Key Points
Cav-1–deficient T cells preferentially differentiate into Tregs, which translates into lower GVHD severity in mice.
Reduced TCR:Lck clustering in Cav-1–deficient T cells is responsible for reduced TCR downstream signaling events promoting Treg differentiation.
Abstract
Caveolin-1 (Cav-1) is a key organizer of membrane specializations and a scaffold protein that regulates signaling in multiple cell types. We found increased Cav-1 expression in human and murine T cells after allogeneic hematopoietic cell transplantation. Indeed, Cav-1−/− donor T cells caused less severe acute graft-versus-host disease (GVHD) and yielded higher numbers of regulatory T cells (Tregs) compared with controls. Depletion of Tregs from the graft abrogated this protective effect. Correspondingly, Treg frequencies increased when Cav-1−/− T cells were exposed to transforming growth factor-β/T-cell receptor (TCR)/CD28 activation or alloantigen stimulation in vitro compared with wild-type T cells. Mechanistically, we found that the phosphorylation of Cav-1 is dispensable for the control of T-cell fate by using a nonphosphorylatable Cav-1 (Y14F/Y14F) point-mutation variant. Moreover, the close proximity of lymphocyte-specific protein tyrosine kinase (Lck) to the TCR induced by TCR-activation was reduced in Cav-1−/− T cells. Therefore, less TCR/Lck clustering results in suboptimal activation of the downstream signaling events, which correlates with the preferential development into a Treg phenotype. Overall, we report a novel role for Cav-1 in TCR/Lck spatial distribution upon TCR triggering, which controls T-cell fate toward a regulatory phenotype. This alteration translated into a significant increase in the frequency of Tregs and reduced GVHD in vivo.
Introduction
Acute graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic cell transplantation (allo-HCT). The disease occurs in 50% to 60% of patients undergoing allo-HCT, and severe GVHD is associated with a mortality of above 60%.1 A hallmark of acute GVHD is the activation of alloreactive donor T cells via foreign major histocompatibility complex (MHC)2 and minor antigens.3 T cells with a T-cell receptor (TCR) recognizing mismatched MHC or minor antigens with a sufficient affinity are then activated. Binding of two TCRs to bivalent antigens within the allowed geometry results in a rearrangement of the TCR structure that is required for TCR phosphorylation, and subsequent downstream signaling leading to T-cell activation.4,5 The phosphorylation of the immunoreceptor tyrosine-based activation motifs at the cytoplasmic tails of the TCR complex is mediated by the lymphocyte-specific protein tyrosine kinase (Lck).6 TCR signal transduction requires the formation and stability of plasma membrane raft microdomains.7 Caveolin-1 (Cav-1) is a key organizer of membrane specializations that coordinates membrane and protein traffic.8-10 Lipid rafts that are stabilized and promoted by Cav-1 have been called “caveolar-lipid rafts” and can serve as platforms for signal transduction.11-13 In addition to this structural role orchestrating the assembly and the activity of multimolecular signaling complexes, Cav-1 binds a wide array of signal transducers through interactions with its phosphorylated tyrosine 14.14,15 Several of the proteins identified as Cav-1–binding partners have been suggested to play a role in TCR-regulated membrane dynamics and intracellular signaling.16-18
We show here that Cav-1 deficiency in donor T cells reduced GVHD in mice undergoing allo-HCT predominantly through differentiation of Cav-1−/− donor cells into regulatory T cells (Tregs), which are known to dramatically decrease GVHD.19,20 Microarray gene expression analysis showed that Foxp3 gene expression was upregulated upon exposure of Cav-1–deficient T cells to alloantigen in vitro compared with wild-type (WT) T cells. Detailed analysis of the molecular mechanism underlying this phenomenon revealed that in the absence of Cav-1, Lck failed to be in close proximity to the cytoplasmic tails of the TCR upon TCR triggering, leading to reduced TCR phosphorylation and reduced activation of downstream signaling cascades, such as mitogen-activated protein kinase. These findings link sub-optimal TCR activation in the absence of Cav-1 to the development of a regulatory phenotype and may open novel avenues to promote a Treg phenotype for therapeutic interventions against acute GVHD and other T-cell–mediated diseases.
Materials and methods
Human subjects
We collected all samples after approval by the Ethics Committee of the Albert-Ludwigs University, Freiburg, Germany (Protocol number: 274/14) and after written informed consent. Intestinal tissue biopsies were collected in a prospective manner from individuals undergoing allo-HCT (see supplemental Tables 1 and 2, available on the Blood Web site). GVHD grading was performed on the basis of histopathology according to a published staging system.21,22
Mice
C57BL/6 (H-2b, Thy-1.2) and BALB/c (H-2d, Thy-1.2) mice were purchased from the local stock of the animal facility at Freiburg University Medical Center. Cav1−/− C57BL/6 mice had been previously described.23 All animal protocols (G-12/34, G-13/114) were approved by the University Committee on the Use and Care of Laboratory Animals at Albert-Ludwigs University.
Human CD4 T-cell and Treg isolation, and culture
Human naïve CD4+ T cells and Tregs were purified from nonmobilized peripheral blood (PB) apheresis products (Memorial Blood Center, St. Paul, MN) in a two-step procedure, in which CD25+ cells were initially enriched from peripheral blood mononuclear cells by AutoMACS with GMPgrade anti-CD25 microbeads (75 mL / 2 × 108 cells). CD25high (containing Tregs) and CD25low (containing naïve CD4 T cells) fractions were stained with CD4/CD45RA/CD25/CD127 and sorted via FACSAria as naïve Treg (CD4+25++45RA+127−) or naïve CD4 T cells (CD4+25−45RA+127+). Purified CD4+ T cells and Tregs were stimulated and expanded for 14 days as reported previously.24 To assess Cav-1 expression, cells were re-stimulated with anti-CD3/28 beads for 3 days and stained anti–Cav-1 (D46G3; Cell Signaling), directly conjugated to phycoerythrin (# S10467; Life Technologies).
Statistical analysis
Normally distributed data were compared using a two-sided unpaired Student t test. If the data did not meet the criteria of normality, the Mann–Whitney U test was performed. Data are presented as mean ± standard error of the mean (SEM). Power analysis was performed to assess the sample size in the mouse GVHD survival experiments. A sample size of at least n = 10 per group was determined capable of detecting, with 80% power, an effect size of at least 1.06 with P < .05. Differences in animal survival (Kaplan–Meier survival curves) were analyzed by a log-rank test. P < .05 was considered significant.
All other methods are described in supplemental Methods.
