Key Points
Lower GVHD after haploidentical transplant with posttransplant cyclophosphamide compared with HLA-matched unrelated donor transplant.
Comparable overall survival after haploidentical compared with matched unrelated donor transplant for AML.
Abstract
We studied adults with acute myeloid leukemia (AML) after haploidentical (n = 192) and 8/8 HLA-matched unrelated donor (n = 1982) transplantation. Haploidentical recipients received calcineurin inhibitor (CNI), mycophenolate, and posttransplant cyclophosphamide for graft-versus-host disease (GVHD) prophylaxis; 104 patients received myeloablative and 88 received reduced intensity conditioning regimens. Matched unrelated donor transplant recipients received CNI with mycophenolate or methotrexate for GVHD prophylaxis; 1245 patients received myeloablative and 737 received reduced intensity conditioning regimens. In the myeloablative setting, day 30 neutrophil recovery was lower after haploidentical compared with matched unrelated donor transplants (90% vs 97%, P = .02). Corresponding rates after reduced intensity conditioning transplants were 93% and 96% (P = .25). In the myeloablative setting, 3-month acute grade 2-4 (16% vs 33%, P < .0001) and 3-year chronic GVHD (30% vs 53%, P < .0001) were lower after haploidentical compared with matched unrelated donor transplants. Similar differences were observed after reduced intensity conditioning transplants, 19% vs 28% (P = .05) and 34% vs 52% (P = .002). Among patients receiving myeloablative regimens, 3-year probabilities of overall survival were 45% (95% CI, 36-54) and 50% (95% CI, 47-53) after haploidentical and matched unrelated donor transplants (P = .38). Corresponding rates after reduced intensity conditioning transplants were 46% (95% CI, 35-56) and 44% (95% CI, 0.40-47) (P = .71). Although statistical power is limited, these data suggests that survival for patients with AML after haploidentical transplantation with posttransplant cyclophosphamide is comparable with matched unrelated donor transplantation.
Introduction
An unrelated adult donor who is HLA-matched to the recipient at the allele-level (at HLA-A, -B, -C, and -DRB1) is considered the best alternative in the absence of an HLA-matched sibling for patients needing hematopoietic cell transplantation.1,2 However, using unrelated donors is limited by (1) a prolonged time to identify and schedule donation for some unrelated donors allowing some patients to relapse before transplantation can be performed,3,4 and (2) limited availability of fully HLA-matched unrelated donors for the non-Caucasian population.5 Alternative donors are used for transplantation in patients without a fully-matched unrelated donor including unrelated umbilical cord blood and grafts from haploidentical related donors. However, haploidentical transplantation performed using extensive in vivo or ex vivo T-cell depletion to prevent graft-versus-host disease (GVHD) is associated with higher nonrelapse mortality and delayed immune reconstitution.6-8 A more recent strategy for haploidentical related donor transplantation, which has had some success, is transplantation of T-cell replete grafts with intensive immune suppression.9-12 A different strategy to control GVHD is the administration of posttransplantation cyclophosphamide, which targets alloreactive T cells generated early after an HLA-mismatched transplant, relatively sparing regulatory T cells and leaving unaffected the nondividing hematopoietic stem cells.13-15 Patients treated in this manner usually receive a bone marrow (BM) graft and standard posttransplant immune suppression with a calcineurin inhibitor (CNI) and mycophenolate mofetil. This approach has been studied at several institutions and in a national clinical trial conducted by the Blood and Marrow Transplant Clinical Trials Network (BMT CTN).16-18 Further, reports from single institutions suggest similar outcomes after haploidentical transplantation using the above stated approach compared with HLA-matched related or unrelated donor transplants.19-21 The current analysis compares transplantation outcomes after haploidentical donor transplantation using the posttransplant cyclophosphamide approach to that after 8/8 HLA-matched unrelated donor transplantation in adults with acute myeloid leukemia (AML).
Patients and methods
Data source and inclusion criteria
Data were obtained from the Center for International Blood and Marrow Transplant Research (CIBMTR). The CIBMTR is a voluntary network of over 300 transplant centers worldwide. Participating centers report consecutive transplants and patients are followed longitudinally until death or lost to follow-up. Included in this analysis are adults with AML (de novo or secondary) who received their first allogeneic hematopoietic cell transplant between 2008 and 2012 and reported to the CIBMTR; 98% of transplants were performed in the United States and the remaining 2% in Europe. Recipients of haploidentical transplantation (mismatched at least two or more HLA-loci to donors) received an unmanipulated, predominantly BM graft with GVHD prophylaxis consisting of posttransplant cyclophosphamide, CNI, and mycophenolate mofetil. Recipients of unrelated transplantation (matched at the allele-level at HLA-A, -B, -C, and -DRB1) received predominantly unmanipulated, peripheral blood and GVHD prophylaxis consisting of a CNI and methotrexate or mycophenolate mofetil. The Institutional Review Boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
End points
The primary end point was overall survival (OS); death from any cause was considered an event and surviving patients were censored at last contact. Neutrophil recovery was defined as achieving absolute neutrophil count ≥500/µL for 3 consecutive days and platelet recovery defined as achieving platelet counts ≥20 000/µL for at least 7 days, unsupported by transfusion. Acute GVHD22 and chronic GVHD23 were graded using standard criteria. Nonrelapse mortality was defined as death in continuous complete remission. Relapse was defined as morphologic, cytogenetic, or molecular leukemia recurrence.
Statistical methods
Separate analyses were undertaken for patients receiving myeloablative and reduced-intensity transplantations.24 OS was calculated using the Kaplan–Meier estimator.25 Rates of hematopoietic recovery, acute and chronic GVHD, nonrelapse mortality, and relapse were calculated using the cumulative incidence estimator to accommodate competing risks.26 Death was the competing risk for acute and chronic GVHD. Leukemia relapse was the competing risk for nonrelapse mortality, and nonrelapse mortality the competing risk for leukemia relapse. Multivariate models for transplantation outcomes were built using Cox regression models.27 Variables tested included: donor type (haploidentical vs unrelated donors), patient age (21-50 vs 51-70 years), recipient performance score (90-100 vs 80 or lower), recipient cytomegalovirus serostatus (positive vs negative), disease risk index28 (low or intermediate vs high risk), de novo AML vs secondary AML, and interval from diagnosis to transplant (≤12 vs >12 months). Variables were fit using the backward selection method and those that retained a P-value of .05 or less were considered significant and held in models, with the exception of the variable for donor type, which was held in all steps of model building. None of the variables violated proportionality assumptions and there were no first order interactions. P values are two-sided and analyses were performed with SAS version 9.3 (Cary, NC).
Results
Patient, disease, and transplant characteristics
Table 1 shows the characteristics of patients and their disease for myeloablative regimens. Compared with HLA-matched unrelated donor transplant recipients, haploidentical transplant recipients were more likely to report performance scores of 80 or lower, and more likely to report favorable and adverse cytogenetics. There were no other differences in patient- and disease-characteristics between the donor types including disease status and disease risk index. Disease risk index28 is a validated tool that used cytogenetics and disease status at transplantation to risk-stratify patients undergoing allogeneic transplantation. Low disease risk index was assigned to those transplanted in complete remission with favorable cytogenetics. Intermediate disease risk index was assigned to those with intermediate cytogenetics transplanted in complete remission. All other combinations were assigned as high disease risk index (favorable or intermediate cytogenetics transplanted in relapse and adverse cytogenetics transplanted in remission or relapse). Although non-total body irradiation (TBI) regimens were predominantly used for both donor types, the regimens varied by donor type. For recipients of haploidentical transplants, 43% received busulfan and cyclophosphamide, 13% received busulfan, thiotepa, and fludarabine, and 22% received melphalan, thiotepa, and fludarabine. The remaining patients received TBI and cyclophosphamide (3%) and TBI and fludarabine (19%). All patients received tacrolimus or cyclosporine with mycophenolate and posttransplant cyclophosphamide. For HLA-matched unrelated donor transplants, 42% of patients received busulfan and fludarabine, 32% received busulfan, and cyclophosphamide, and 26% received TBI and cyclophosphamide. Eighty-four percent received tacrolimus or cyclosporine with methotrexate, 9% received tacrolimus or cyclosporine with mycophenolate, and 7% received tacrolimus or cyclosporine with sirolimus.
Patient and disease characteristics: myeloablative regimens
| . | Donor type . | . | |
|---|---|---|---|
| Variable . | Haploidentical . | Unrelated . | P . |
| Number | 104 | 1245 | |
| Age, y | .94 | ||
| 21-50 | 60 (58%) | 709 (57%) | |
| 51-70 | 44 (42%) | 536 (43%) | |
| Sex, male | 47 (45%) | 631 (51%) | .28 |
| Performance score | .002 | ||
| 90-100 | 48 (46%) | 787 (63%) | |
| ≤80 | 50 (48%) | 425 (34%) | |
| Not reported | 6 (6%) | 33 (3%) | |
| Recipient CMV serostatus | .56 | ||
| Positive | 48 (46%) | 786 (63%) | |
| Negative | 23 (22%) | 438 (35%) | |
| Not reported | 33 (32%) | 21 (2%) | |
| Disease status at transplantation | .13 | ||
| First complete remission | 48 (46%) | 786 (63%) | |
| Second complete remission | 21 (20%) | 255 (20%) | |
| Relapse | 35 (34%) | 310 (25%) | |
| Disease risk index | .62 | ||
| Low risk index | 5 (5%) | 62 (5%) | |
| Intermediate risk index | 66 (63%) | 843 (68%) | |
| High risk index | 33 (32%) | 340 (27%) | |
| Secondary AML | 17 (16%) | 179 (14%) | .58 |
| De novo AML | 87 (84%) | 1066 (86%) | — |
| Cytogenetics* | .01 | ||
| Favorable | 12 (12%) | 74 (6%) | |
| Intermediate | 66 (63%) | 951 (76%) | |
| Adverse | 23 (22%) | 178 (14%) | |
| Not reported | 3 (3%) | 42 (3%) | |
| Conditioning regimen | N/A† | ||
| TBI + cyclophosphamide | 3 (3%) | 324 (26%) | |
| TBI + fludarabine | 20 (19%) | — | |
| Busulfan + cyclophosphamide | 45 (43%) | 401 (32%) | |
| Melphalan + thiotepa + fludarabine | 23 (22%) | — | |
| Busulfan + fludarabine | — | 233 (19%) | |
| Busulfan + fludarabine + ATG | — | 287 (23%) | |
| Busulfan + thiotepa + fludarabine | 13 (13%) | — | |
| GVHD prophylaxis | N/A† | ||
| Tacrolimus/CSA + MMF | 100 (96%) | 114 (9%) | |
| Tacrolimus/CSA + MTX | — | 1048 (84%) | |
| Tacrolimus/CSA + sirolimus | — | 83 (7%) | |
| Posttransplant cyclophosphamide | 104 (100%) | — | |
| Graft type | <.001 | ||
| BM | 85 (82%) | 231(19%) | |
| Peripheral blood | 19 (18%) | 1014 (81%) | |
| Interval between diagnosis and transplant | .82 | ||
| ≤12 mo | 80 (77%) | 970 (78%) | |
| >12 mo | 24 (23%) | 275 (22%) | |
| Transplant period | <.001 | ||
| 2009-2010 | 25 (24%) | 578 (47%) | |
| 2011-2012 | 79 (76%) | 667 (53%) | |
| Follow up, median (range), mo | 30 (7-59) | 36 (9 -64) | |
| . | Donor type . | . | |
|---|---|---|---|
| Variable . | Haploidentical . | Unrelated . | P . |
| Number | 104 | 1245 | |
| Age, y | .94 | ||
| 21-50 | 60 (58%) | 709 (57%) | |
| 51-70 | 44 (42%) | 536 (43%) | |
| Sex, male | 47 (45%) | 631 (51%) | .28 |
| Performance score | .002 | ||
| 90-100 | 48 (46%) | 787 (63%) | |
| ≤80 | 50 (48%) | 425 (34%) | |
| Not reported | 6 (6%) | 33 (3%) | |
| Recipient CMV serostatus | .56 | ||
| Positive | 48 (46%) | 786 (63%) | |
| Negative | 23 (22%) | 438 (35%) | |
| Not reported | 33 (32%) | 21 (2%) | |
| Disease status at transplantation | .13 | ||
| First complete remission | 48 (46%) | 786 (63%) | |
| Second complete remission | 21 (20%) | 255 (20%) | |
| Relapse | 35 (34%) | 310 (25%) | |
| Disease risk index | .62 | ||
| Low risk index | 5 (5%) | 62 (5%) | |
| Intermediate risk index | 66 (63%) | 843 (68%) | |
| High risk index | 33 (32%) | 340 (27%) | |
| Secondary AML | 17 (16%) | 179 (14%) | .58 |
| De novo AML | 87 (84%) | 1066 (86%) | — |
| Cytogenetics* | .01 | ||
| Favorable | 12 (12%) | 74 (6%) | |
| Intermediate | 66 (63%) | 951 (76%) | |
| Adverse | 23 (22%) | 178 (14%) | |
| Not reported | 3 (3%) | 42 (3%) | |
| Conditioning regimen | N/A† | ||
| TBI + cyclophosphamide | 3 (3%) | 324 (26%) | |
| TBI + fludarabine | 20 (19%) | — | |
| Busulfan + cyclophosphamide | 45 (43%) | 401 (32%) | |
| Melphalan + thiotepa + fludarabine | 23 (22%) | — | |
| Busulfan + fludarabine | — | 233 (19%) | |
| Busulfan + fludarabine + ATG | — | 287 (23%) | |
| Busulfan + thiotepa + fludarabine | 13 (13%) | — | |
| GVHD prophylaxis | N/A† | ||
| Tacrolimus/CSA + MMF | 100 (96%) | 114 (9%) | |
| Tacrolimus/CSA + MTX | — | 1048 (84%) | |
| Tacrolimus/CSA + sirolimus | — | 83 (7%) | |
| Posttransplant cyclophosphamide | 104 (100%) | — | |
| Graft type | <.001 | ||
| BM | 85 (82%) | 231(19%) | |
| Peripheral blood | 19 (18%) | 1014 (81%) | |
| Interval between diagnosis and transplant | .82 | ||
| ≤12 mo | 80 (77%) | 970 (78%) | |
| >12 mo | 24 (23%) | 275 (22%) | |
| Transplant period | <.001 | ||
| 2009-2010 | 25 (24%) | 578 (47%) | |
| 2011-2012 | 79 (76%) | 667 (53%) | |
| Follow up, median (range), mo | 30 (7-59) | 36 (9 -64) | |
ATG, anti-thymocyte globulin; CMV, cytomegalovirus; CSA, cyclosporine; MMF, mycophenolate; MTX, methotrexate.
Favorable = t(8;21); inv(16) and t(15;17); adverse = complex karyotypes ≥4 abnormalities; intermediate = all others.
N/A = not applicable; P-values are not shown as conditioning regimens and GVHD prophylaxis are “packages” and therefore differ by donor source.
Table 2 shows the characteristics of patients and their disease for reduced intensity transplant conditioning regimens. Compared with HLA-matched unrelated donor transplant, recipients of haploidentical transplants were younger, more likely to report performance scores higher than 80, and were more likely to be in second complete remission. There were no differences in cytogenetics or disease risk index. As with myeloablative transplants, conditioning regimen and GVHD prophylaxis varied by donor type. All haploidentical transplant recipients received TBI 200 cGy with cyclophosphamide and fludarabine. Almost all patients received tacrolimus or cyclosporine with mycophenolate and all received posttransplant cyclophosphamide for GVHD prophylaxis. Among recipients of unrelated donor transplants, 39% received busulfan or melphalan and fludarabine with in vivo T-cell depletion, 39% received busulfan or melphalan and fludarabine, and the remaining 21% received TBI 200 cGy, cyclophosphamide, and fludarabine. GVHD prophylaxis included tacrolimus or cyclosporine with methotrexate for 49%, tacrolimus or cyclosporine with mycophenolate for 43%, and tacrolimus or cyclosporine with sirolimus for 8% of patients.
Patient, disease, and transplant characteristics: reduced intensity regimens
| . | Donor type . | . | |
|---|---|---|---|
| Variable . | Haploidentical . | Unrelated . | P . |
| Number | 88 | 737 | |
| Age, y | <.001 | ||
| 21-50 | 19 (22%) | 41 (5%) | |
| 51-0 | 69 (78%) | 696 (95%) | |
| Sex, male | 51 (58%) | 415 (56%) | .77 |
| Performance score | .03 | ||
| 90-100 | 57 (65%) | 426 (58%) | |
| ≤80 | 23 (26%) | 300 (41%) | |
| Not reported | 8 (9%) | 11 (1%) | |
| Recipient CMV serostatus | .91 | ||
| Positive | 60 (68%) | 504 (68%) | |
| Negative | 28 (32%) | 229 (31%) | |
| Not reported | — | 4 (< 1%) | |
| Disease status at transplantation | <.001 | ||
| First complete remission | 43 (46%) | 447 (61%) | |
| Second complete remission | 31 (35%) | 128 (17%) | |
| Relapse | 14 (16%) | 162 (22%) | |
| Disease risk index | .11 | ||
| Low risk index | 4 (5%) | 13 (2%) | |
| Intermediate risk index | 68 (77%) | 542 (74%) | |
| High risk index | 16 (18%) | 182 (25%) | |
| Secondary AML | 20 (23%) | 182 (25%) | .68 |
| De novo AML | 68 (77%) | 555 (75%) | — |
| Cytogenetics* | .16 | ||
| Favorable | 4 (5%) | 23 (2%) | |
| Intermediate | 73 (83%) | 586 (80%) | |
| Adverse | 10 (11%) | 99 (13%) | |
| Not reported | 1 (1%) | 29 (4%) | |
| Conditioning regimen | N/A† | ||
| TBI + cyclophosphamide + fludarabine | 88 (100%) | 158 (21%) | |
| Busulfan/melphalan + fludarabine + ATG | __ | 291 (39%) | |
| Busulfan/melphalan + fludarabine | __ | 288 (39%) | |
| GVHD prophylaxis | N/A† | ||
| Tacrolimus/CSA + MMF | 85 (97%) | 315 (43%) | |
| Tacrolimus/CSA + MTX | — | 362 (49%) | |
| Tacrolimus/CSA + sirolimus | 3 (3%) | 60 (8%) | |
| Posttransplant cyclophosphamide | 88 (100%) | — | |
| Interval between diagnosis and transplant | .01 | ||
| ≤12 mo | 57 (65%) | 569 (77%) | |
| >12 mo | 31 (35%) | 168 (23%) | |
| Graft type | <.001 | ||
| BM | 77 (88%) | 80 (11%) | |
| Peripheral blood | 11 (13%) | 657 (89%) | |
| Transplant period | .83 | ||
| 2008-2010 | 48 (55%) | 404 (55%) | |
| 2011-2012 | 40 (45%) | 333 (45%) | |
| Follow up, median (range), mo | 39 (12-73) | 37 (7-75) | |
| . | Donor type . | . | |
|---|---|---|---|
| Variable . | Haploidentical . | Unrelated . | P . |
| Number | 88 | 737 | |
| Age, y | <.001 | ||
| 21-50 | 19 (22%) | 41 (5%) | |
| 51-0 | 69 (78%) | 696 (95%) | |
| Sex, male | 51 (58%) | 415 (56%) | .77 |
| Performance score | .03 | ||
| 90-100 | 57 (65%) | 426 (58%) | |
| ≤80 | 23 (26%) | 300 (41%) | |
| Not reported | 8 (9%) | 11 (1%) | |
| Recipient CMV serostatus | .91 | ||
| Positive | 60 (68%) | 504 (68%) | |
| Negative | 28 (32%) | 229 (31%) | |
| Not reported | — | 4 (< 1%) | |
| Disease status at transplantation | <.001 | ||
| First complete remission | 43 (46%) | 447 (61%) | |
| Second complete remission | 31 (35%) | 128 (17%) | |
| Relapse | 14 (16%) | 162 (22%) | |
| Disease risk index | .11 | ||
| Low risk index | 4 (5%) | 13 (2%) | |
| Intermediate risk index | 68 (77%) | 542 (74%) | |
| High risk index | 16 (18%) | 182 (25%) | |
| Secondary AML | 20 (23%) | 182 (25%) | .68 |
| De novo AML | 68 (77%) | 555 (75%) | — |
| Cytogenetics* | .16 | ||
| Favorable | 4 (5%) | 23 (2%) | |
| Intermediate | 73 (83%) | 586 (80%) | |
| Adverse | 10 (11%) | 99 (13%) | |
| Not reported | 1 (1%) | 29 (4%) | |
| Conditioning regimen | N/A† | ||
| TBI + cyclophosphamide + fludarabine | 88 (100%) | 158 (21%) | |
| Busulfan/melphalan + fludarabine + ATG | __ | 291 (39%) | |
| Busulfan/melphalan + fludarabine | __ | 288 (39%) | |
| GVHD prophylaxis | N/A† | ||
| Tacrolimus/CSA + MMF | 85 (97%) | 315 (43%) | |
| Tacrolimus/CSA + MTX | — | 362 (49%) | |
| Tacrolimus/CSA + sirolimus | 3 (3%) | 60 (8%) | |
| Posttransplant cyclophosphamide | 88 (100%) | — | |
| Interval between diagnosis and transplant | .01 | ||
| ≤12 mo | 57 (65%) | 569 (77%) | |
| >12 mo | 31 (35%) | 168 (23%) | |
| Graft type | <.001 | ||
| BM | 77 (88%) | 80 (11%) | |
| Peripheral blood | 11 (13%) | 657 (89%) | |
| Transplant period | .83 | ||
| 2008-2010 | 48 (55%) | 404 (55%) | |
| 2011-2012 | 40 (45%) | 333 (45%) | |
| Follow up, median (range), mo | 39 (12-73) | 37 (7-75) | |
Favorable = t(8;21); inv(16) and t(15;17); adverse = complex karyotypes ≥4 abnormalities; intermediate = all others.
N/A = not applicable; P-values are not shown as conditioning regimens, and GVHD prophylaxis are “packages” and therefore differ by donor source.
Hematopoietic recovery
Neutrophil and platelet recovery rates were not different after haploidentical compared with HLA-matched unrelated donor transplants except that neutrophil recovery after myeloablative HLA-matched unrelated donor was higher compared with haploidentical transplants. The day 30 incidence of neutrophil recovery after haploidentical and HLA-matched unrelated donor myeloablative transplantation were 90% (95% CI, 84-94) and 97% (95% CI, 96-98), respectively; P = .02. The corresponding 6-month incidence of platelet recovery was 88% (95% CI, 78-93) and 92% (95% CI, 91-94); P = .19. The day 30 incidence of neutrophil recovery after haploidentical and HLA-matched unrelated donor reduced intensity transplantation were 93% (95% CI, 87-97) and 96% (95% CI, 95-97), respectively; P = .24. The corresponding 6-month incidence of platelet recovery was 88% (95% CI, 79-93) and 93% (95% CI, 91-94); P = .15.
Acute and chronic GVHD
Acute and chronic GVHD rates were lower after haploidentical transplantation compared with HLA-matched unrelated donor transplantation (Tables 3 and 4). BM was the predominant graft for haploidentical transplants and peripheral blood, the predominant graft for unrelated donor transplants. Chronic GVHD rates are generally higher with transplantation of peripheral blood. Therefore, we compared chronic GVHD rates in the subset of patients transplanted with BM. There were no differences in 3-year rates of chronic GVHD after haploidentical and unrelated donor transplantation with myeloablative regimens (30% [95% CI, 21-39]; n = 85 vs 36% [95% CI, 30-43]; n = 231) or with reduced intensity regimens (34% [95% CI, 24-44]; n = 77 vs 30% [95% CI, 20-41]; n = 80).
Probabilities of acute and chronic GVHD, nonrelapse mortality, relapse, and OS by donor type
| . | Donor type . | . | |
|---|---|---|---|
| Outcome . | Haploidentical . | Unrelated . | P . |
| Myeloablative transplants | |||
| Grade 2-4 acute GVHD at day 90 | 16% (10-24) | 33% (30-35) | <.0001 |
| Grade 3-4 acute GVHD at day 90 | 7% (3-13) | 13% (11-15) | .02 |
| Chronic GVHD | |||
| At 12 mo | 28% (20-37) | 45% (42-47) | .0005 |
| At 36 mo | 30% (21-39) | 53% (50-56) | <.0001 |
| Nonrelapse mortality | |||
| At 12 mo | 12% (7-19) | 14% (12-16) | .56 |
| At 36 mo | 14% (8-22) | 20% (18-22) | .14 |
| Relapse | |||
| At 12 mo | 41% (33-50) | 32% (30-35) | .04 |
| At 36 mo | 44% (34-53) | 39% (37-42) | .37 |
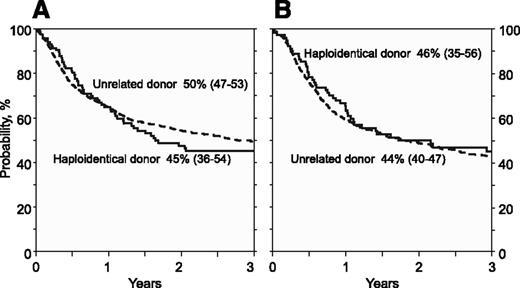
| OS | |||
| At 12 mo | 65% (56-73) | 65% (63-68) | .98 |
| At 36 mo | 45% (36-54) | 50% (47-53) | .38 |
| Reduced intensity transplants | |||
| Grade 2-4 acute GVHD at day 90 | 19% (12-28) | 28% (25-31) | .05 |
| Grade 3-4 acute GVHD at day 90 | 2% (0-7) | 11% (8-13) | <.0001 |
| Chronic GVHD | |||
| At 12 mo | 27% (19-37) | 43% (40-47) | .001 |
| At 36 mo | 34% (24-44) | 52% (48-55) | .002 |
| Nonrelapse mortality | |||
| At 12 mo | 6% (2-12) | 16% (13-18) | .0001 |
| At 36 mo | 9% (4-16) | 23% (19-26) | .0001 |
| Relapse | |||
| At 12 mo | 43% (32-53) | 34% (31-38) | .12 |
| At 36 mo | 58% (46-68) | 42% (38-45) | .006 |
| OS | |||
| At 12 mo | 64% (53-73) | 60% (56-63) | .46 |
| At 36 mo | 46% (35-56) | 44% (40-47) | .71 |
| . | Donor type . | . | |
|---|---|---|---|
| Outcome . | Haploidentical . | Unrelated . | P . |
| Myeloablative transplants | |||
| Grade 2-4 acute GVHD at day 90 | 16% (10-24) | 33% (30-35) | <.0001 |
| Grade 3-4 acute GVHD at day 90 | 7% (3-13) | 13% (11-15) | .02 |
| Chronic GVHD | |||
| At 12 mo | 28% (20-37) | 45% (42-47) | .0005 |
| At 36 mo | 30% (21-39) | 53% (50-56) | <.0001 |
| Nonrelapse mortality | |||
| At 12 mo | 12% (7-19) | 14% (12-16) | .56 |
| At 36 mo | 14% (8-22) | 20% (18-22) | .14 |
| Relapse | |||
| At 12 mo | 41% (33-50) | 32% (30-35) | .04 |
| At 36 mo | 44% (34-53) | 39% (37-42) | .37 |
| OS | |||
| At 12 mo | 65% (56-73) | 65% (63-68) | .98 |
| At 36 mo | 45% (36-54) | 50% (47-53) | .38 |
| Reduced intensity transplants | |||
| Grade 2-4 acute GVHD at day 90 | 19% (12-28) | 28% (25-31) | .05 |
| Grade 3-4 acute GVHD at day 90 | 2% (0-7) | 11% (8-13) | <.0001 |
| Chronic GVHD | |||
| At 12 mo | 27% (19-37) | 43% (40-47) | .001 |
| At 36 mo | 34% (24-44) | 52% (48-55) | .002 |
| Nonrelapse mortality | |||
| At 12 mo | 6% (2-12) | 16% (13-18) | .0001 |
| At 36 mo | 9% (4-16) | 23% (19-26) | .0001 |
| Relapse | |||
| At 12 mo | 43% (32-53) | 34% (31-38) | .12 |
| At 36 mo | 58% (46-68) | 42% (38-45) | .006 |
| OS | |||
| At 12 mo | 64% (53-73) | 60% (56-63) | .46 |
| At 36 mo | 46% (35-56) | 44% (40-47) | .71 |
Multivariate analysis: risks of acute and chronic GVHD, nonrelapse mortality, relapse, and OS by donor type
| . | Transplant conditioning regimen intensity . | |
|---|---|---|
| Outcome . | Myeloablative* . | Reduced intensity† . |
| Hazard ratio (95% CI) . | Hazard ratio (95% CI) . | |
| Grade 2-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.40 (0.26-0.62) | 0.68 (0.46-1.00) |
| P <.0001 | P = .05 | |
| Grade 3-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.47 (0.23-0.94) | 0.20 (0.06-0.64) |
| P = .03 | P = .006 | |
| Chronic GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.46 (0.32-0.67) | 0.51 (0.35-0.75) |
| P <.0001 | P = .0006 | |
| Nonrelapse mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.76 (0.45-1.28) | 0.41 (0.20-0.83) |
| P = .31 | P = .01 | |
| Relapse | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.12 (0.83-1.52) | 1.36 (1.00-1.85) |
| P = .46 | P = .05 | |
| Overall mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.08 (0.82-1.43) | 0.94 (0.70-1.27) |
| P = .58 | P = .70 | |
| . | Transplant conditioning regimen intensity . | |
|---|---|---|
| Outcome . | Myeloablative* . | Reduced intensity† . |
| Hazard ratio (95% CI) . | Hazard ratio (95% CI) . | |
| Grade 2-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.40 (0.26-0.62) | 0.68 (0.46-1.00) |
| P <.0001 | P = .05 | |
| Grade 3-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.47 (0.23-0.94) | 0.20 (0.06-0.64) |
| P = .03 | P = .006 | |
| Chronic GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.46 (0.32-0.67) | 0.51 (0.35-0.75) |
| P <.0001 | P = .0006 | |
| Nonrelapse mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.76 (0.45-1.28) | 0.41 (0.20-0.83) |
| P = .31 | P = .01 | |
| Relapse | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.12 (0.83-1.52) | 1.36 (1.00-1.85) |
| P = .46 | P = .05 | |
| Overall mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.08 (0.82-1.43) | 0.94 (0.70-1.27) |
| P = .58 | P = .70 | |
Nonrelapse mortality risk adjusted for performance score; relapse risk adjusted for disease risk index; overall mortality adjusted for patient age and disease risk index.
Nonrelapse mortality risk adjusted for disease risk index; relapse risk adjusted for performance score, disease risk index, and secondary AML; overall mortality adjusted for disease risk index and secondary AML.
Nonrelapse mortality and relapse
Among recipients of myeloablative regimens, nonrelapse mortality risks were not different after haploidentical compared with HLA-matched unrelated donor transplantation (Figure 1A; Tables 3 and 4). One-year relapse rates were lower after unrelated HLA-matched transplants but at 3-years there were no differences by donor type (Figure 2A). Independent of donor type, nonrelapse mortality risks were higher for patients with performance scores of 80 or lower compared with those with scores of 90 or 100 (HR, 1.39; 95% CI, 1.07-1.80; P = .01). Disease risk index was associated with relapse; relapse risks were higher for patients with high disease risk index compared with low or intermediate disease risk index (HR, 3.17; 95% CI, 2.66-3.78; P < .0001). The effect of disease risk index on relapse was independent of donor type. Interval between diagnosis and transplant was not associated with relapse (HR, 0.85; 95% CI, 0.68-1.06; P = .14) or nonrelapse mortality (HR, 0.84; 95% CI, 0.53-1.34; P = .46).
Nonrelapse mortality. (A) The cumulative incidence of nonrelapse mortality by donor type after myeloablative conditioning regimen, adjusted for performance score. (B) The cumulative incidence of nonrelapse mortality by donor type after reduced intensity conditioning regimen, adjusted for disease risk index.
Nonrelapse mortality. (A) The cumulative incidence of nonrelapse mortality by donor type after myeloablative conditioning regimen, adjusted for performance score. (B) The cumulative incidence of nonrelapse mortality by donor type after reduced intensity conditioning regimen, adjusted for disease risk index.
Relapse. (A) The cumulative incidence of relapse by donor type after myeloablative conditioning regimen, adjusted for disease risk index. (B) The cumulative incidence of relapse by donor type after reduced intensity conditioning regimen, adjusted for performance score, disease risk index, and secondary AML.
Relapse. (A) The cumulative incidence of relapse by donor type after myeloablative conditioning regimen, adjusted for disease risk index. (B) The cumulative incidence of relapse by donor type after reduced intensity conditioning regimen, adjusted for performance score, disease risk index, and secondary AML.
Among recipients of reduced intensity regimens, nonrelapse mortality risks were lower and relapse risks higher after haploidentical compared with HLA-matched unrelated donor transplantation, Figures 1B and 2B; Tables 3 and 4. Independent of donor type, high disease risk index was associated with higher nonrelapse mortality (HR, 1.54; 95% CI, 1.09-2.17; P = .01). Relapse risks were higher in patients with performance scores 80 or lower (HR, 1.31; 95% CI, 1.05-1.63; P = .01), secondary AML (HR, 1.41; 95% CI, 1.11-1.78; P = .004), and high disease risk index (HR, 1.96; 95% CI, 1.55-2.47; P < .0001). The effects of performance score, disease risk index, and secondary AML were independent of donor type. Interval between diagnosis and transplant was not associated with relapse (HR, 0.95; 95% CI, 0.73-1.22; P = .66) or nonrelapse mortality (HR, 1.12; 95% CI, 0.79-1.58; P = .52).
OS
Among recipients of myeloablative and reduced intensity transplant conditioning regimens, overall mortality risks were not different after haploidentical compared with HLA-matched unrelated donor transplantation (Tables 3 and 4; Figure 3A-B). Among recipients of myeloablative transplantations, mortality was higher for patients aged 51 to 70 years compared with those aged 21 to 50 years (HR, 1.26; 95% CI, 1.08-1.47; P = .003) and high disease risk index (HR, 2.31; 95% CI, 1.97-2.70; P < .0001). Among recipients of reduced intensity conditioning transplantations, high disease risk index (HR, 1.74; 95% CI, 1.43-2.12; P < .0001) and secondary AML (HR, 1.29; 95% CI, 1.06-1.61; P = .01) were associated with higher mortality. Mortality risks were not associated with the interval between diagnosis and transplant after myeloablative (HR, 0.95; 95% CI, 0.78-1.15; P = .59) or reduced intensity transplants (HR, 1.05; 95% CI, 0.84-1.30; P = .68). The 3-year probabilities of chronic GVHD-free/leukemia-free survival after myeloablative HLA-matched unrelated donor and haploidentical transplants were 12% (95% CI, 10-14) and 24% (95% CI, 16-33), P = .008, respectively. The corresponding probabilities after reduced intensity conditioning transplants were 10% (95% CI, 8-13) and 18% (95% CI, 11-27), P = .09.
Overall survival. (A) The probability of OS by donor type after myeloablative conditioning regimen, adjusted for age and disease risk index. (B) The probability of OS by donor type after reduced intensity conditioning regimen, adjusted for disease risk index and secondary AML.
Overall survival. (A) The probability of OS by donor type after myeloablative conditioning regimen, adjusted for age and disease risk index. (B) The probability of OS by donor type after reduced intensity conditioning regimen, adjusted for disease risk index and secondary AML.
In vivo T-cell depletion with anti-thymocyte globulin (ATG) was not employed for haploidentical transplants. On the other hand, 23% of HLA-matched myeloablative transplant and 39% of reduced intensity transplant regimens included ATG. We tested for an effect of ATG on survival and found none. Compared with haploidentical transplants, mortality risks for non-ATG and ATG-containing myeloablative HLA-matched unrelated donor transplants were HR, 0.81, P = .19, and HR, 0.83, P = .45, respectively. The corresponding risks with reduced intensity HLA-matched unrelated donor transplants were HR, 1.10, P = .58 and HR, 1.18, P = .33.
Transplant center effect
Haploidentical transplantations were performed at 19 transplant centers compared with the over 80 centers that performed HLA-matched unrelated donor transplantations. Therefore, to ensure that the survival rates reported in the current analysis were not driven by center expertise for one donor type or the other, we performed a subset analysis limited to centers that performed both haploidentical and unrelated donor transplantations (n = 890). Consistent with the main analysis, we did not observe differences in mortality risks in either the myeloablative (HR, 0.88; 95% CI, 0.65-1.21; P = .44) or reduced intensity conditioning (HR, 1.02; 95% CI, 0.71-1.46, P = .92) after haploidentical and unrelated donor transplants.
Causes of death
Fifty-five of 104 (53%) recipients of myeloablative haploidentical and 608 of 1245 (49%) HLA-matched unrelated donor transplant recipients have died. The most common cause of death in both groups was recurrent leukemia; 41 (75%) and 380 (63%) after haploidentical and HLA-matched unrelated donor transplantation, respectively. Among recipients of haploidentical transplants, the remaining 14 deaths were attributed to the following: graft failure (n = 4, 7%), GVHD (n = 4, 7%), infection (n = 3, 5%), organ failure (n = 1, 2%), and not reported (n = 2, 4%). Among the other 228 deaths in recipients of unrelated donor transplants, the most common cause was GVHD (n = 134, 22%). Other causes included graft failure (n = 25, 4%), infection (n = 25, 4%), interstitial pneumonitis (n = 6, 1%), organ failure (n = 11, 2%), and others (n = 27, 4%).
Forty-eight of 88 (55%) recipients of reduced intensity haploidentical and 418 of 737 (56%) HLA-matched unrelated donor transplant recipients have also died. Although the most common cause of death in both groups was recurrent leukemia, 40 (83%) and 262 (63%), respectively, this was more likely after haploidentical compared with HLA-matched unrelated donor transplantation (P = .004). The remaining 8 deaths after haploidentical transplantation were attributed to: GVHD (n = 4, 8%), infection (n = 3, 6%), and graft failure (n = 1, 3%). The remaining 156 deaths after HLA-matched unrelated donor transplantation were attributed to: GVHD (n = 113, 27%) infection (n = 8, 2%), graft failure (n = 9, 2%), organ failure (n = 7, 2%), and other causes (n = 19, 4%).
Subset analysis
Subset analysis included haploidentical transplants with BM and HLA-matched unrelated donor transplants with peripheral blood grafts and CNI with methotrexate, the predominant graft, and GVHD prophylaxis for unrelated donor transplants. The characteristics of this population are shown in supplemental Tables 1 and 2 on the Blood Web site. In the myeloablative setting, recipients of haploidentical transplants (n = 85) received tacrolimus (n = 51) or cyclosporine (n = 30) with mycophenolate, and posttransplant cyclophosphamide or posttransplant cyclophosphamide alone (n = 4) for GVHD prophylaxis. Transplant conditioning regimens included TBI-containing (20%), busulfan and cyclophosphamide (38%), melphalan and thiotepa (27%), and busulfan and fludarabine (15%). Recipients with unrelated donor transplants (n = 834) received tacrolimus (n = 756) or cyclosporine (n = 78) with methotrexate for GVHD prophylaxis. Most patients received busulfan and cyclophosphamide (37%) or busulfan and fludarabine (39%), and the remaining 23% received TBI and cyclophosphamide. In the reduced intensity setting, recipients of haploidentical transplants (n = 74) received BM grafts and tacrolimus with mycophenolate and posttransplant cyclophosphamide for GVHD prophylaxis. Transplant conditioning regimen included TBI (200 cGy), cyclophosphamide, and fludarabine. Recipients unrelated donor transplants (n = 303) received tacrolimus (n = 289) or cyclosporine (n = 14) with methotrexate for GVHD prophylaxis. Most patients (92%) received busulfan or melphalan and fludarabine, and the remaining received TBI (200 cGy), cyclophosphamide, and fludarabine.
The results of multivariate analyses are shown Table 5. Consistent with the main analyses, after myeloablative transplantation, there are no differences in survival, transplant-related mortality, or relapse by donor type and grade 2-4 acute GVHD, 3-4 acute GVHD, and chronic GVHD risks are higher after HLA-matched unrelated donor transplantation. After reduced intensity conditioning transplantation, consistent with the main analyses, there are no differences in survival, relapse risks are higher after haploidentical transplantation and grade 3-4 acute GVHD, and chronic GVHD risks are higher after HLA-matched unrelated donor transplantation. In contrast to the main analyses, there were no differences in transplant-related mortality or grade 2-4 acute GVHD risks after haploidentical and unrelated donor transplantation.
Multivariate analysis (subset): risks of acute and chronic GVHD, nonrelapse mortality, relapse, and OS by donor type
| . | Transplant conditioning regimen intensity . | |
|---|---|---|
| Outcome . | Myeloablative* . | Reduced intensity† . |
| Hazard ratio (95% CI) . | Hazard ratio (95% CI) . | |
| Grade 2-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.37 (0.23-0.61) | 0.71 (0.44-1.15) |
| P = .0001 | P = .16 | |
| Grade 3-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.33 (0.14-0.81) | 0.21 (0.05-0.86) |
| P = .02 | P = .03 | |
| Chronic GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.44 (0.29-0.66) | 0.45 (0.28-0.71) |
| P = .0001 | P = .0006 | |
| Nonrelapse mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.93 (0.54-1.61) | 0.59 (0.27-1.29) |
| P = .83 | P = .19 | |
| Relapse | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.28 (0.911.81) | 1.53 (1.08-2.22) |
| P = .16 | P = .02 | |
| Overall mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.19 (0.87-1.61) | 1.06 (0.76-1.51) |
| P = .28 | P = .70 | |
| . | Transplant conditioning regimen intensity . | |
|---|---|---|
| Outcome . | Myeloablative* . | Reduced intensity† . |
| Hazard ratio (95% CI) . | Hazard ratio (95% CI) . | |
| Grade 2-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.37 (0.23-0.61) | 0.71 (0.44-1.15) |
| P = .0001 | P = .16 | |
| Grade 3-4 acute GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.33 (0.14-0.81) | 0.21 (0.05-0.86) |
| P = .02 | P = .03 | |
| Chronic GVHD | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.44 (0.29-0.66) | 0.45 (0.28-0.71) |
| P = .0001 | P = .0006 | |
| Nonrelapse mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 0.93 (0.54-1.61) | 0.59 (0.27-1.29) |
| P = .83 | P = .19 | |
| Relapse | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.28 (0.911.81) | 1.53 (1.08-2.22) |
| P = .16 | P = .02 | |
| Overall mortality | ||
| Matched unrelated donor | 1.00 | 1.00 |
| Haploidentical donor | 1.19 (0.87-1.61) | 1.06 (0.76-1.51) |
| P = .28 | P = .70 | |
BM recipients of haploidentical donor transplants and peripheral blood recipient of matched unrelated donor transplant who received tacrolimus or cyclosporine with methotrexate for GVHD prophylaxis are shown above.
Nonrelapse mortality risk adjusted for performance score; relapse risk adjusted for disease risk index; overall mortality adjusted for patient age and disease risk index.
Nonrelapse mortality risk adjusted for disease risk index; relapse risk adjusted for performance score, disease risk index and secondary AML; overall mortality adjusted for disease risk index and secondary AML.
Discussion
Allogeneic stem cell transplantation is a lifesaving procedure for many patients with hematologic malignancies and, through the use of HLA-matched and mismatched related/unrelated donors and umbilical cord blood, this treatment is now theoretically available to virtually all patients in need of transplantation. Although there are several reports that have shown comparable survival after HLA-matched adult unrelated donor and HLA-mismatched umbilical cord blood transplantation,29-32 there are few reports that compare outcomes after haploidentical transplantation to that after HLA-matched unrelated donor transplantation,19-21 the accepted standard when an HLA-matched sibling is lacking. Therefore, the primary objective of this analysis was to compare survival and other transplantation outcomes after haploidentical donor transplantation that used the posttransplant cyclophosphamide approach, the most widely adopted practice in the United States. In the absence of a randomized trial, we used data collected by a large observational registry to compare outcomes by donor type, adjusting for patient and disease characteristics associated with transplantation outcomes. The data confirm that in both the myeloablative and reduced intensity setting, OS after haploidentical donor transplantation was comparable to that after HLA-matched unrelated donor transplantation for AML. Survival after transplantation was adjusted for age and disease risk index, factors associated with survival and independent of donor type. We used disease risk index, a validated tool that incorporates disease status at transplantation and cytogenetic risk as a composite end point.28 Our observations were confirmed by adjusting for disease status and cytogenetic risk separately (data not shown). Recipients of reduced intensity haploidentical transplants received a uniform conditioning regimen (low dose TBI with cyclophosphamide and fludarabine). In this setting, we observed lower nonrelapse mortality risks compared with the more intensive regimen of an alkylating agent with fludarabine for HLA-matched unrelated donor transplants. However, any advantage derived from lower mortality risks with the very low intensity regimen for haploidentical transplantation was negated by higher relapse risks in this group. In the myeloablative setting, an effect of donor type on nonrelapse mortality or relapse risks was not seen.
Acute and chronic GVHD were substantially lower after haploidentical transplantation. Consequently, chronic GVHD-free/leukemia-free survival was higher after myeloablative haploidentical transplantation. The lower chronic GVHD-free/leukemia-free survival after HLA-matched unrelated donor transplantation is in part explained by the use of peripheral blood grafts. The observed difference in GVHD rates may be attributed to several factors. First, the predominant graft used for haploidentical transplantation was BM and for unrelated donor transplantation, peripheral blood, which is associated with higher GVHD rates relative to BM in the setting of HLA-matched sibling and unrelated donor transplantations. Second, GVHD prophylaxis for haploidentical transplantation included posttransplant cyclophosphamide, a strategy associated with low GVHD rates. Whether the observed low rate of GVHD in the current analyses was solely explained by donor source or use of posttransplant cyclophosphamide or the combination of both cannot be determined by this analysis. The BMT CTN has an on-going study (BMT CTN 1301, NCT02345850) that randomizes recipients of HLA-matched related and unrelated donor transplantation to 3 specified interventions, one of which is the transplantation of BM followed by posttransplant cyclophosphamide and a second is the use of BM with conventional CNI-based prophylaxis. Upon completion of this trial, we may better understand the effects of the posttransplant cyclophosphamide approach for GVHD prophylaxis relative to the standard approach, which is CNI-based prophylaxis.
There were other differences between the treatment groups. Notably, neutrophil recovery after HLA-matched unrelated donor transplantation was better than after haploidentical donor transplantation with myeloablative conditioning regimens. Although we did not observe differences in disease risk index, differences in graft type and/or heterogeneity of conditioning regimens may have contributed to this. The limited number of patients in the current analysis prevents us from exploring this further. Donor-specific HLA antibodies are associated with graft failure after haploidentical and unrelated donor transplantation.33-35 We do not have these data and are unable to test in the current analyses.
The current analysis has several limitations. First, we used data reported to an observation registry, which makes it impossible to study donor choices or the choice of other treatments patients received. Although some of the patients may not have had a suitably HLA-matched adult unrelated donor, others may have been offered haploidentical transplantation based on institutional preference. Second, although the analysis was limited to AML and disease risk index was not different between donor groups, recipients of haploidentical donor transplantation reported poor performance scores and a longer time from diagnosis to transplantation for recipients of reduced intensity conditioning regimens. This is in part explained by a higher proportion of patients transplanted in first complete remission with HLA-matched unrelated donors. Although every attempt was made to adjust for the differences on transplantation outcomes, there may be several unknown or unmeasured factors we did not adjust for. Third, the heterogeneity of myeloablative conditioning regimens and the use of BM for haploidentical transplants vs peripheral blood for unrelated donor transplants prevents us from being able to segregate the impact of donor vs anatomic source of stem cells. Fourth, haploidentical transplants were performed at 19 transplant centers, whereas unrelated donor transplants were performed at several more centers representing clinical practice across small-, mid-, and large-sized transplant centers. Although we were not able to identify a center effect, it remains to be seen whether the results observed in the current analyses will hold true if these transplants are performed more widely. Fifth, we observed survival differences of about 5% between the donor groups. Therefore, to conduct a comparative study with 80% power (α level of 5%) to detect a 5% difference in survival, we would need ∼1500 patients in each of the 4 donor groups.
A randomized trial is the gold standard for comparing outcomes between donor types. In the absence of such a trial, a carefully controlled analysis that considered pertinent clinical characteristics such as age, performance score, cytomegalovirus serostatus, leukemia type (de novo vs secondary AML), disease risk index, and the interval between diagnosis and transplantation was performed to study outcomes after transplantation by donor type. Haploidentical donor transplantation with a GVHD prophylaxis regimen with CNI, mycophenolate, and posttransplant cyclophosphamide is an acceptable approach for AML. Based on the results of a multicenter phase 2 trial,16,36 there is an ongoing trial that randomizes patients to haploidentical BM or umbilical cord blood grafts (BMT CTN 1101; NCT01597778). It is perhaps timely to plan a trial that randomizes patients to haploidentical or unrelated HLA-matched grafts for hematologic malignancy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The CIBMTR is supported by a Public Health Service grant/cooperative agreement (U24-CA076518) from the National Institutes of Health National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; a grant/cooperative agreement from the National Heart, Lung, and Blood Institute (5U10HL069294) and National Cancer Institute; a contract with Health Resources and Services Administration (HHSH250201200016C); grants from the Office of Naval Research (N00014-13-1-0039 and N00014-14-1-0028); and grants from the following: Actinium Pharmaceuticals; Allos Therapeutics, Inc.; Amgen, Inc.; anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Celgene Corporation; Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Gentium SpA; Genzyme Corporation; GlaxoSmithKline; Health Research, Inc., Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Jeff Gordon Children’s Foundation; Kiadis Pharma; Medac GmbH; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; PerkinElmer, Inc.; Remedy Informatics; Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; St. Baldrick’s Foundation; StemCyte; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; TerumoBCT; Teva Neuroscience, Inc.; THERAKOS, Inc.; University of Minnesota; University of Utah; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration, or any other agency of the US government.
Authorship
Contribution: S.O.C., A.A.B., R.E.C., E.J.F., M.E., and M.-J.Z. designed the study; J.C. and M.-J.Z. prepared and analyzed data; S.O.C. drafted the first manuscript; M.-J.Z., A.A.B., A.B., F.R.A., O.S.A., P.A., J.H.A., J.C., S.M.D., M.M.H., D.H.F., L.L., R.N., M.-A.P., P.V.O., S.R.P., D.L.P., M.R.R., O.T.H.R., V.R., R.V., D.J.W., R.E.C., E.J.F., and M.E. reviewed and interpreted data, and edited the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan O. Ciurea, Department of Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 423, Houston, TX 77030; e-mail: sciurea@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal