Key Points
Infusion of platelets containing FVIII triggers neither a primary nor memory anti-FVIII immune response in hemophilia A mice.
Total body irradiation plus platelet-FVIII infusion suppresses anti-FVIII immune response.
Abstract
Evidence shows that factor VIII (FVIII) ectopically expressed in platelets (2bF8) is therapeutic in FVIIInull mice even with anti-FVIII inhibitory antibodies (inhibitors). If current efforts to generate platelets in vitro succeed, genetically manipulated platelets containing FVIII may be used therapeutically in hemophilia A patients with inhibitors. One important concern is the immunogenicity of platelet-derived FVIII. To address this concern, we infused 2bF8 transgenic (2bF8Tg) platelets into naïve FVIIInull mice weekly for 8 weeks. No anti-FVIII antibodies were detected in the infused animals during the study course. We then explored whether platelet-derived FVIII is immunogenic in FVIIInull mice with inhibitors. The 2bF8Tg platelets were transfused into rhF8-primed FVIIInull mice, resulting in no augmentation of anti-FVIII antibodies. To investigate whether preconditioning affects the immune response, animals were sublethally irradiated and subsequently transfused with 2bF8Tg platelets. No anti-FVIII antibodies were detected in the recipients after platelet infusions. Following further challenge with rhF8, the inhibitor titer in this group was significantly lower than in naïve FVIIInull mice utilizing the same immunization protocol. Thus, our data demonstrate that infusion of platelets containing FVIII triggers neither primary nor memory anti-FVIII immune response in FVIIInull mice and that sublethal irradiation plus 2bF8Tg platelet infusion suppresses anti-FVIII immune response in FVIIInull mice.
Introduction
Recent studies from our group and others using a transgenic approach have demonstrated that factor VIII (FVIII) ectopically targeted to platelets can restore hemostasis in hemophilia A (HA; FVIIInull) mice even in the presence of inhibitory antibodies (inhibitors) directed against FVIII.1-3 Utilizing lentivirus-mediated platelet-specific FVIII gene delivery to hematopoietic stem cells (HSCs), we have demonstrated that therapeutic levels of platelet-FVIII are sustainable and that inhibitor titers decline with time in transduced animals with preexisting anti-FVIII immunity after gene therapy.4 Our further studies show that platelet gene therapy can not only correct the hemophilic phenotype, but also induce FVIII-specific immune tolerance.5 The highly efficient clinical efficacy of platelet-derived FVIII has been further confirmed in an HA dog model6 and our study using human cells.7
In our platelet gene therapy model, HSCs are transduced ex vivo with lentivirus carrying the transgene 2bF8, in which FVIII expression is directed by the platelet-specific glycoprotein αIIb gene promoter, and transplanted into the recipient. Sufficient preconditioning must be employed to create space for therapeutic engraftment of the transduced HSCs.4 It is not clear whether preconditioning affects the potential for an immune response or immune tolerance in the context of platelet-derived FVIII. Furthermore, if current efforts8-13 to generate platelets or megakaryocytes in vitro succeed, genetically manipulated platelets containing FVIII may be used therapeutically as a potential transfusion alternative, in HA patients even with inhibitors. One important concern that has not been explored, however, is the immunogenicity of platelet-derived FVIII.
In the current study, we investigated (1) the immune response in naïve HA mice after platelet-derived FVIII infusion; (2) the immunogenicity of platelet-derived FVIII in HA mice with preexisting anti-FVIII immunity; and (3) whether preconditioning affects the immune response in HA mice. The 2bF8 genetically manipulated platelets from transgenic mice, in which FVIII is sequestrated in platelets, were used as a source of platelet-derived FVIII and infused into FVIIInull mice under various conditions. We show that infusion of platelets containing FVIII triggers neither a primary nor memory anti-FVIII immune response in HA mice. Furthermore, infusion of platelet-derived FVIII into HA mice preconditioned with a nonmyeloablative conditioning regimen can suppress the anti-FVIII immune response.
Methods
Mice
Mice were housed in a pathogen-free facility, and all animal studies were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. FVIIInull mice, which were a kind gift from H. Kazazian (University of Pennsylvania School of Medicine), contained a targeted disruption of exon 17 of the FVIII gene.14 The 2bF8 transgenic mice were generated in the Transgenic Core Facility of the Blood Research Institute and Medical College of Wisconsin using lentivirus-mediated transgenesis as reported previously.15,16 The 2bF8 lentiviral vector (LV) was generated as described in our previous report.17 The 2bF8 transgene was crossed onto the FVIIInull background (2bF8Tg), which were used as donors for platelet infusion. FVIIInull and 2bF8Tg mice used in this study were on a 129/SV × C57BL/6 mixed genetic background. Isoflurane or ketamine was used for anesthesia. Blood samples were collected from a retro-orbital plexus, tail, or vena cava blood draw as described in our previous report.2
Platelet isolation and infusion
Platelets were isolated as previously described.2 Briefly, 200 μL of blood was collected via eye bleeds from 2bF8Tg mice and transferred into a 1.5-mL microtube containing Tyrode buffer with 0.01 M sodium citrate and 50 ng/mL prostaglandin E1 (Sigma, St. Louis, MO). Platelets recovered from a soft spin were washed, resuspended in Tyrode buffer, and infused into FVIIInull mice weekly in a volume of 300 μL per mouse via retro-orbital venous administration for a total of 4 weeks (1 round) or 8 weeks (2 rounds). A sublethal 660-cGy total body irradiation (TBI) as described in our previous report4 was employed on some animals before platelet infusion to investigate whether preconditioning would affect the immune response to the infusion of platelets that contain FVIII. Platelets were collected from some of the infused recipients 1 week after infusion, and the level of functional platelet-FVIII activity was determined by a chromogenic assay on platelet lysates using the Coatest SP4 FVIII Kit (DiaPharma, Franklin, OH) as described in our previous reports.2,4 Blood cell counts were determined using the Vet ABC Hematology Analyzer (Scil Animal Care Company, Gurnee, IL).