Results
Cav-1 expression is upregulated upon homeostatic signals, allorecognition, and tissue damage in T cells
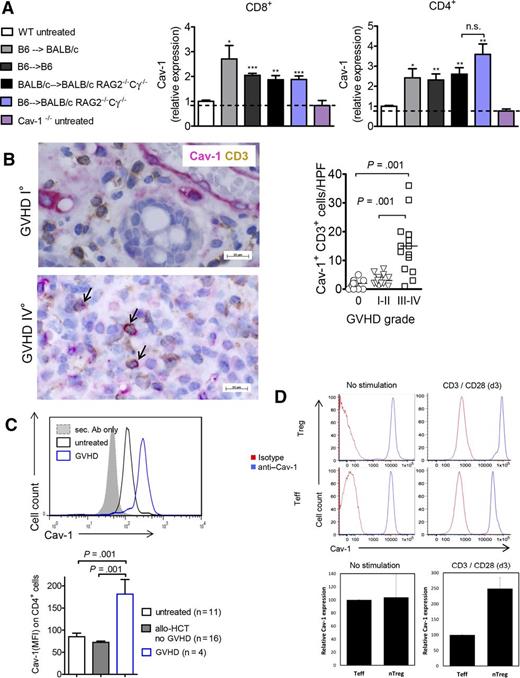
As a first step, we aimed to delineate the signals that could contribute to the expression of Cav-1 in donor T cells including recognition of allogeneic host cells, lymphopenia-driven proliferation, or pro-inflammatory cytokines provoked by irradiation-induced tissue damage. CD4+ and CD8+ T cells from untreated mice expressed low to absent levels of Cav-1 that were comparable to those of Cav-1−/− T cells (Figure 1A). Transfer of T cells in a MHC class I and II mismatch GVHD model resulted in upregulation of Cav-1 expression on the donor-derived (H-2b+) T cells (Figure 1A). A similar increase was also seen when a syngeneic HCT was performed (C57BL/6 into C57BL/6), indicating that Cav-1 upregulation was at least partly related to total body irradiation-induced tissue damage (Figure 1A). To avoid total body irradiation-induced tissue damage, we transplanted WT BALB/c bone marrow (BM) and purified T cells into non-irradiated BALB/c Rag2−/−Cγ−/− mice. The homeostatic lymphopenia-related stimuli in non-irradiated Rag2−/−Cγ−/− mice were sufficient to cause increased Cav-1 expression in the T cells (Figure 1A). The expression of Cav-1 did not significantly increase further when both homeostatic stimuli and alloantigen were present (Figure 1A, blue bars).
Stimuli leading to Cav-1 expression in murine and human T cells. (A) Expression of Cav-1 in CD4+ or CD8+ T cells derived from mouse spleens under the indicated conditions as measured by flow cytometry (pooled from 2 experiments, n = 8). The mean fluorescence intensity (MFI) measured for Cav-1 on CD4+ or CD8+ T cells derived from untreated WT mice was set as “1,” and all values were set in relation to this group. The dotted line indicates the background MFI for Cav-1 that is detected in Cav-1−/− CD4+ or CD8+ T cells. WT BM and purified T cells were transplanted as follows: C57BL/6 cells into irradiated BALB/c mice (allogeneic), C57BL/6 cells into irradiated C57BL/6 mice (syngeneic), C57BL/6 cells into non-irradiated BALB/c Rag2−/−Cγ−/− mice (allogeneic and lymphopenia), and BALB/c cells into non-irradiated BALB/c Rag2−/−Cγ−/− mice (syngeneic and lymphopenia). Untreated WT or Cav-1−/− mice were used as controls. The analyses of the T cells were performed on day 7 after HCT. *P < .05; **P < .01; ***P < .001. (B) The number of CD3+ Cav-1+ T cells per HPF is shown within human intestinal biopsies detected by immunohistochemistry. The arrows indicate Cav-1/CD3 double positive cells. Samples from 18 patients were analyzed and different time points after allo-HCT (supplemental Table 1). (C) Expression of Cav-1 in CD4+ T cells derived from the PB of healthy controls and patients who underwent allo-HCT without or with acute GVHD. Representative histogram (top) and bar diagram of multiple patients (bottom). The human blood samples were analyzed at multiple time points after allo-HCT (supplemental Table 2). (D) Expression of Cav-1 in in vitro expanded naïve CD4+ T cells and naïve Tregs derived from human PB re-stimulated with anti-CD3/28 beads for 3 days. Representative histogram for effector T cells (Teff) and Treg cells (top) and quantification for both Treg and conventional CD4+ T cells (n = 3 each) (bottom). The blue lines are the anti–Cav-1 stained cells and the red lines are the isotype-stained cells. The Cav-1 MFI values were normalized in the Teff group (100%). HPF, high power field, n.s., not significant. Ab, antibody; nTreg, naturally occurring Treg.
Stimuli leading to Cav-1 expression in murine and human T cells. (A) Expression of Cav-1 in CD4+ or CD8+ T cells derived from mouse spleens under the indicated conditions as measured by flow cytometry (pooled from 2 experiments, n = 8). The mean fluorescence intensity (MFI) measured for Cav-1 on CD4+ or CD8+ T cells derived from untreated WT mice was set as “1,” and all values were set in relation to this group. The dotted line indicates the background MFI for Cav-1 that is detected in Cav-1−/− CD4+ or CD8+ T cells. WT BM and purified T cells were transplanted as follows: C57BL/6 cells into irradiated BALB/c mice (allogeneic), C57BL/6 cells into irradiated C57BL/6 mice (syngeneic), C57BL/6 cells into non-irradiated BALB/c Rag2−/−Cγ−/− mice (allogeneic and lymphopenia), and BALB/c cells into non-irradiated BALB/c Rag2−/−Cγ−/− mice (syngeneic and lymphopenia). Untreated WT or Cav-1−/− mice were used as controls. The analyses of the T cells were performed on day 7 after HCT. *P < .05; **P < .01; ***P < .001. (B) The number of CD3+ Cav-1+ T cells per HPF is shown within human intestinal biopsies detected by immunohistochemistry. The arrows indicate Cav-1/CD3 double positive cells. Samples from 18 patients were analyzed and different time points after allo-HCT (supplemental Table 1). (C) Expression of Cav-1 in CD4+ T cells derived from the PB of healthy controls and patients who underwent allo-HCT without or with acute GVHD. Representative histogram (top) and bar diagram of multiple patients (bottom). The human blood samples were analyzed at multiple time points after allo-HCT (supplemental Table 2). (D) Expression of Cav-1 in in vitro expanded naïve CD4+ T cells and naïve Tregs derived from human PB re-stimulated with anti-CD3/28 beads for 3 days. Representative histogram for effector T cells (Teff) and Treg cells (top) and quantification for both Treg and conventional CD4+ T cells (n = 3 each) (bottom). The blue lines are the anti–Cav-1 stained cells and the red lines are the isotype-stained cells. The Cav-1 MFI values were normalized in the Teff group (100%). HPF, high power field, n.s., not significant. Ab, antibody; nTreg, naturally occurring Treg.
Next, we investigated whether Cav-1 expression in human T cells was also upregulated in patients suffering from GVHD. To this end, double-staining for CD3 and Cav-1 was performed on human intestinal biopsies. A significant increase in double-positive cells for all CD3+ T cells was found in patients (supplemental Table 1) suffering from GVHD at grades 3 to 4 compared with GVHD grades 0 to 2 (Figure 1B). We also found CD3− cells expressed high levels of Cav-1, which most probably are nonhematopoietic cells, eg, endothelial cells. Additionally Cav-1 expression on human CD4+ T cells was higher in patients who had developed GVHD compared with patients who had undergone allo-HCT but did not develop GVHD (Figure 1C; supplemental Table 2). PB-derived human Tregs stimulated with CD3/CD28 beads displayed high levels of Cav-1 expression (Figure 1D). Altogether, these findings support the concept that multiple activation signals accompanying GVHD, including tissue damage, homeostatic expansion, and encounters with foreign MHC, lead to upregulation of Cav-1 expression in donor T cells.
Lack of Cav-1 expression of the donor T cells decreases GVHD
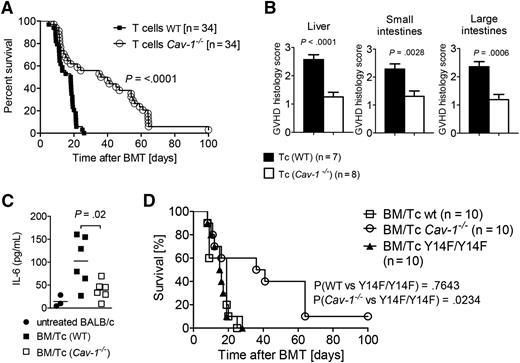
To analyze whether Cav-1 has a functional role in T cells in vivo, such as in the activation of donor T cells during the priming phase of GVHD, we transferred WT or Cav-1−/− T cells (C57BL/6) into irradiated BALB/c recipients in a classical MHC class I and II mismatch GVHD model. Survival as an indicator of GVHD severity was significantly improved in mice that received Cav-1−/− T cells compared with WT T cells (Figure 2A). Consistent with the increase in the survival rate, the histopathological GVHD severity scores were lower in mice receiving Cav-1−/− T cells (Figure 2B). Interleukin (IL)-6 plays a central role in GVHD and has recently been shown to be a targetable factor.25 We observed significantly reduced IL-6 levels in the serum of mice that received Cav-1−/− T cells compared with WT T cells (Figure 2C). These data indicate that lack of Cav-1 in donor T cells significantly reduces GVHD severity, pointing to a functional role for Cav-1 in T-cell activation upon allo-HCT.
Mice receiving Cav-1−/− T cells show prolonged survival. (A) Recipients were transplanted with 5 × 106 BM cells and 3 × 105 T cells (Cav-1+/+ or Cav-1−/−) each. Survival was monitored for 100 days. The experiment was repeated 4 times (n = 34 per group). (B) On day 7 after allo-HCT, GVHD target organs such as the liver (left), small intestine (middle), and large intestine (right) were isolated. A histopathological scoring was performed by an experienced pathologist who was single-blinded (pooled from 2 experiments, n = 7 or 8 as indicated). (C) On days 7 to 10 after allo-HCT, recipient mice were analyzed for IL-6 serum levels (pooled from 2 experiments, n = 3 or 6 as indicated, and each data point represents a mouse). (D) BALB/c WT recipients treated as in (A) were subsequently transplanted with 5 × 106 BM cells and 3 × 105 T cells from either Y14F/Y14F transgenic, C57BL/6 WT, or Cav-1−/− mice. Survival was monitored for 100 days. (Y14F/Y14F vs WT, P = .7643; Y14F/Y14F vs Cav-1−/−, P = .0234; pooled from 2 experiments, n = 10). Tc, T cells.
Mice receiving Cav-1−/− T cells show prolonged survival. (A) Recipients were transplanted with 5 × 106 BM cells and 3 × 105 T cells (Cav-1+/+ or Cav-1−/−) each. Survival was monitored for 100 days. The experiment was repeated 4 times (n = 34 per group). (B) On day 7 after allo-HCT, GVHD target organs such as the liver (left), small intestine (middle), and large intestine (right) were isolated. A histopathological scoring was performed by an experienced pathologist who was single-blinded (pooled from 2 experiments, n = 7 or 8 as indicated). (C) On days 7 to 10 after allo-HCT, recipient mice were analyzed for IL-6 serum levels (pooled from 2 experiments, n = 3 or 6 as indicated, and each data point represents a mouse). (D) BALB/c WT recipients treated as in (A) were subsequently transplanted with 5 × 106 BM cells and 3 × 105 T cells from either Y14F/Y14F transgenic, C57BL/6 WT, or Cav-1−/− mice. Survival was monitored for 100 days. (Y14F/Y14F vs WT, P = .7643; Y14F/Y14F vs Cav-1−/−, P = .0234; pooled from 2 experiments, n = 10). Tc, T cells.
The structural role of Cav-1 but not its signaling ability is causative for the protection from GVHD
Besides its structural role, Cav-1 has the ability to transduce signals through interactions with its phosphorylated tyrosine 14,14,15 as for example in toll-like receptor 4/MyD88-dependent signaling.26 Therefore, we aimed to clarify which of these functions are causative for the protection from GVHD induced by Cav-1−/− T cells. To this end, we generated a mouse model carrying a Cav-1 point-mutation variant, which cannot be phosphorylated (Y14F/Y14F). When T cells purified from Cav-1Y14F/Y14F mice were transferred in our MHC class I and II mismatched model, they caused GVHD that was just as severe as that caused by the WT T cells. Once again, a complete lack of Cav-1 in T cells was protective (Figure 2D). These data clearly indicate that Cav-1 phosphorylation is not involved in the improved survival of mice transferred with Cav-1−/− T cells.
Foxp3 is upregulated in Cav-1−/− T cells upon contact with alloantigen
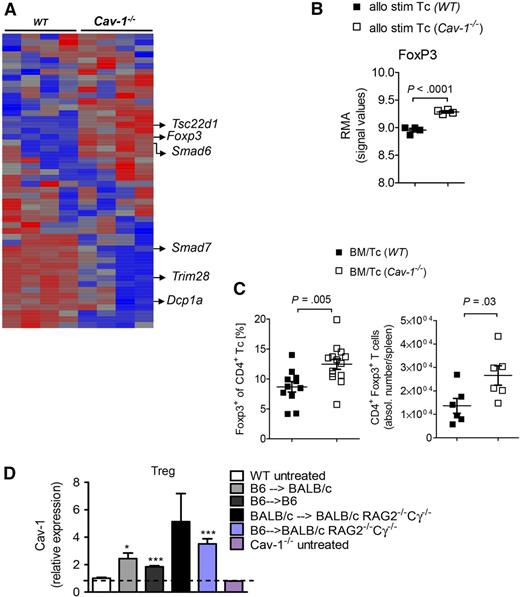
To understand why Cav-1–deficient T cells caused less severe GVHD, we next decided to examine differences in the gene expression profiles of Cav-1−/− and WT T cells in the context of allogeneic activation. Therefore, purified T cells were exposed to irradiated (40 Gy) allogeneic dendritic cells (DCs) (BALB/c) for 48 hours, DCs were depleted (<5%), and RNA was isolated from the remaining CD4+ T cells. Differentially regulated genes were identified using a microarray-based analysis. Several genes related to the transforming growth factor (TGF)-β signaling pathway in T cells, such as FoxP3, Smad6, Smad7, Dcp1a, and Tsc22d1 were differentially regulated in Cav-1−/− T cells when compared with WT T cells (Figure 3A-B). Foxp3 downregulates Smad7 expression, an inhibitor of the TGF-β signaling cascade in T cells, thereby creating a positive feedback loop that potentially stabilizes Tregs.27 Indeed, Smad7 expression was significantly reduced in Cav-1−/− T cells when compared with control T cells (Figure 3A). The absence of the transcription repressor Trim28 in T cells promotes the generation of Tregs.28,29 Trim28 expression was significantly downregulated in Cav-1−/− T cells compared with WT T cells (Figure 3A). Considered together, these results point to differential regulation of the Treg-related transcriptome in Cav-1−/− T cells upon allogeneic activation. To understand if this difference has relevance for the T-cell phenotype observed in vivo, we next analyzed the abundance of CD4+FoxP3+ cells in mice undergoing allo-HCT. We found increased relative and absolute numbers of Tregs in the spleens of mice receiving Cav-1−/− T cells compared with those receiving WT T cells (Figure 3C). These data indicate a shift toward a Treg phenotype of Cav-1−/− T cells compared with WT T cells upon exposure to alloantigen. Cav-1 upregulation in Tregs was seen in response to tissue damage, homeostatic expansion, and foreign MHC (Figure 3D). Interestingly, expansion of Tregs in immunodeficient mice was sufficient for Cav-1 upregulation on Tregs when analyzed on day 7.
Significant increase in transcription frequency and intracellular protein expression of FoxP3 after allo-BMT using Cav-1−/− T cells. (A) BALB/c BM-derived DCs were irradiated with 40 Gy and cocultured with isolated WT or Cav-1−/− T cells (n = 4 per group). After 2 days of coculture, cells were harvested and applied to CD11c depletion. The CD11c− T-cell fraction was then further analyzed for differentially transcribed genes by microarray analysis. The RMA was calculated (mean ± SEM). (B) The RMA values for FoxP3 are selectively displayed (n = 4 per group). (C) BALB/c recipient mice were irradiated with a total of 9 Gy and subsequently transplanted with 5 × 106 BM plus 3 × 105 WT or Cav-1−/− T cells. Recipients were euthanized 7 days post–allo-BMT and analyzed by flow cytometry, using specific staining against CD4 and FoxP3. Relative Treg proportions of all CD4+ T cells in the spleen are shown (mean ± SEM; Cav-1+/+, n = 12 and Cav-1−/−, n = 14) (left). Absolute Treg numbers in the spleen are displayed (mean ± SEM; Cav-1+/+, n = 6 and Cav-1−/−, n = 6) (right). (D) Cav-1 expression in Treg cells was analyzed at day 7 after BMT. Teffs were obtained from sort-purified naive CD4+CD25−CD127+CD45RA+ T cells that have been stimulated with anti–CD3-loaded KT64/86 and expanded 7 to 8 days in vitro. The MFI was normalized to the value obtained for Treg cells derived from untreated WT mice. The dotted line indicates the background MFI for Cav-1 that is detected in Cav-1−/− Treg cells. RMA, robust multi-array average; stim, stimulated. *P < .05; ***P < .001.
Significant increase in transcription frequency and intracellular protein expression of FoxP3 after allo-BMT using Cav-1−/− T cells. (A) BALB/c BM-derived DCs were irradiated with 40 Gy and cocultured with isolated WT or Cav-1−/− T cells (n = 4 per group). After 2 days of coculture, cells were harvested and applied to CD11c depletion. The CD11c− T-cell fraction was then further analyzed for differentially transcribed genes by microarray analysis. The RMA was calculated (mean ± SEM). (B) The RMA values for FoxP3 are selectively displayed (n = 4 per group). (C) BALB/c recipient mice were irradiated with a total of 9 Gy and subsequently transplanted with 5 × 106 BM plus 3 × 105 WT or Cav-1−/− T cells. Recipients were euthanized 7 days post–allo-BMT and analyzed by flow cytometry, using specific staining against CD4 and FoxP3. Relative Treg proportions of all CD4+ T cells in the spleen are shown (mean ± SEM; Cav-1+/+, n = 12 and Cav-1−/−, n = 14) (left). Absolute Treg numbers in the spleen are displayed (mean ± SEM; Cav-1+/+, n = 6 and Cav-1−/−, n = 6) (right). (D) Cav-1 expression in Treg cells was analyzed at day 7 after BMT. Teffs were obtained from sort-purified naive CD4+CD25−CD127+CD45RA+ T cells that have been stimulated with anti–CD3-loaded KT64/86 and expanded 7 to 8 days in vitro. The MFI was normalized to the value obtained for Treg cells derived from untreated WT mice. The dotted line indicates the background MFI for Cav-1 that is detected in Cav-1−/− Treg cells. RMA, robust multi-array average; stim, stimulated. *P < .05; ***P < .001.
Improved survival of mice receiving Cav-1−/− T cells is caused by Tregs in the graft
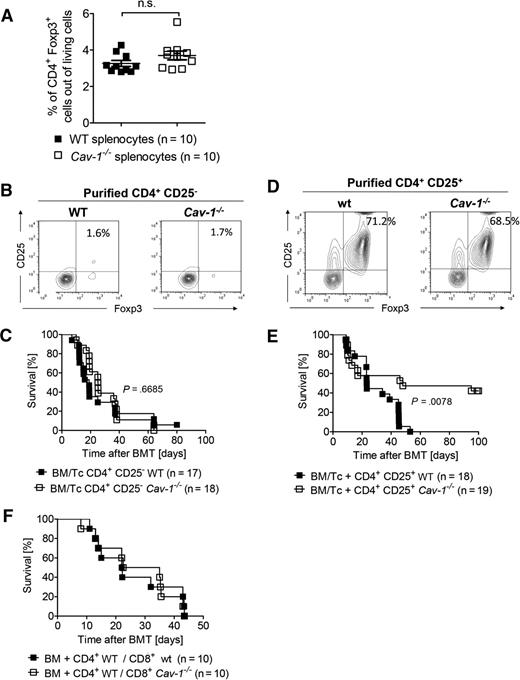
To investigate if alloantigen exposure was driving the increased Treg frequencies, which were found when Cav-1−/− T cells were transplanted (Figure 3C), or in contrast, if they were already increased in the graft a priori, we next determined the Treg frequencies in the spleens of Cav-1−/− and WT mice under steady state conditions. Importantly, comparable Treg numbers were found in Cav-1−/− and in WT mice (Figure 4A). To clarify if conventional T cells lacking Cav-1 expression had an intrinsic defect causing less severe GVHD, we performed allo-HCT experiments with infusion of T cells that were depleted of Tregs. Magnetic-activated cell sorting-based depletion achieved a frequency of CD4+CD25+FoxP3+ cells below 2% (Figure 4B). Transfer of these Treg-depleted T-cell grafts resulted in comparable survival of the recipients infused with Cav-1−/− CD4+ T cells to those receiving WT CD4+ T cells (Figure 4C). These results indicate that (1) WT and Cav-1−/− Treg-depleted T-cell grafts are equally potent at inducing GVHD, and (2) an initial pool of Cav-1−/− Tregs is needed in order to improve survival upon allo-HCT. To formally demonstrate the latter conclusion, we next adoptively transferred Treg cells purified from Cav-1−/− or control mice. After 2 days, WT conventional T cells were transferred to induce GVHD. The purity of Cav-1−/− and WT Treg preparations were comparable (Figure 4D). Protection from GVHD was markedly improved in mice receiving Cav-1−/− Tregs compared with mice receiving WT Tregs (Figure 4E). Moreover, when only the transferred CD8+ T cells were Cav-1 deficient, no protection from GVHD was observed (Figure 4F). These findings indicate that the observed improvement in survival during GVHD upon transferring Cav-1−/− T cells compared with WT T cells was due to the presence of Tregs in the graft. Importantly, these data suggest that either Cav-1−/− Tregs are more potent at reducing GVHD compared with WT Treg cells or that the in vivo generation of Tregs during allogeneic stimulation is favored in the absence of Cav-1 expression, since improved survival becomes evident after 20 days posttransplantation.
The presence of Tregs in the graft is crucial for the improved survival of mice receiving Cav-1−/− T cells. (A) Splenocytes isolated from untreated WT and Cav-1−/− mice were stained for CD4 and Foxp3, and analyzed by flow cytometry (mean ± SEM; WT, n = 10 and CAV-1−/−, n = 10; pooled from 2 experiments). (B) CD4+CD25− were isolated from C57BL/6 WT or Cav-1−/− donor splenocytes. The purity of CD4+CD25− fraction is shown (1 of 4 comparable results). (C) Irradiated BALB/c recipient mice received 5 × 106 BM cells isolated from C57BL/6 WT mice and 2 days later received 3 × 105 CD4+CD25− conventional T cells from either WT or Cav-1−/− mice. Survival was monitored for 100 days. Data were pooled from 2 independent experiments (WT control group, n = 17 and Cav-1−/− group, n = 18). (D) Donor cells were purified as in (B) and the enrichment of the CD4+CD25+ fraction is shown (1 of 4 comparable results). (E) Irradiated BALB/c recipient mice were transplanted with 5 × 106 BM cells isolated from C57BL/6 WT mice plus 3 × 105 CD4+ CD25+ Tregs from either WT C57BL/6 or Cav-1−/− mice. Two days later, recipients received 3 × 105 CD4+ and CD8+ T cells from C57BL/6 mice. Survival was monitored for 100 days. Data were pooled from 2 independent experiments (P = .0078; WT control group, n = 18 and Cav-1−/− group, n = 19). (F) Irradiated BALB/c recipient mice were transplanted with 5 × 106 BM cells isolated from C57BL/6 WT mice. In addition, 2 × 105 CD4+ WT cells and 1 × 105 CD8+ T cells from either WT or Cav-1−/− mice (C57BL/6) were also injected. The experiment was performed once (n = 10 per group). n.s., not significant.
The presence of Tregs in the graft is crucial for the improved survival of mice receiving Cav-1−/− T cells. (A) Splenocytes isolated from untreated WT and Cav-1−/− mice were stained for CD4 and Foxp3, and analyzed by flow cytometry (mean ± SEM; WT, n = 10 and CAV-1−/−, n = 10; pooled from 2 experiments). (B) CD4+CD25− were isolated from C57BL/6 WT or Cav-1−/− donor splenocytes. The purity of CD4+CD25− fraction is shown (1 of 4 comparable results). (C) Irradiated BALB/c recipient mice received 5 × 106 BM cells isolated from C57BL/6 WT mice and 2 days later received 3 × 105 CD4+CD25− conventional T cells from either WT or Cav-1−/− mice. Survival was monitored for 100 days. Data were pooled from 2 independent experiments (WT control group, n = 17 and Cav-1−/− group, n = 18). (D) Donor cells were purified as in (B) and the enrichment of the CD4+CD25+ fraction is shown (1 of 4 comparable results). (E) Irradiated BALB/c recipient mice were transplanted with 5 × 106 BM cells isolated from C57BL/6 WT mice plus 3 × 105 CD4+ CD25+ Tregs from either WT C57BL/6 or Cav-1−/− mice. Two days later, recipients received 3 × 105 CD4+ and CD8+ T cells from C57BL/6 mice. Survival was monitored for 100 days. Data were pooled from 2 independent experiments (P = .0078; WT control group, n = 18 and Cav-1−/− group, n = 19). (F) Irradiated BALB/c recipient mice were transplanted with 5 × 106 BM cells isolated from C57BL/6 WT mice. In addition, 2 × 105 CD4+ WT cells and 1 × 105 CD8+ T cells from either WT or Cav-1−/− mice (C57BL/6) were also injected. The experiment was performed once (n = 10 per group). n.s., not significant.
Cav-1 regulates both TGF-β and TCR signals resulting in increased generation of Tregs
In a further step, we investigated whether Cav-1−/− Tregs exhibit enhanced suppressive activity compared with WT Tregs. To this end, purified Tregs from Cav-1−/− or control mice were cocultured for 3 days with carboxyfluorescein diacetate succinimidyl ester-labeled conventional WT T cells. As shown in Figure 5A, the regulatory capacity of Tregs with respect to the suppression of conventional T-cell expansion in vitro was independent of Cav-1 expression.
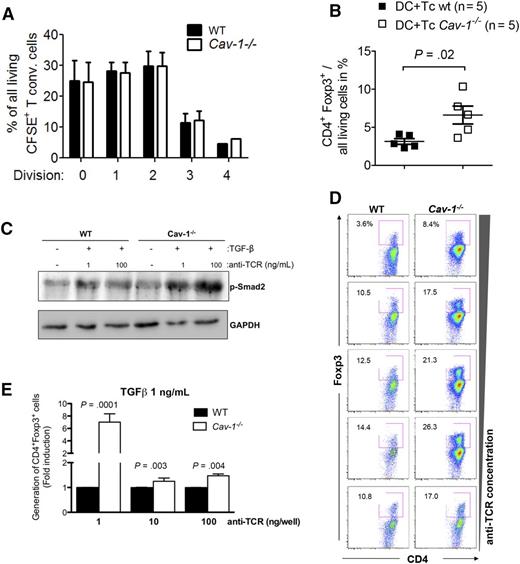
Increased potential of Cav-1−/− T cells to develop into Tregs. (A) 1 × 105 Treg (CD4+CD25+) were cocultured with 1 × 105 carboxyfluorescein diacetate succinimidyl ester-labeled conventional T cells (CD4+CD25−) stimulated with CD3/CD28 beads for 48 hours. T-cell proliferation was assayed by flow cytometry (mean ± SEM, pooled from 5 experiments). (B) BMDCs from BALB/c mice were cocultured with C57BL/6 WT or Cav-1−/− T cells at a 1:2 ratio (2.5 × 106 DC + 5 × 106 T cells) and the level of Foxp3 expression was analyzed by flow cytometry. Data are pooled from 5 independent repetitions (mean ± SEM, n = 5). (C) Purified CD4+ T cells were stimulated with 1 μg/mL (low) or 10 μg/mL (high) anti-TCR (145-2C11) antibody for 5 minutes in the presence of 1 μg/mL TGF-β for 30 minutes. After cell lysis, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and examined by WB for anti–phospho-Smad2 (p-Smad2) and anti-GAPDH (1 of 3 comparable experiments is shown). (D) Purified CD4+CD25− T cells were incubated for 72 hours in the presence of increasing amounts of plate-bound anti-CD3 antibodies under Treg-generating conditions (IL-2 and blocking antibodies against IL-4 and interferon-γ). Cells were harvested and stained with cell viability dye and antibodies against CD4, CD25, and Foxp3 (1 of 3 comparable experiments is shown). (E) Cells were treated as in (D) with the addition of exogenous TGF-β (1 ng/mL). The pooled results of at least 3 independently performed experiments are shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. BMDC, bone marrow derived dendritic cells; CSFE, carboxyfluorescein diacetate succinimidyl ester; WB, western blot.
Increased potential of Cav-1−/− T cells to develop into Tregs. (A) 1 × 105 Treg (CD4+CD25+) were cocultured with 1 × 105 carboxyfluorescein diacetate succinimidyl ester-labeled conventional T cells (CD4+CD25−) stimulated with CD3/CD28 beads for 48 hours. T-cell proliferation was assayed by flow cytometry (mean ± SEM, pooled from 5 experiments). (B) BMDCs from BALB/c mice were cocultured with C57BL/6 WT or Cav-1−/− T cells at a 1:2 ratio (2.5 × 106 DC + 5 × 106 T cells) and the level of Foxp3 expression was analyzed by flow cytometry. Data are pooled from 5 independent repetitions (mean ± SEM, n = 5). (C) Purified CD4+ T cells were stimulated with 1 μg/mL (low) or 10 μg/mL (high) anti-TCR (145-2C11) antibody for 5 minutes in the presence of 1 μg/mL TGF-β for 30 minutes. After cell lysis, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and examined by WB for anti–phospho-Smad2 (p-Smad2) and anti-GAPDH (1 of 3 comparable experiments is shown). (D) Purified CD4+CD25− T cells were incubated for 72 hours in the presence of increasing amounts of plate-bound anti-CD3 antibodies under Treg-generating conditions (IL-2 and blocking antibodies against IL-4 and interferon-γ). Cells were harvested and stained with cell viability dye and antibodies against CD4, CD25, and Foxp3 (1 of 3 comparable experiments is shown). (E) Cells were treated as in (D) with the addition of exogenous TGF-β (1 ng/mL). The pooled results of at least 3 independently performed experiments are shown. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. BMDC, bone marrow derived dendritic cells; CSFE, carboxyfluorescein diacetate succinimidyl ester; WB, western blot.
Next, we sought to investigate if Tregs were preferentially generated from Cav-1−/− or WT CD4+ T cells. Exposure of purified T cells (C57BL/6) to allogeneic DCs (BALB/c) led to increased CD4+Foxp3+ Treg frequencies in Cav-1−/− T cells when compared with WT T cells (Figure 5B). Viability was comparable between Cav-1−/− and WT T cells exposed to allogeneic DCs (supplemental Figure 1). Taken together, Cav-1 deficiency does not affect thymic Treg development, as Cav-1−/− mice do not have increased Treg frequencies a priori. However, Tregs lacking Cav-1 appear to be either preferentially generated from CD4+ T cells or expanded upon exposure to alloantigen.
On the one hand, Cav-1 negatively regulates TGF-β signaling in multiple cell types30,31 by molecular mechanisms that seem to be cell-type specific. Thus, we first analyzed if Tregs were differentially generated from WT and Cav-1−/− CD4+CD25− naïve T cells at different TGF-β concentrations. At all TGF-β concentrations tested, we observed increased CD4+Foxp3+ Treg frequencies in the cultures of Cav-1−/− T cells compared with WT T cells (supplemental Figure 1B). These data, together with the results of the microarray-based comparison of WT and Cav-1–deficient T cells (Figure 3A), suggest that Cav-1 also functions as a negative regulator of TGF-β signaling in T cells. In accordance, the levels of p-Smad2 upon TCR and TGF-β stimulation were increased in Cav-1−/− CD4+ T cells compared with WT cells (Figure 5C). Because TGF-β mediates growth-inhibitory effects on most target cells via activation of the canonical SMAD-signaling pathway, we next measured proliferation of Tregs upon TCR/CD28 stimulation in the presence of increasing concentrations of TGF-β. Although proliferation of WT Foxp3+Treg is only slightly inhibited by TGF-β, the proliferation of Cav-1−/−Foxp3+ T cells is clearly reduced with increasing concentrations of TGF-β (supplemental Figure 1C).
On the other hand, low TCR signaling due to low antigen density or affinity or to premature termination of the antigen-presenting cell (APC):T-cell interaction directly correlates with Treg generation, whereas high TCR signaling promotes Teff differentiation.32-34 Therefore, we investigated whether generation of Tregs was enhanced in the absence of Cav-1 under conditions of variegated TCR signaling strength. First, we tested if Treg generation depends on TCR strength in the in vitro system of TGF-β–driven Treg induction. To this end, purified CD4+CD25− cells from lymph nodes of WT mice were stimulated with increasing concentrations of plate-bound anti-CD3 antibodies, and the generation of Foxp3-expressing cells was analyzed after 3 days. Our results showed that TGF-β driven differentiation into Foxp3+Treg cells was optimal at low concentrations of anti-TCR, whereas increased TCR concentrations reduced the percentage of generated Tregs (supplemental Figure 2A). To study the role of Cav-1 in Treg generation independently of TGF-β signaling, purified CD4+CD25− cells from WT and Cav-1−/− mice were compared in the absence of exogenous TGF-β. Tregs were more efficiently generated in the absence of Cav-1 expression at all anti-TCR concentrations tested (Figure 5D). The ratios (induced Tregs/conventional T cells) changed with the concentration of the stimulus within one given genotype (WT or Cav-1−/− mice). However, when the ratio of the Cav-1−/− sample was divided by the ratio of the WT sample for a given concentration of the stimulus, the resultant ratio was constantly 1.59 ± 0.06. These analyses thus indicate that independent of the concentration of the anti-TCR stimulus, 1.59 more induced Tregs were generated in the absence of Cav-1. Altogether, these data suggest that in the absence of Cav-1, TCR signaling might be reduced, therefore favoring differentiation toward a regulatory phenotype. Moreover, increasing concentrations of TGF-β enhanced the difference between WT and Cav-1−/−, indicating that TCR and TGF-β signaling pathways cooperate to promote the generation of Tregs (Figure 5E and supplemental Figure 2B). Thus, TGF-β signaling is enhanced in the absence of Cav-1, which most probably facilitates Treg generation by negative regulation of TCR-induced proliferation. Altogether, these data indicate that Cav-1 influences both TGF-β and TCR-induced signals leading to the generation of Tregs.
Cav-1 orchestrates TCR signaling in CD4+ T cells
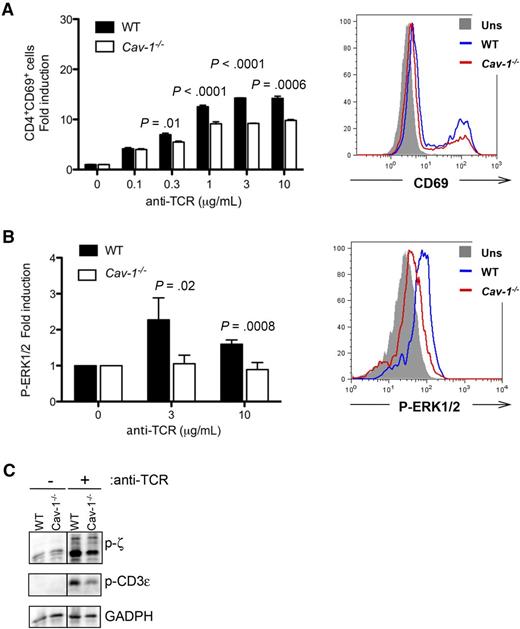
Our results suggest that TCR-signaling strength is not equally integrated in Cav-1−/− compared with WT cells. To test this, we stimulated CD4+ T cells with increasing concentrations of anti-TCR antibodies and assayed the level of the early activation marker CD69 by flow cytometry. Our data showed that in the absence of Cav-1, upregulation of CD69 is defective in CD4+ T cells (Figure 6A and supplemental Figure 3). Moreover, induction of phospho-ERK1/2 upon TCR triggering was also reduced in Cav-1−/− compared with control CD4+ T cells (Figure 6B). To further investigate the molecular mechanism by which Cav-1 controls TCR-signaling strength, naïve CD4+ T cells were purified from WT and Cav-1−/− mice and stimulated ex vivo, after which the cells were lysed and TCR phosphorylation was analyzed. As shown in Figure 6C, phosphorylation of the TCR, measured as phosphorylation of its subunits CD3ε and ζ, was reduced in Cav-1−/− compared with WT controls. These data indicate that Cav-1 might play a role in regulating the molecular interaction between Lck and the TCR upon T-cell stimulation.
Deficient TCR triggering in CD4+ cells lacking Cav-1. (A) Purified conventional CD4+ T cells were incubated for 16 hours in the presence of the indicated amounts of plate-bound anti-TCR (145-2C11) antibodies. Cells were harvested, stained with antibodies against CD4 and CD69, and analyzed by flow cytometry. Results are representative of 4 independently performed experiments. (B) Purified conventional CD4+ T cells were stimulated with the indicated amounts of anti-TCR antibodies for 5 minutes. Cells were fixed, intracellularly stained with an antibody against phospho-ERK1/2, and analyzed by flow cytometry. Two independently performed experiments are shown pooled in the left panel. (C) Purified CD4+ T cells were stimulated with 3 μg/mL anti-TCR (145-2C11) antibody for 5 minutes. After cell lysis, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and examined by WB for anti–phospho-ζ (Y-142), anti–phospho-ε, and anti-GAPDH (1 of 3 comparable experiments is shown). P-ERK, phospho-extracellular signal-regulated kinase.
Deficient TCR triggering in CD4+ cells lacking Cav-1. (A) Purified conventional CD4+ T cells were incubated for 16 hours in the presence of the indicated amounts of plate-bound anti-TCR (145-2C11) antibodies. Cells were harvested, stained with antibodies against CD4 and CD69, and analyzed by flow cytometry. Results are representative of 4 independently performed experiments. (B) Purified conventional CD4+ T cells were stimulated with the indicated amounts of anti-TCR antibodies for 5 minutes. Cells were fixed, intracellularly stained with an antibody against phospho-ERK1/2, and analyzed by flow cytometry. Two independently performed experiments are shown pooled in the left panel. (C) Purified CD4+ T cells were stimulated with 3 μg/mL anti-TCR (145-2C11) antibody for 5 minutes. After cell lysis, proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and examined by WB for anti–phospho-ζ (Y-142), anti–phospho-ε, and anti-GAPDH (1 of 3 comparable experiments is shown). P-ERK, phospho-extracellular signal-regulated kinase.
Cav-1 is required for optimal clustering of Lck and the TCR in response to activation
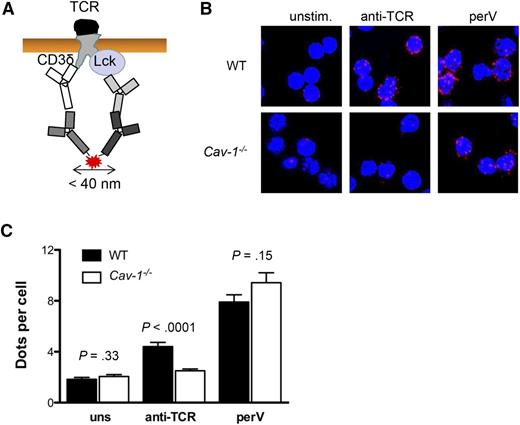
Cav-1 promotes the formation and stability of caveolar-lipid rafts within the plasma membrane. In addition, Lck localization in these domains is crucial for its function, coupling the TCR to downstream signaling. Therefore, we looked at whether Cav-1 regulates the Lck and TCR encounter upon TCR triggering in CD4+ T cells and in turn TCR phosphorylation. To this end, we established a proximity ligation assay (PLA) between the cytoplasmic tail of CD3δ and Lck (supplemental Figure 4). This assay allowed us to investigate if the cytoplasmic tails of the TCR are in close proximity (<40 nm) to Lck upon TCR activation (Figure 7A). In the resting state, the TCR and Lck are to some extent close to each other but no differences were observed between Cav-1−/− and WT CD4+ cells. Upon TCR triggering with specific antibodies, the TCR and Lck clustered in WT cells (Figure 7B). However, Cav-1−/− CD4+ cells failed to relocalize the TCR and Lck into close proximity (Figure 7B-C). As a positive control, cells were stimulated with pervanadate, which inhibits phosphatases and leads to massive TCR phosphorylation and the recruitment of Lck via its SH2 domain. Under these conditions, no significant differences were observed between Cav-1−/− and WT CD4+ T cells (Figure 7B-C). Altogether, these data demonstrate that Cav-1 promotes the localization of the activated TCR and Lck within close proximity, facilitating TCR phosphorylation and downstream signaling.
Cav-1 is required for optimal clustering of Lck and the TCR upon activation. (A) Scheme of the PLA between Lck and the TCR. Proximity between Lck and CD3δ results in red fluorescent signals. (B) Purified conventional CD4+ T cells were either unstimulated, stimulated with 10 μg/mL anti-TCR antibody (145-2C11), or 1 mM pervanadate at 37°C for 5 minutes. PLA between the TCR (CD3δ) and Lck was performed. Representative pictures of an experiment of 3 independently performed repetitions are shown. (C) Quantification of the experiment shown in (B). Mean ± SEM was plotted (n = 3 per group). perV, pervanadate; uns, unstimulated.
Cav-1 is required for optimal clustering of Lck and the TCR upon activation. (A) Scheme of the PLA between Lck and the TCR. Proximity between Lck and CD3δ results in red fluorescent signals. (B) Purified conventional CD4+ T cells were either unstimulated, stimulated with 10 μg/mL anti-TCR antibody (145-2C11), or 1 mM pervanadate at 37°C for 5 minutes. PLA between the TCR (CD3δ) and Lck was performed. Representative pictures of an experiment of 3 independently performed repetitions are shown. (C) Quantification of the experiment shown in (B). Mean ± SEM was plotted (n = 3 per group). perV, pervanadate; uns, unstimulated.
Discussion
Multiple preclinical19,20 and early clinical studies35-37 have shown the potential of Treg cells to suppress GVHD. A better understanding of Treg biology and the requirements for conventional T cells to develop into Treg cells could help to more efficiently use Tregs as a cellular therapy option in patients. Multiple signals contribute to GVHD and the activation of donor T cells, including recognition of allogeneic host cells, lymphopenia-driven proliferation, or pro-inflammatory cytokines provoked by irradiation-induced tissue damage. Herein, we observed that Cav-1 was upregulated on Tregs equally after syngeneic and allogeneic transplantation. Conversely, CD4+ T cells isolated from recipients with GVHD, express significantly higher levels of Cav-1 compared with those without GVHD and healthy controls. This discrepancy could be due to species-related differences and/or to the time point of the analysis. Although in the mice experiments all samples were analyzed on day 7 after BM transplantation (BMT), the human samples for flow cytometry-based analysis (supplemental Table 2) were collected at later time points (34 to 159 days).
In the periphery, Tregs are generated from CD4+-naïve T cells as a result of the stimulation of their TCR in the presence of TGF-β.32-34 On the one hand, the strength of TCR signaling is related to the generation of Tregs in vivo, with low TCR signals due to low density or low affinity of the antigen or to premature termination of the APC:T-cell interaction, directly correlating with the conversion to Tregs.32-34 On the other hand, induction of Foxp3 expression and conversion to Tregs requires TGF-β.32 TGF-β also ensures that Foxp3 expression is maintained in ex vivo derived Tregs during continued TCR activation.32 In contrast, once the cells are generated in vivo, TGF-β is solely a regulator of Treg expansion, but is not required for the maintenance of tolerance or for the expression of Foxp3.38
Cav-1 is involved in regulating the strength of TCR and TGF-β signaling. Firstly, Cav-1 is required for optimal TCR-induced proliferation and cytokine production of CD8+ T cells.10 Secondly, Cav-1 is a negative regulator of TGF-β signaling via the activation of SMAD proteins in several types of cells.30,31 Based on the connection of Cav-1 and TGF-β/Tregs, we tested if the transfer of T cells deficient in Cav-1 expression is protective in a GVHD scenario. Although Tregs lacking Cav-1 expression were not more suppressive in vitro on a cell-to-cell basis, they were more protective in mice developing GVHD. Higher frequencies and total numbers of Tregs were found in mice receiving Cav-1−/− T cells than WT T cells. We were able to mimic this higher frequency of Tregs in vitro when allogeneic DCs or CD3/CD28/TGF-β were used. However, a preexisting population of Cav-1−/− Tregs is essential to see the protective effect, because the transfer of Treg-depleted grafts from Cav-1−/− mice abrogated the survival benefit seen when total Cav-1−/− T cells were transferred compared with WT T cells. These results indicate that natural Tregs are needed in the first days after allo-BMT for protection against GVHD. Consistently, in none of the in vivo experiments in which GVHD protection by Cav-1−/− donors was observed, the protective effect became evident before day 20 after allo-BMT. Altogether, these data suggest that a preexisting population of Cav-1−/− Tregs is essential to allow for their more vigorous generation and/or expansion during GVHD.
To understand the functional role of Cav-1 in GVHD, we analyzed the changes in gene expression profiles between Cav-1−/− and WT T cells in the context of allogeneic activation. Among all differentially expressed genes, a substantial fraction were genes related to the TGF-β signaling pathway, including FoxP3, Smad6, Smad7, Dcp1a, and Tsc22d1, suggesting hyperactive TGF-β signaling in Cav-1−/− T cells. TGF-β drives the differentiation into Treg cells and has anti-proliferative effects on multiple cell types. To further investigate the impact of Cav-1 in TGF-β–driven Treg generation, we analyzed the generation of Foxp3+ Tregs from primary WT and Cav-1−/− CD4+ T cells in vitro. At each of the TGF-β concentrations tested, the frequencies of CD4+Foxp3+ Tregs were increased in Cav-1−/− T cells compared with WT T cells, suggesting that Cav-1 is a negative regulator of TGF-β signaling during the activation of T cells. Consistent with the reported growth-inhibitory effects of TGF-β, we found that proliferation of Cav-1−/− Foxp3+ T cells was more strongly reduced than the proliferation of WT cells upon exposure to increasing concentrations of TGF-β. TGF-β mediates its growth-inhibitory effects via activation of the canonical SMAD signaling pathway on most target cells. Consistently, the levels of p-Smad2 measured after TCR and TGF-β stimulation were increased in Cav-1−/− CD4+ T cells compared with WT cells. Altogether, these data favor a scenario in which TGF-β signaling is enhanced in the absence of Cav-1, promoting the generation of Tregs.
Several reports have connected the strength of TCR signaling to the generation of peripheral Tregs in vivo.32-34,39 High TCR signaling on its own can promote Teff differentiation,32-34 whereas low TCR activation as a result of low density or low affinity of the antigen, or to premature termination of the APC:T-cell interaction shows a direct correlation with Treg generation.32-34 In this study, we found that Treg generation was enhanced in the absence of Cav-1 when T cells were stimulated with anti-CD3 antibodies. Importantly, phosphorylation of the TCR was also reduced in Cav-1−/− when compared with WT controls, indicating that Cav-1 plays a role in the very early steps of controlling TCR activation upon antigen-induced triggering. Cav-1 regulates the stability of plasma membrane raft microdomains,8,10-12 including the polarization of these domains toward the interface between DCs and CD8+ T cells.10
Lateral organization and compartmentalization of the plasma membrane is critical for Lck localization and its function, coupling the triggered TCR to downstream signaling. Because of this, we aimed to investigate if the absence of Cav-1 impacted the relative distribution of Lck and the TCR prior to and after stimulation. Cav-1−/− CD4+ cells failed to efficiently bring the TCR and Lck in close proximity upon TCR activation. This finding supports the idea that, in the absence of Cav-1, the nanoscale organization of the plasma membrane, most likely including the formation or stability of the raft microdomains,11,12 is altered, affecting TCR/ Lck proximity and in turn, productive TCR phosphorylation and activation of downstream signaling pathways. In the absence of Cav-1, the strength of TCR signaling is reduced, and triggered CD4+ cells preferentially convert into a Treg phenotype. Importantly, these differentially regulated events at the cellular level were connected to a protective phenotype of the transferred donor T cells in an alloantigen-driven inflammation model.
In conclusion, our data demonstrate that in CD4+ T cells, Cav-1 regulates both TCR and TGF-β signaling pathways, which cooperate to promote the generation of Tregs in vivo and which directly translate into increased protection from the massive alloantigen-driven inflammation of GVHD. On a molecular level, our findings indicate a central role for Cav-1 in regulating the lateral organization of the plasma membrane upon TCR triggering, which is required for the close proximity between the activated TCR and Lck, facilitating TCR phosphorylation and downstream signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by grants from the Else Kröner Fresenius Stiftung (2013_A04) (R.Z. and S.M.) and (DFG SFB1160 [P14 to R.Z. and P5 to S.M.]); R.Z. is funded by a Heisenberg Professorship (DFG ZE 872/3-1) and ERC Consolidator grant (681012 GvHDCure); K.S.R. is funded by an International Graduate Academy fellowship; K.S. is funded by the Federal Ministry of Education and Research grant (BMBF 01EO1303); and B.R.B. is funded by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL11879).
Authorship
Contribution: A.S. designed experiments, performed experiments, and helped to write the manuscript; F.A.H., J.M., T.N., K.S.R., A.P., S.A.W., A.-K.H., W.M., K.F., M.F., G.P., and A.-K.R. performed experiments and analyzed data; P.A. performed BMT experiments; D.P. performed microarray analyses and helped to interpret the data; M.C.G. and M.A.d.P. generated the Y14F/Y14F point-mutation mice; A.S.-G. analyzed GVHD severity and provided histology of human samples; J.D. helped to analyze experiments and assisted with the manuscript; K.I.H. performed experiments and analyzed data; B.R.B. analyzed data and helped to write the manuscript; K.S. helped to design experiments and analyzed data; and S.M. and R.Z. developed the concept of the study, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Robert Zeiser, Department of Hematology and Oncology, Freiburg University Medical Center, Albert-Ludwigs University, Hugstetter Strasse 55, 79106 Freiburg, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de; and Susana Minguet, University of Freiburg, Faculty of Biology, BIOSS Centre for Biological Signalling Studies, Freiburg, Germany; e-mail: susana.minguet@biologie.uni-freiburg.de.
References
Author notes
A.S. and F.A.H. are co-first authors.
S.M. and R.Z. are co-senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal