study, it is apparent from the ALC data that the addition of ofatumumab to ibrutinib treatment played a role in eliminating peripheral blood lymphocytes regardless of the sequence administered. Further, in vitro CDC observed with ofatumumab is unaffected by ibrutinib.34 Because ibrutinib induces an egress of lymphocytes from tissues into the peripheral blood where complement is abundant, its combination with an anti-CD20 monoclonal antibody that mediates CDC may be effective and contribute to the benefit shown in this study.
In conclusion, we demonstrate that the combination of ibrutinib and ofatumumab exhibited clinical activity and an acceptable safety profile in heavily pretreated patients with relapsed/refractory CLL/SLL in all 3 administration sequences investigated. Unexpectedly, we observed mild and time-limited neuropathy with the combination of ofatumumab and ibrutinib. Moving forward, the long-term benefit of adding a CD20 antibody to ibrutinib compared with ibrutinib monotherapy will require phase 3 studies that assess both efficacy and also added toxicity of the combination treatment. Such phase 3 trials using rituximab are currently ongoing at this time.
Presented in part as a poster presentation at the 2014 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, May 30 to June 3, 2014, and as an oral presentation at the 2012 ASCO Annual Meeting, Chicago, IL, June 1 to 5, 2012.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients who participated in this study and their families. Editorial assistance was provided by Maoko Naganuma Carter, CMPP (funded by Pharmacyclics, Inc), after the first draft was generated by J.C.B. and S.M.J. In addition, the authors acknowledge Ahmed Hamdy, MD, and Raquel Izumi, PhD, who contributed to design of the study and initial management of the trial at Pharmacyclics but left before completion of the work.
This study was funded by Pharmacyclics, Inc. Additional research funding was provided by the Conquer Cancer Foundation Young Investigator Award, the American Society of Hematology Scholar Award, the Leukemia and Lymphoma Society Specialized Centers of Research grant, and a National Institutes of Health National Cancer Institute grants K12 CA133250 and P50 CA140158.
Authorship
Contribution: S.M.J. and J.C.B. conceived the study, oversaw it, reviewed the data, and wrote the first draft of the manuscript; J.A.J., J.M.F., L.A.A., K.J.M., J.A.W., K.A.B., and M.R.G. enrolled patients on the study, reviewed drafts of the paper, and approved the final version; A.S.R. contributed to the initial statistical design of the paper; G.L., N.A.H., K.S., and A.J.J. coordinated correlative and prognostic factor assessment, reviewed drafts of the paper, and approved the final version; V.N. and N.H. reviewed the CT scans to assess response at different time points during the study, reviewed drafts of the paper, and approved the final version; M.S. was the coordinator for the study, reviewed drafts of the paper, and approved the final version; B.M., J.-S.W., J.K.N., and D.F.J. contributed to coordinating the trial at Pharmacyclics, reviewing data, reviewing drafts of the paper, and approved the final version; and S.M.J. and J.C.B. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: B.M., J.-S.W., J.K.N., and D.F.J. are employees of Pharmacyclics and have financial interest in the development of Ibrutinib. J.C.B., J.A.J., and S.M.J. have received support for performance of clinical research as part of this trial or others by Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Samantha Jaglowski, Division of Hematology, The Ohio State University, A354 Starling-Loving Hall, 320 W 10th Ave, Columbus, OH 43210; e-mail: samantha.jaglowski@osucmc.edu.