Immune response studies
To establish the inhibitor model, 6- to 8-week-old FVIIInull mice were immunized via intravenous injection of recombinant human B-domain–deleted FVIII (rhF8, Xyntha; Pfizer Inc., New York, NY) at a dose of 50 U/kg (full dose) weekly for a total of 4 weeks, and inhibitor titers were monitored. Animals were randomly assigned to the treatment groups. This immunization protocol was also employed on platelet-infused animals to investigate whether immune tolerance/suppression was induced after platelet-derived FVIII infusion. For the low-dose rhF8 immunization, animals were infused with rhF8 intravenously at a dose of 2 U/kg weekly. To determine the titer of anti-FVIII antibodies, blood samples were collected from animals 1 week after rhF8 or platelet infusion, and the plasmas were isolated as previously described.2 Inhibitory and total anti-FVIII antibodies were determined by Bethesda and enzyme-linked immunosorbent assay (ELISA) assay, respectively, as described in our previous reports.1
T-cell proliferation assay
CD4+ T cells were isolated from rhF8-primed FVIIInull mouse spleens using the Miltenyi CD4+ T-cell Isolation Kit (Miltenyi Biotec Inc., San Diego, CA) and labeled with the cell proliferation tracer carboxyfluorescein succinimidyl ester (CFSE; Life Technologies, Carlsbad, CA) following the protocols provided by the manufacturer. Dendritic cells from naïve FVIIInull mouse spleens were used as antigen-presenting cells. Cells were cultured in completed RPMI 1640 media containing rhF8 (10 U/mL), 2bF8Tg platelets (1.5 × 108/mL), or concanavalin A (which is known for its ability to stimulate T-cell proliferation18 ) (Sigma) in duplicates for 96 hours. Cells were harvested and stained with the anti-mouse CD4+ and anti-mouse T-cell receptor β antibodies (eBioscience, San Diego, CA) to identify CD4+ T cells and analyzed by flow cytometry for CFSE expression. The details are provided in the supplemental Materials and Methods (available on the Blood Web site).
Statistical analysis
Data are presented as the mean ± standard error of the mean. Statistical comparisons of experimental groups were evaluated by 2-tailed Student t test if data distribution passed the Normality test (Shapiro-Wilk) using SigmaPlot 13.0 (Systat Software Inc., San Jose, CA) . The Mann-Whitney rank sum test was used for comparison if data distribution failed in the normality test. A value of P < .05 was considered statistically significant.
Results
The immunogenicity of platelet-FVIII in naïve FVIIInull mice
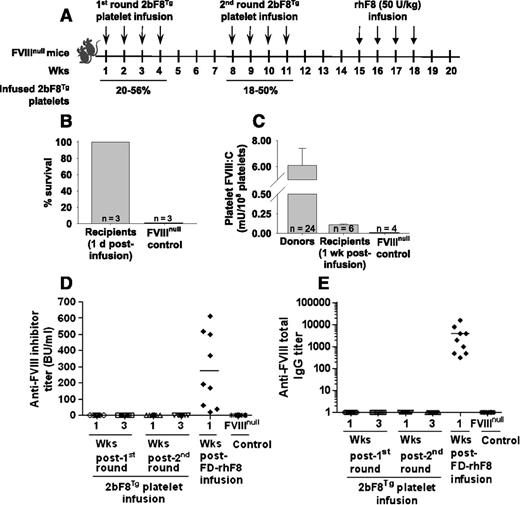
We have recently generated 2 new lines of 2bF8 transgenic mice, LV17Tg and LV18Tg, by lentivirus-mediated transgenesis. In the LV17Tg line, the levels of platelet-FVIII expression are 3 mU/108 platelets in heterozygous and 6 mU/108 platelets in homozygous animals. In the LV18Tg line, the level of platelet-FVIII expression is 6 mU/108 platelets in heterozygous mice. In our platelet transfusion studies, homozygous LV17Tg or heterozygous LV18Tg mice were used as donors for platelet collections. To investigate whether platelet-derived FVIII can act as an immunogen in FVIIInull mice, we infused 2bF8Tg platelets with platelet-FVIII level of 6 mU/108 platelets into naïve FVIIInull mice without any preconditioning weekly for 4 weeks each round for 2 rounds with a 4-week interval. A time table of infusion studies for naïve FVIIInull mice is depicted in Figure 1A. The transgenic platelets were transfused to a level between 18% and 56% of total platelets upon infusion. With weekly transfusion of such amount of platelets, the platelet count (before each transfusion) was not significantly changed (supplemental Figure 1). All animals survived the tail-clip challenge even when the test was performed 1 day after platelet infusion (Figure 1B). The level of platelet-FVIII in the infused animals was still detectable (0.11 ± 0.01 mU/108 platelets; n = 6) 1 week after infusion of 2bF8Tg platelets (Figure 1C). Importantly, neither inhibitory nor noninhibitory anti-FVIII antibodies were detected in the infused mice during the study course (Figure 1D-E), demonstrating that infusion of platelets containing FVIII does not trigger an anti-FVIII immune response in FVIIInull mice. All animals developed antibodies following further challenge with rhF8 at a dose of 50 U/kg by intravenous injection weekly for 4 weeks (Figure 1D-E).
The immunogenicity of platelets that contain FVIII in naïve FVIIInull mice. Platelets isolated from transgenic mice (2bF8Tg), in which FVIII expression was driven by the platelet-specific αIIb promoter, were infused into FVIIInull mice without any preconditioning. The transgenic platelets were transfused to a level between 18% and 56% of total platelets upon infusion. (A) Schematic diagram of platelet transfusion in naïve FVIIInull mice. (B) Tail-clipping test on FVIIInull mice after transfusion of 2bF8Tg platelets. Tail-clipping test was performed 1 day after platelet infusion to assess phenotypic correction of FVIIInull coagulation defect. (C) The levels of platelet-FVIII in transfused recipients. Platelets were collected 1 week after platelet infusion, and functional FVIII activity levels were determined by a chromogenic assay on platelet lysates. (D) The inhibitor titers in recipients after infusion of 2bF8Tg platelets or rhF8. The anti-FVIII inhibitor titers were determined by Bethesda assay. (E) The total anti-FVIII immunoglobulin (Ig) G titers in recipients after infusion of 2bF8Tg platelets or rhF8. The total anti-FVIII IgG titers were determined by ELISA assay. These results demonstrate that transfusion of platelets containing FVIII into naïve FVIIInull mice neither elicits an anti-FVIII immune response nor induces immune suppression. FD, full dose; Wks, weeks.
The immunogenicity of platelets that contain FVIII in naïve FVIIInull mice. Platelets isolated from transgenic mice (2bF8Tg), in which FVIII expression was driven by the platelet-specific αIIb promoter, were infused into FVIIInull mice without any preconditioning. The transgenic platelets were transfused to a level between 18% and 56% of total platelets upon infusion. (A) Schematic diagram of platelet transfusion in naïve FVIIInull mice. (B) Tail-clipping test on FVIIInull mice after transfusion of 2bF8Tg platelets. Tail-clipping test was performed 1 day after platelet infusion to assess phenotypic correction of FVIIInull coagulation defect. (C) The levels of platelet-FVIII in transfused recipients. Platelets were collected 1 week after platelet infusion, and functional FVIII activity levels were determined by a chromogenic assay on platelet lysates. (D) The inhibitor titers in recipients after infusion of 2bF8Tg platelets or rhF8. The anti-FVIII inhibitor titers were determined by Bethesda assay. (E) The total anti-FVIII immunoglobulin (Ig) G titers in recipients after infusion of 2bF8Tg platelets or rhF8. The total anti-FVIII IgG titers were determined by ELISA assay. These results demonstrate that transfusion of platelets containing FVIII into naïve FVIIInull mice neither elicits an anti-FVIII immune response nor induces immune suppression. FD, full dose; Wks, weeks.
The immunogenicity of platelet-FVIII in FVIIInull mice with inhibitors
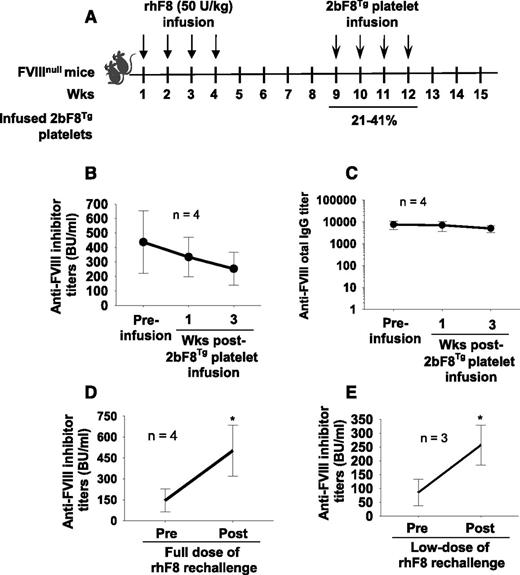
To explore the immune response after platelet-derived FVIII transfusion in FVIIInull mice with preexisting anti-FVIII immunity, FVIIInull mice were immunized with rhF8 to induce anti-FVIII antibody development. After the inhibitor model was established and antibody titers passed the peak time, which occurs 3 to 4 weeks after the last immunization based on our experience, 2bF8Tg platelets were transfused into rhF8-primed FVIIInull mice, and anti-FVIII antibody titers were monitored. A time table is depicted in Figure 2A. There was no significant augmentation of FVIII-specific antibodies as determined by Bethesda assay for inhibitory antibodies (Figure 2B) and ELISA assay for total anti-FVIII IgG (Figure 2C). Instead, the inhibitor titers appeared to decline with the time after platelet infusion from 421 ± 224 BU/mL before platelet infusion to 252 ± 114 BU/mL at 3 weeks after transfusion (a 40% reduction), which was not significantly different from the titers in rhF8-primed FVIIInull mice without platelet infusion at similar time points (395 ± 106 BU/mL at week 8 and 224 ± 73 BU/mL at week 16, a 43% reduction; n = 7).
The immunogenicity of platelets that contain FVIII in FVIIInull mice with preexisting anti-FVIII immunity. FVIIInull mice were immunized with rhF8 (50 U/kg per week × 4) to induce anti-FVIII antibody development. The 2bF8Tg platelets were infused into rhF8-primed mice. The transgenic platelets were transfused to a level between 21% and 41% of total platelets upon infusion. (A) Schematic diagram of platelet infusion in FVIIInull mice with preexisting anti-FVIII immunity. (B) Anti-FVIII inhibitor titers in rhF8-primed FVIIInull mice after transfusion of 2bF8Tg platelets. (C) Anti-FVIII total IgG titers in rhF8-primed FVIIInull mice after transfusion of 2bF8Tg platelets. (D) Anti-FVIII inhibitor titers in rhF8-primed FVIIInull mice after rechallenge with full dose of rhF8 (50 U/kg per week × 4). (E) Anti-FVIII inhibitor titers in rhF8-primed FVIIInull mice after rechallenge with low dose of rhF8 (2 U/kg per week × 4). These results show that transfusion of platelets containing FVIII does not trigger an anti-FVIII memory response in FVIII-primed FVIIInull mice. *P < .05.
The immunogenicity of platelets that contain FVIII in FVIIInull mice with preexisting anti-FVIII immunity. FVIIInull mice were immunized with rhF8 (50 U/kg per week × 4) to induce anti-FVIII antibody development. The 2bF8Tg platelets were infused into rhF8-primed mice. The transgenic platelets were transfused to a level between 21% and 41% of total platelets upon infusion. (A) Schematic diagram of platelet infusion in FVIIInull mice with preexisting anti-FVIII immunity. (B) Anti-FVIII inhibitor titers in rhF8-primed FVIIInull mice after transfusion of 2bF8Tg platelets. (C) Anti-FVIII total IgG titers in rhF8-primed FVIIInull mice after transfusion of 2bF8Tg platelets. (D) Anti-FVIII inhibitor titers in rhF8-primed FVIIInull mice after rechallenge with full dose of rhF8 (50 U/kg per week × 4). (E) Anti-FVIII inhibitor titers in rhF8-primed FVIIInull mice after rechallenge with low dose of rhF8 (2 U/kg per week × 4). These results show that transfusion of platelets containing FVIII does not trigger an anti-FVIII memory response in FVIII-primed FVIIInull mice. *P < .05.
To ensure that the immune system was not exhausted in the inhibitor model that we generated, some animals were immunized with 4 more doses of rhF8 at 50 U/kg. As shown in Figure 2D, anti-FVIII inhibitor titers significantly increased 3.4-fold from 146 ± 84 BU/mL to 503 ± 183 BU/mL after mice were rechallenged with 4 doses of rhF8. To investigate whether a low dose of rhF8 would stimulate a memory response in rhF8-primed animals, we immunized some animals with 4 doses of rhF8 at 2 U/kg, which is equivalent to the level of plt-F8 achieved upon 2bF8Tg platelet infusion (∼33% infused 2bF8Tg platelets). As shown in Figure 2E, the inhibitor titers significantly increased in rhF8-primed animals from 85 ± 47.7 BU/mL to 256.7 ± 72.2 BU/mL after 4 doses of low-dose rhF8 immunizations.
Because the anti-FVIII immune response is CD4+ T-cell dependent,19 we developed an in vitro CFSE-labeled T-cell proliferation assay to determine if FVIII sequestered in platelets would stimulate rhF8-primed CD4+ T-cell proliferation. Because the CFSE label is inherited by daughter cells after cell division with half of the fluorescent label from its parent cell, the percent of daughter cells that contain less intensity of CFSE can thereby be determined by fluorescence-activated cell sorter. In our preliminary studies, we found that, using our CFSE-labeled T-cell proliferation protocol, there were no CFSE-labeled daughter CD4+ T cells detectable when CD4+ T cells were isolated from animals after only 1 or 2 doses of rhF8 immunization following rhF8 restimulation in vitro (data not shown). Thus, we used CD4+ T cells from FVIIInull mice after 4 full doses of rhF8 immunization for in vitro studies. As shown in Figure 3A, T-cell proliferation was dose dependent with a plateau of 10 to 15 U/mL of rhF8. A set of representative histograms of CFSE-labeled T-cell proliferation is shown in Figure 3B. The percentage of daughter CD4+ T cells (D-CFSE) in the condition cocultured with 2bF8Tg platelets (3.9 ± 0.5%) was not significantly different from the control group (5.8 ± 0.9%) in which no rhF8 was added (0 U/mL of rhF8). In contrast, there were 12.9 ± 0.7% of daughter CD4+ T cells in the group restimulated with 10 U/mL of rhF8, which was significantly higher than the control without rhF8 restimulation (P < .01; Figure 3C). Taken together, these data demonstrate that platelets containing FVIII do not elicit an anti-FVIII memory response.
Ex vivo T-cell proliferation assay assesses the immunogenicity of platelet-containing FVIII. The CFSE-labeled CD4+ T-cell proliferation assay was used to elucidate whether platelets that contain recombinant FVIII would stimulate rhF8-primed CD4+ T-cell proliferation. CD4+ T cells isolated from rhF8-primed FVIIInull splenocytes were labeled with CFSE and cocultured with dendritic cells from FVIIInull spleens in the presence of rhF8 or 2bF8Tg platelets for 96 hours. The daughter CD4+ T cells (D-CFSE) were analyzed by flow cytometry. (A) The rhF8 dose-response curve in the CFSE-labeled CD4+ T-cell proliferation assay. (B) Representative histograms from flow cytometry analysis of daughter CD4+ T cells. (C) The graph of daughter CD4+ T cells after coculture with rhF8 or 2bF8Tg platelets. Concanavalin A (ConA) was used as a positive control for T-cell proliferation. These results show that platelets that contain FVIII do not stimulate FVIII-primed CD4+ T-cell proliferation in vitro. plts, platelets.
Ex vivo T-cell proliferation assay assesses the immunogenicity of platelet-containing FVIII. The CFSE-labeled CD4+ T-cell proliferation assay was used to elucidate whether platelets that contain recombinant FVIII would stimulate rhF8-primed CD4+ T-cell proliferation. CD4+ T cells isolated from rhF8-primed FVIIInull splenocytes were labeled with CFSE and cocultured with dendritic cells from FVIIInull spleens in the presence of rhF8 or 2bF8Tg platelets for 96 hours. The daughter CD4+ T cells (D-CFSE) were analyzed by flow cytometry. (A) The rhF8 dose-response curve in the CFSE-labeled CD4+ T-cell proliferation assay. (B) Representative histograms from flow cytometry analysis of daughter CD4+ T cells. (C) The graph of daughter CD4+ T cells after coculture with rhF8 or 2bF8Tg platelets. Concanavalin A (ConA) was used as a positive control for T-cell proliferation. These results show that platelets that contain FVIII do not stimulate FVIII-primed CD4+ T-cell proliferation in vitro. plts, platelets.
The effect of preconditioning on the immune response in platelet-FVIII infusion
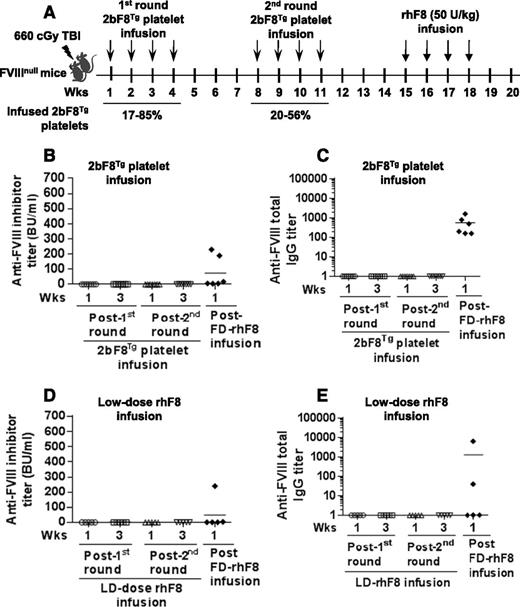
To investigate whether preconditioning affects the anti-FVIII immune response, animals were preconditioned with a sublethal 660-cGy TBI, and the first of 8 weekly 2bF8Tg platelet transfusions was administered 24 hours later. The time table for this study is depicted in Figure 4A. After the 660-cGy TBI conditioning, leukocyte and platelet counts and the hemoglobin level were significantly decreased, reaching their lowest levels at 7 to 14 days. Although the platelet number and hemoglobin level recovered, the leukocyte number was still lower on day 21 after irradiation than the preirradiated time point (supplemental Figure 2). Neither inhibitory nor noninhibitory anti-FVIII antibodies were detected in recipients after 2bF8Tg platelet infusion (Figure 4B-C). Following further challenge with rhF8 intravenously at 50 U/kg weekly for 4 weeks, both the inhibitor titer (75 ± 43 BU/mL) and noninhibitor titer (570 ± 230) in this group were significantly lower than in the naïve FVIIInull mice utilizing the same infusion protocol but without preconditioning (276 ± 76 BU/mL, Figure 1D; and 4035 ± 1712, Figure 1E; P < .05). These results indicate that 660-cGy TBI plus 2bF8Tg platelet infusion may suppress the anti-FVIII immune response.
The immune response in FVIIInull mice that were preconditioned with a nonmyeloablative regimen followed by an early phase of platelet-FVIII or low-dose rhF8 transfusion. FVIIInull mice were preconditioned with 660-cGy TBI. Twenty-four hours later, animals were infused with 2bF8Tg platelets or low-dose rhF8 (2 U/kg) weekly for a total of 8 infusions and subsequently challenged with full-dose rhF8 (50 U/kg per week × 4). (A) Schematic diagram of the transfusion in preconditioned FVIIInull mice. The transgenic platelets were transfused to a level between 17% and 85% of total platelets upon infusion in the first round and between 20% and 56% in the second round. (B) The titers of anti-FVIII inhibitors in animals that received 2bF8Tg platelet transfusion. (C) The titers of total anti-FVIII IgG in animals that received 2bF8Tg platelet transfusion. (D) The titers of anti-FVIII inhibitors in animals that received low-dose rhF8 infusion. (E) The titers of total anti-FVIII IgG in animals that received low-dose rhF8 infusion. These results demonstrate that transfusion of 2bF8Tg platelets or low-dose rhF8 together with 660-cGy TBI preconditioning suppresses the anti-FVIII immune response in FVIIInull mice. LD, low dose.
The immune response in FVIIInull mice that were preconditioned with a nonmyeloablative regimen followed by an early phase of platelet-FVIII or low-dose rhF8 transfusion. FVIIInull mice were preconditioned with 660-cGy TBI. Twenty-four hours later, animals were infused with 2bF8Tg platelets or low-dose rhF8 (2 U/kg) weekly for a total of 8 infusions and subsequently challenged with full-dose rhF8 (50 U/kg per week × 4). (A) Schematic diagram of the transfusion in preconditioned FVIIInull mice. The transgenic platelets were transfused to a level between 17% and 85% of total platelets upon infusion in the first round and between 20% and 56% in the second round. (B) The titers of anti-FVIII inhibitors in animals that received 2bF8Tg platelet transfusion. (C) The titers of total anti-FVIII IgG in animals that received 2bF8Tg platelet transfusion. (D) The titers of anti-FVIII inhibitors in animals that received low-dose rhF8 infusion. (E) The titers of total anti-FVIII IgG in animals that received low-dose rhF8 infusion. These results demonstrate that transfusion of 2bF8Tg platelets or low-dose rhF8 together with 660-cGy TBI preconditioning suppresses the anti-FVIII immune response in FVIIInull mice. LD, low dose.
We then investigated whether infusion of the low-dose FVIII together with 660-cGy TBI would have a similar suppressive function on FVIII immune response. A similar time table as shown in Figure 4A was used. As shown in Figure 4D-E, no antibodies were detected in recipients during low-dose rhF8 infusion. Following further full-dose rhF8 immunization, 2 of 5 mice developed antibodies with inhibitor titers of 3.8 and 240 BU/mL. The incidence of anti-FVIII antibody development and the inhibitor titers in this group were significantly lower than in the control group, in which all animals developed various levels of antibodies with a range of 1.8 to 2000 BU/mL (424 ± 77 BU/mL; n = 27) when they were immunized with the full dose of rhF8 without preconditioning (Figure 5D).
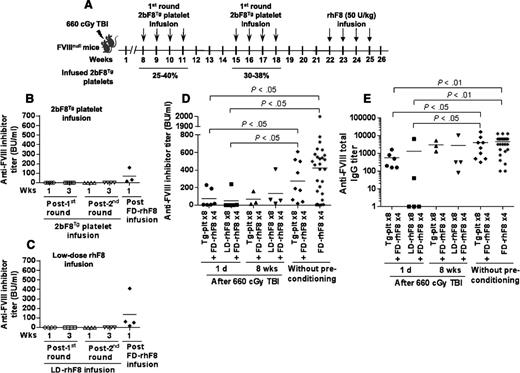
The immune response in FVIIInull mice that were preconditioned with a nonmyeloablative regimen, allowing an 8-week recovery before transfusion of platelet-FVIII or low-dose rhF8. FVIIInull mice were preconditioned with 660-cGy TBI. Eight weeks later, animals were transfused with 2bF8Tg platelets or low-dose rhF8 (2 U/kg). After 8 doses of infusion with either 2bF8Tg platelets or low-dose rhF8, animals were further challenged with full-dose rhF8 immunization. The anti-FVIII inhibitor titers from different time points were determined by Bethesda assay. (A) Schematic diagram of transfusion in FVIIInull mice that were preconditioned with 660-cGy TBI followed by 8 weeks of recovery. The transgenic platelets were transfused to a level between 25% and 40% of total platelets upon infusion. (B) The inhibitor titers in the animals that received 2bF8Tg platelet infusion. (C) The inhibitor titers in animals that received low-dose rhF8 infusion. (D) Comparison of inhibitor titers among 6 groups of animals under various conditions. (E) Comparison of total anti-FVIII IgG titers among 6 groups of animals under various conditions. These results suggest that the immune suppression induced by 2bF8Tg platelet or low-dose rhF8 transfusion occurs during the early phase of BM recovery following 660-cGy TBI.
The immune response in FVIIInull mice that were preconditioned with a nonmyeloablative regimen, allowing an 8-week recovery before transfusion of platelet-FVIII or low-dose rhF8. FVIIInull mice were preconditioned with 660-cGy TBI. Eight weeks later, animals were transfused with 2bF8Tg platelets or low-dose rhF8 (2 U/kg). After 8 doses of infusion with either 2bF8Tg platelets or low-dose rhF8, animals were further challenged with full-dose rhF8 immunization. The anti-FVIII inhibitor titers from different time points were determined by Bethesda assay. (A) Schematic diagram of transfusion in FVIIInull mice that were preconditioned with 660-cGy TBI followed by 8 weeks of recovery. The transgenic platelets were transfused to a level between 25% and 40% of total platelets upon infusion. (B) The inhibitor titers in the animals that received 2bF8Tg platelet infusion. (C) The inhibitor titers in animals that received low-dose rhF8 infusion. (D) Comparison of inhibitor titers among 6 groups of animals under various conditions. (E) Comparison of total anti-FVIII IgG titers among 6 groups of animals under various conditions. These results suggest that the immune suppression induced by 2bF8Tg platelet or low-dose rhF8 transfusion occurs during the early phase of BM recovery following 660-cGy TBI.
To investigate whether a different stage of bone marrow (BM) reconstitution after the 660-cGy TBI would affect the immune response to platelet-FVIII or low-dose rhF8, we started the infusion 8 weeks after irradiation. As shown in supplemental Figure 3, the platelet number and the hemoglobin level recovered by 8 weeks after irradiation, but the number of leukocytes was still lower than the pre–time point. When 2bF8Tg platelet or low-dose rhF8 infusion started at 8 weeks after irradiation (as depicted in Figure 5A), none of the infused animals developed anti-FVIII inhibitors during 2 rounds of infusion (Figure 5B-C). However, when animals were further challenged with a full dose of rhF8 infusion, all animals developed various levels of anti-FVIII antibodies, including inhibitors determined by Bethesda assay and total IgG measured by ELISA. The levels in this group were not significantly different from those obtained from the group without preconditioning but with platelet infusion for 8 times and the naïve control animals after rhF8 immunization without preconditioning (Figure 5D-E), indicating that anti-FVIII immune response was gradually restored while BM was recovering after 660-cGy TBI. Our additional studies showed that the humoral immune response in older animals that were irradiated with 660 cGy and allowed to recover for 6 months was comparable to the control mice without irradiation (see supplemental Figure 4). These results suggest that immune suppression established by 2bF8Tg platelet or low-dose rhF8 infusion only occurs during the early phase (<8 weeks) of BM reconstitution.
Discussion
Inhibitory antibody development against FVIII is not only a significant complication in protein replacement therapy in clinical care,20-23 but also a major concern in gene therapy of HA.24-26 In the clinic, up to 35% of severe HA patients develop inhibitors after FVIII protein infusion, rendering routine FVIII treatment useless. Previous studies from our group and others have demonstrated that ectopically targeted FVIII expression to platelets results in transgenic FVIII protein storage together with endogenous von Willebrand factor, a carrier protein for FVIII, in platelet α-granules and that this platelet-derived FVIII can still function even in the presence of anti-FVIII inhibitors in FVIIInull mice.1,3,4,27 In the current study, we explored the immunogenicity of platelet-derived FVIII in HA mice with or without preexisting anti-FVIII immunity by transfusion of platelets that contain recombinant FVIII.
Transfusion of platelets containing FVIII provides a better therapeutic effect than recombinant FVIII protein for HA mice. Our previous studies have demonstrated that transfusion of genetically modified platelets containing FVIII fully rescued the hemophilia phenotype in FVIIInull mice using a tail-clip model when transgenic platelets were infused up to 30% of total platelets upon infusion.1 In that study, the level of plt-F8 from transgenic animals [2bF8Tg(ES)] generated by ES (embryonic stem cell)–mediated transgenesis was only 0.74 mU/108 platelets, which corresponds to ∼1.2% of FVIII in normal wild-type mouse whole blood, assuming there are 1 × 109 platelets per mL. Thus, infusion of 2bF8Tg(ES) transgenic platelets to 30% would correspond to as little as 0.4% of plasma FVIII. In contrast, only 25% of animals were protected from tail clipping when rhF8 was infused to a plasma level of 0.5%, demonstrating that restoring hemostasis in FVIIInull mice via transfusion of genetically modified platelets containing FVIII is highly effective. In our current study, the phenotypic correction assessment was performed 1 day after platelet infusion, and all animals that received 2bF8Tg platelet infusion survived tail clipping, confirming the high clinical efficacy of transfusion of platelets that contain FVIII in HA mice.
Sequestering FVIII within platelets will significantly extend the half-life of FVIII protein. The median half-life of FVIII protein in severe HA patients after rhF8 infusion is 11.8 hours28 and only 3.3 hours in FVIIInull mice.29 In contrast, the life span of platelets in humans is 8 to 10 days30-32 and 4 to 5 days in mice.33,34 When FVIII expression is targeted to platelets, FVIII protein is stored in platelet α-granules together with its carrier protein von Willebrand factor,1,17 sequestered and carried by platelets, circulating in blood, and protected from protease degradation for the life span of platelets. Thus, the half-life of platelet-FVIII theoretically would be 18-fold longer in humans and 32-fold in mice, respectively, when compared with the intravenous infusion of rhF8. Indeed, our results show that platelet-FVIII was still detectable 7 days after transfusion of 2bF8Tg platelets (Figure 1C). If transgenic platelets were transfused to 30% of total platelets upon infusion, the level of platelet-FVIII in recipient animals would be 1.8 mU/108 platelets, which corresponds to 3% of plasma FVIII in normal wild-type animals. With a half-life of 3.3 hours,29 3% of infused rhF8 in plasma would be almost completely gone within 24 hours.
Platelets contain a large number of bioactive proteins that participate in myriad physiological and pathological processes including hemostasis and immunity.35 Because transfused platelets containing FVIII are highly effective in correcting the HA phenotype, it is important to explore how the immune system responds to FVIII after transfusion of platelet-derived FVIII. When FVIII expression is targeted to platelets, FVIII is stored within platelet α-granules, limiting exposure to the immune system. However, there are at least 2 potential pathways by which platelet-FVIII could potentially be exposed to the immune system. One scenario is at the site of injury, in which FVIII is released from activated platelets, participates in blood coagulation, and is consumed locally within the clot. The other is when aged platelets undergo apoptosis and are phagocytosed by macrophages.34,36,37 In both scenarios, the chance of FVIII entering blood circulation should be minimal. Although macrophages are antigen-presenting cells, evidence suggests that apoptotic cells are phagocytosed by macrophages via mechanisms that are actively anti-inflammatory and tolerogenic (eg, releasing immunosuppressant transforming growth factor and interleukin 10).38-40 Thus, the incidence of anti-FVIII inhibitor development after transfusion of platelets that contain FVIII should be minimized. Indeed, none of the FVIIInull mice developed inhibitors after transfusion of 2bF8Tg platelets even after 2 rounds with a total of 8 infusions. It has been reported that injury (bleeds/trauma) could be one of the risk factors for inhibitor development in HA patients.41-43 In our preliminary studies, blood samples were collected from some of the platelet-infused animals (n = 6) weekly by retro-orbital venous bleeds, which could represent a minor injury model. Notably, no anti-FVIII antibodies were detected in recipients during the entire study course of platelet infusion (data from early time points not shown).
Our previous studies have demonstrated that lentivirus-mediated platelet-specific gene delivery of FVIII to HSCs not only restores hemostasis, but also induces immune tolerance in FVIIInull mice.5 Our current study shows that infusion of 2bF8Tg platelets into FVIIInull mice without preconditioning neither triggers an immune response nor induces immune tolerance to FVIII. However, anti-FVIII immune tolerance, which was evidenced by significant reduction of the levels of anti-FVIII inhibitor titers after full-dose rhF8 immunization (Figure 5D), did occur when the animals were preconditioned with a nonmyeloablative conditioning, 660-cGy TBI, followed by 2bF8Tg platelet or low-dose rhF8 infusion 24 hours after conditioning. The function of the anti-FVIII immune response was restored if the platelet or the low-dose rhF8 infusion started 8 weeks after the 660-cGy TBI. A lack of detectable anti-FVIII antibodies after low-dose rhF8 infusion could be because of the ignorance of CD4+ T cells to such a low level of FVIII. Our data suggest that immune suppression development only occurs during the early phase (<2 months) of BM reconstitution, in which the immune system is recovering.
Although the mechanism of immune suppression induction by 2bF8Tg platelet or low-dose FVIII infusion in the early phase of BM reconstitution is still unclear, it might be because of the induction of antigen-specific regulatory T cells. The immune system undergoes rapid reconstitution after preconditioning followed by bone marrow transplantation (BMT). Although in our current study the animals did not receive BMT after irradiation, the BM was rapidly reconstituted by the remaining endogenous cells, as indicated by blood cell count. Recent studies have demonstrated that regulator T cells play a critical role in establishing tolerance to self-major histocompatibility complex class II antigen after TBI followed by BMT.44 Hori et al showed that UV irradiation prior to antigen immunization can induce antigen-specific immune tolerance in mice.45 Further studies to explore the underlying mechanisms of immune suppression after platelet-FVIII or low-dose rhF8 infusion together with preconditioning is a subject under investigation by our group.
Immune response to FVIII in the inhibitor model could be more complex than in the noninhibitor model because the immune system has already been mounted by FVIII immunization. It has been shown that even a relatively low dose of FVIII can recall a substantial memory response.19,46 Indeed, for the inhibitor model, our studies showed that even infusion of 2 U/kg rhF8 could trigger an anti-FVIII memory response, resulting in significant augmentation of inhibitor titers. Of note, the titers of both inhibitory and noninhibitory anti-FVIII antibodies in rhF8-primed FVIIInull mice did not significantly augment after infusion of platelets that contain FVIII. Instead, it appears that antibody titers declined with time, suggesting that FVIII is well sequestered within platelets, avoiding exposure to the immune system. How the apoptotic platelets that contain FVIII impact the immune system will need to be further explored in the future.
In summary, our studies demonstrate that transfusion of platelets that are genetically modified to express functional FVIII does not elicit anti-FVIII antibody development in naïve FVIIInull mice. The combination of preconditioning with infusion of platelets that contain FVIII can suppress the immune response to FVIII. Transfusion of platelets containing FVIII does not trigger an anti-FVIII memory response in rhF8-primed FVIIInull mice. Although our goal is to develop a gene therapy protocol to cure HA patients even in the presence of inhibitors, and our studies have demonstrated platelet gene therapy is a promising approach,1,2,4-7,17 transfusion of genetically modified platelets could be an alternative treatment approach if the availability of gene therapy is limited by certain circumstances. Transfusion of platelets containing FVIII would be more clinically effective with a lower possibility of trigging an anti-FVIII immune response than rhF8 in HA patients and patients with inhibitors, if in vitro platelet production becomes possible.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute R01-HL102035 (Q.S.) and P01-HL44612 (R.R.M.), the Hemophilia Association of New York (Q.S.), and the Children’s Hospital of Wisconsin Foundation (Q.S.), and the Midwest Athletes Against Childhood Cancer (MACC Fund) (Q.S.). Y.C. was a recipient of the National Hemophilia Foundation Judith Graham Pool Postdoctoral Research Fellowship.
Authorship
Contribution: Y.C. designed the study, performed experiments, and analyzed data; J.C. and X.L. performed experiments and analyzed data; J.A.S. performed experiments, analyzed data, and commented on the manuscript; C.K.B. and R.R.M contributed to study design and made comments to the manuscript; J.H. provided the administrative support; and Q.S. designed and conducted research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qizhen Shi, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Rd, Milwaukee, WI 53226; e-mail: qizhen.shi@bcw.edu; and Jianda Hu, Department of Hematology, Union Hospital, No. 11 Xinquan Rd, Fuzhou, Fujian 350001, China; e-mail: jdhu@medmail.com.cn.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal