Abstract
The widespread use of multiplex molecular diagnostics has led to a significant increase in the detection of respiratory viruses in patients undergoing cytotoxic chemotherapy and hematopoietic cell transplantation (HCT). Respiratory viruses initially infect the upper respiratory tract and then progress to lower respiratory tract disease in a subset of patients. Lower respiratory tract disease can manifest itself as airflow obstruction or viral pneumonia, which can be fatal. Infection in HCT candidates may require delay of transplantation. The risk of progression differs between viruses and immunosuppressive regimens. Risk factors for progression and severity scores have been described, which may allow targeting treatment to high-risk patients. Ribavirin is the only antiviral treatment option for noninfluenza respiratory viruses; however, high-quality data demonstrating its efficacy and relative advantages of the aerosolized versus oral form are lacking. There are significant unmet needs, including data defining the virologic characteristics and clinical significance of human rhinoviruses, human coronaviruses, human metapneumovirus, and human bocavirus, as well as the need for new treatment and preventative options.
Clinical significance
The epidemiology of respiratory virus infections among hematopoietic cell transplantation (HCT) recipients and patients undergoing cytotoxic chemotherapy closely parallels the occurrence of infections in the community, although infections in immunocompromised hosts are notable for prolonged viral shedding, higher rates of pneumonia, late airflow obstruction, and mortality.1,2 These effects are well documented for respiratory syncytial virus (RSV), parainfluenzaviruses (PIVs), influenza viruses, and, to some extent, human metapneumovirus (HMPV), whereas less information is available on human rhinoviruses (HRVs), human coronaviruses (HCoVs), and human bocavirus (HBoV). Other viruses can manifest as pulmonary infection; however, management of these viruses is beyond the scope of this article.
One key feature of respiratory viruses in immunocompromised patients is higher rates of progression from upper respiratory tract infection (URTI) to lower respiratory tract infection (LRTI). Assessing risk factors for progression has been a focus of recent research to identify subjects who may benefit from early treatment. The best data exist for RSV, where lymphopenia, smoking history, high-dose total body irradiation (TBI), recipient age, APACHE II score, and presence of copathogens are significant risk factors for progression.3,4 Recently, scores have been proposed to increase the precision of severity predictions for RSV and influenza viruses5-7 ; however, these scores have not yet been validated. A meta-analysis of risk factors for progression of PIV identified cytopenias and high-dose steroids, among other factors.8 Limited data on risk factors for progression of HMPV, HCoV, HRV, or HBoV exist.
LRTI can manifest as viral pneumonia and/or an airflow obstruction syndrome1,9 ; risk factors for mortality and airflow obstruction have been examined for RSV, PIV, influenza, and HMPV.2,10 Mortality rates for LRTI with respiratory viruses range from 15% to 40%; mortality-associated risk factors include stem cell source, oxygen requirement, high-dose steroid use, APACHE II score, and cytopenias.11-16 Prolonged shedding, lasting for weeks or months, is a hallmark of respiratory virus infections in immunocompromised patients.17,18 Prolonged shedding may result in continued risk for virus transmission and is a rationale for continued isolation.19,20
Case 1
A 10-year-old boy with relapsed T-cell acute lymphoblastic leukemia presented for evaluation 14 days prior to a prescheduled second unrelated matched cord blood transplant. A nasal respiratory swab was sent for multiplex viral polymerase chain reaction (PCR) for routine pretransplant evaluation documented HRV with a cycle threshold (CT) of 27. The patient was asymptomatic, and chest computed tomography was normal. On day −6, the patient began conditioning with treosulfan and fludarabine; TBI (200 cGy) was completed on day −1. Graft-versus-host disease (GVHD) prophylaxis included cyclosporine (day −3) and mycophenolate mofetil (day 0). On day −1, he developed a dry cough without other symptoms; lung examination was normal. On day 0, his cough worsened and nasal congestion developed, although his lungs remained clear with normal oxygen saturations. Cells were infused, but the patient developed respiratory distress 15 minutes into infusion necessitating transfer to the intensive care unit and eventual intubation. Chest radiography showed interval development of diffuse bilateral patchy pulmonary opacities concerning for pulmonary edema. Echocardiogram demonstrated acute systolic cardiac failure. A repeat nasal swab documented HRV (CT = 17). Despite initial improvements in respiratory status, his cardiac dysfunction deteriorated and he died on day +13 posttransplant with multiorgan failure.
Case 2
A 77-year-old female, 6 years after allogeneic HCT for acute myeloid leukemia, presented to the emergency room with 3 days of fever and upper respiratory symptoms, 1 day of shortness of breath, and a new oxygen requirement (5 L/min via nasal cannula). On physical examination, she had diminished and coarse breath sounds and was slightly tachypneic. The patient had chronic GVHD treated with prednisone (<0.5 mg/kg per day) and sirolimus. Absolute lymphocyte count was 0.86 × 109/L and neutrophil count was 5.26 × 109/L. The patient had a smoking history. A nasal swab was positive for RSV with a CT of 22.7. A chest radiograph was normal, whereas chest computed tomography showed scattered foci of ground glass infiltrates and centrilobular nodules. BAL was positive for RSV with a CT of 27.2, and the patient was started on 2 g aerosolized ribavirin every 8 hours. She improved after 2 days and was back on room air on hospital day 5, at which point she was switched to oral ribavirin (600 mg 3 times a day) for an additional 7 days. She was discharged on hospital day 6 and continues to do well as an outpatient.
These 2 cases present real clinical challenges in the management of HCT candidates and recipients with respiratory viral infections. The first case is relatively common and highlights our limited understanding of risk factors for disease severity in HRV infection, the impact of pretransplant infection on posttransplant outcomes, and the risks of asymptomatic shedding. The second case raises several important questions, including the impact of RSV LRTI very late after allogeneic HCT compared with what we know about RSV infection early after transplant and the correlation between radiographic findings and outcomes. These issues are discussed further in the text.
Diagnostic considerations
Multiplex PCR testing is rapidly replacing conventional diagnostic platforms such as viral culture and direct fluorescent antibody tests. PCR is rapid, sensitive, and specific and can potentially quantify viral load, although most commercial assays provide only qualitative results. In the cases presented, CT values are inversely related to viral load. Respiratory viruses are typically detected in samples obtained from the upper respiratory tract (nasal wash or swab) from symptomatic patients. Viruses can, however, be detected in asymptomatic patients, as in case 1.21,22 Once clinical and/or radiographic evidence of LRTI is documented, we routinely perform a bronchoalveolar lavage (BAL) to reliably establish lower respiratory tract involvement and facilitate targeted treatment of the virus and potential copathogens23,24 (supplemental Table 1, available on the Blood Web site). Detection of virus in the lower respiratory tract is associated with poorer outcome.13 In case 1, the rapid clinical deterioration suggested multiple etiologies for respiratory failure but a BAL was never performed to confirm HRV LRTI, highlighting the limitations of empiric management and lack of specific treatments. In case 2, the BAL was positive for RSV with discrepant findings on chest imaging. It is not known how radiographic presentation correlates with outcome. In case 2, infection occurred late posttransplant, emphasizing the importance of primary care physicians in obtaining a full diagnostic workup and considering consultation with a transplant and/or infectious disease specialist.
Treatment
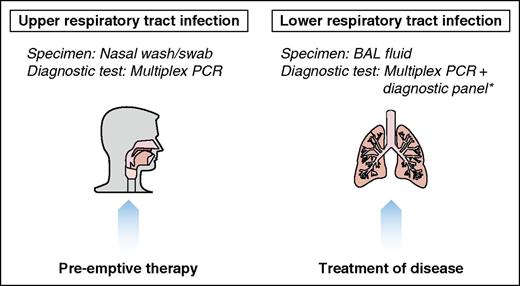
Several strategies exist for prevention and treatment of respiratory viruses in HCT recipients and hematologic malignancy patients (Figure 1). Here, we outline our center’s recommendations for treatment of confirmed respiratory viral infections at the URTI and LRTI stage.
Therapeutic strategies for respiratory viral infections posttransplant. *See supplemental Table 1.
Therapeutic strategies for respiratory viral infections posttransplant. *See supplemental Table 1.
RSV
Ribavirin is a broad-spectrum nucleoside analog with activity against many RNA and DNA viruses that is available in an aerosolized, oral, or IV form. Several retrospective studies, including a pooled analysis, have identified ribavirin in any form as protective against disease progression from URTI to LRTI and mortality in HCT recipients.4,25 In patients with leukemia, multivariate models demonstrated similar effects.26 However, conclusive proof of efficacy from randomized trials remains elusive.27 Oral and IV ribavirin showed promising results in smaller case series28,29 and in a larger study of patients receiving chemotherapy or transplant.30 A recent meta-analysis of nonrandomized studies suggested a possible effect of oral ribavirin; however, lack of randomized controls was a limitation in all studies.31 A randomized trial comparing oral and aerosolized ribavirin in HCT recipients is ongoing.32
In HCT recipients with confirmed RSV LRTI, use of aerosolized ribavirin was associated with decreased mortality, with a lower effect seen with oral or IV ribavirin.11 Efficacy of ribavirin in hematologic malignancy patients with LRTI has also been demonstrated,33,34 although larger studies are lacking. Current international guidelines recommend aerosolized or systemic (oral or IV) ribavirin with IVIG in patients with RSV URTI undergoing allogeneic HCT, allogeneic HCT recipients with risk factors for progression to LRTI, and allogeneic HCT patients with LRTI.20 Lymphopenia is a specific risk factor that has been associated with progression to LRTI in several studies.3,5,35,36,36 At our institution, ribavirin is given to HCT recipients with RSV URTI who have lymphopenia; additional risk factors, such as smoking history and use of high-dose TBI, could be used to further risk-stratify patients.3 Given significant cost increases in aerosolized ribavirin in the United States from $6105 per day in January 2013 to $29 953 per day in September 2015 (average wholesale price)37 and lack of conclusive evidence that aerosolized ribavirin is superior,11 we currently consider the use of oral ribavirin in adults (∼$25 per day depending on dosing strategy). Using an immunodeficiency scoring index developed at the MD Anderson Cancer Center, the patient in case 2 would have moderate risk of LRTI and mortality and potentially an intermediate benefit of ribavirin treatment.5 Interestingly, the patient presented late (>5 years) after transplantation, and the benefit of treatment (including ribavirin) is poorly studied in the late period. In a large MD Anderson Cancer Center analysis, the latest death occurred ∼4 years after HCT,4 but it is unclear how complete the data capture was in this very late posttransplant period. Our patient was transitioned from aerosolized to oral ribavirin as soon as respiratory status improved. Whether such moderate-risk patients or pediatric patients can be treated with oral ribavirin alone is presently poorly defined.
The additional benefit of immunoglobulin products remains controversial. One retrospective review of 280 HCT recipients with RSV URTI or LRTI showed benefit when used with ribavirin4 ; another study in LRTI did not.11 The use of RSV-specific monoclonal antibody palivizumab also appears to have variable efficacy and is very costly in adults. A small nonrandomized unadjusted analysis in pediatric cancer patients with RSV LRTI suggested a beneficial effect of adjunctive palivizumab or IVIG.38 In larger studies of HCT recipients with RSV LRTI, we were unable to demonstrate improved outcomes with adjunctive palivizumab11,12 ; we are not currently utilizing palivizumab for treatment of RSV infection in any immunocompromised patients.
Influenza
Several guidelines have outlined the importance of early influenza treatment in immunosuppressed patients.19,39 Shifting susceptibility patterns of influenza viral strains have now rendered M2 inhibitors (amantadine and rimantadine) ineffective, and neuraminidase inhibitors (NAIs) are now first line for prophylaxis and treatment of influenza. Though early therapy is associated with better outcomes,40 there may be benefits even with delayed treatment.14,41 Available NAIs available in the United States include oral oseltamivir, inhaled zanamivir, and IV peramivir. Several reports in patients with leukemia or HCT recipients demonstrate clinical efficacy with either oseltamivir or inhaled zanamivir.14,42-45 Many mutations causing oseltamivir and peramivir resistance, including the common H275Y mutation in A(H1N1)pdm09 influenza, do not lead to zanamivir resistance46 ; inhaled zanamivir has been used to treat these resistant strains.47,48 IV peramivir used during the 2009 pandemic in severely ill patients was well tolerated, with evidence of recovery in most patients.49 In a randomized trial comparing IV peramivir to oral oseltamivir, clinical outcomes were similar.50 The drug is approved as a single dose; however, this is likely inadequate in immunocompromised hosts, and the optimal duration of therapy has not been determined. Longer treatment courses (10 days) with oseltamivir or zanamivir have also been suggested given the potential for recurrence and the median time for progression from URTI to LRTI.40,51,52
Several case reports describe multidrug resistant strains in immunocompromised patients53,54 and combination therapy has been proposed. Triple-combination antiviral therapy with amantadine, ribavirin, and oseltamivir was given to patients with severe influenza A with oseltamivir or amantadine resistance.55-57 A randomized trial is comparing triple-combination antiviral therapy with oseltamivir alone in high-risk adults.58 Interestingly, a randomized trial in hospitalized patients using oseltamivir/zanamivir combination showed poorer outcomes than with monotherapy oseltamivir.59
Parainfluenza
In several retrospective studies, ribavirin had no impact on viral shedding, symptom and hospitalization length, progression to LRTI, or mortality in patients with PIV.60,61 Our own experience with aerosolized ribavirin suggested a moderate reduction in overall mortality, but not in death due to respiratory failure.13 A recent systematic review evaluated aerosolized or systemic ribavirin in 10 retrospective studies of HCT recipients and hematologic malignancy patients and found no difference in PIV-associated mortality or in progression to LRTI.8 Given lack of evidence of clinical efficacy, we currently do not use ribavirin for PIV infections. The impact of IVIG alone remains to be determined, although IVIG in PIV LRTI cases did not reduce mortality.13
Other respiratory viruses
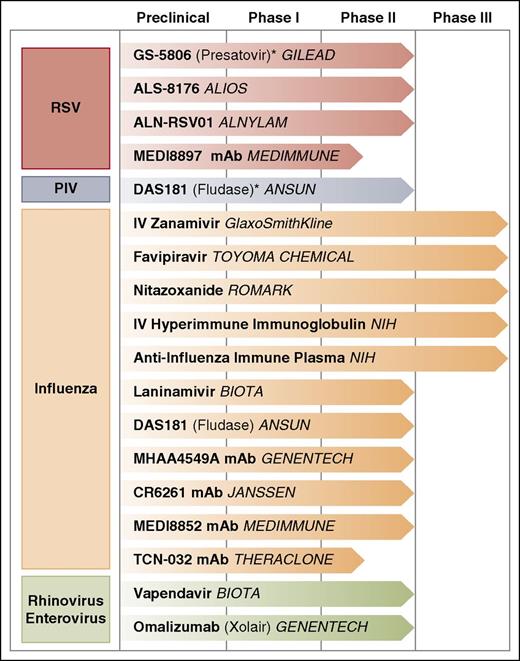
The management of other respiratory viral infections in immunosuppressed patients is generally supportive (Table 1). Ribavirin has shown efficacy against HMPV in vitro62 and in BALB/c mice,63 with anecdotal reports describing ribavirin use in severe infection in immunocompromised hosts (with and without IVIG).64-68 Lack of controlled studies of ribavirin for HMPV therapy and the known toxicities of therapy, including hemolytic anemia, limits its recommendation for use in immunocompromised hosts. The largest nonrandomized series to date did not find an effect of ribavirin for mortality, although a treatment bias cannot be excluded.16 Currently, no approved antiviral agents exist for treatment of HCov, HRV, HBoV, or enteroviruses, although some investigational agents are in the pipeline (Figure 2). The use of IVIG has not been evaluated and is not recommended. In case 1, the patient was presumed to have HRV LRTI given the clinical circumstances; however, no specific therapies directed at HRV could be offered.
Principles of prevention and treatment recommendations for HCT recipients
| Virus . | Prophylaxis . | Asymptomatic shedding . | URTI . | LRTI . |
|---|---|---|---|---|
| RSV | • Infection control procedures • Palivizumab for children ≤2 y (during season) | Isolation | • Isolation • Ribavirin for high-risk situations*,† | • Isolation • Low-risk situations: oral ribavirin† • High-risk situations: aerosolized ribavirin initially followed by oral† • Supportive care |
| Influenza virus | • Infection control procedures • Vaccination of contacts • Vaccination 2-3 weeks before transplant in recipients of nonmyeloablative conditioning‡ • Vaccination of HCT recipients as early as possible, depending on timing of flu season | • Isolation • Oseltamivir or zanamivir | • Isolation • Oseltamivir or zanamivir | • Isolation • Oseltamivir or zanamivir • Consider combination therapy with rimantadine (only Influenza A) and/or ribavirin • Consider IV peramivir if mechanically ventilated • Supportive care |
| Parainfluenza virus | Infection control procedures | Isolation | • Isolation • Consider reduction of steroid dose | • Isolation • Supportive care • Consider ribavirin if mechanically ventilated† |
| Metapneumovirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care • Consider ribavirin if mechanically ventilated† |
| Rhinovirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care |
| Coronavirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care |
| Bocavirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care |
| Virus . | Prophylaxis . | Asymptomatic shedding . | URTI . | LRTI . |
|---|---|---|---|---|
| RSV | • Infection control procedures • Palivizumab for children ≤2 y (during season) | Isolation | • Isolation • Ribavirin for high-risk situations*,† | • Isolation • Low-risk situations: oral ribavirin† • High-risk situations: aerosolized ribavirin initially followed by oral† • Supportive care |
| Influenza virus | • Infection control procedures • Vaccination of contacts • Vaccination 2-3 weeks before transplant in recipients of nonmyeloablative conditioning‡ • Vaccination of HCT recipients as early as possible, depending on timing of flu season | • Isolation • Oseltamivir or zanamivir | • Isolation • Oseltamivir or zanamivir | • Isolation • Oseltamivir or zanamivir • Consider combination therapy with rimantadine (only Influenza A) and/or ribavirin • Consider IV peramivir if mechanically ventilated • Supportive care |
| Parainfluenza virus | Infection control procedures | Isolation | • Isolation • Consider reduction of steroid dose | • Isolation • Supportive care • Consider ribavirin if mechanically ventilated† |
| Metapneumovirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care • Consider ribavirin if mechanically ventilated† |
| Rhinovirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care |
| Coronavirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care |
| Bocavirus | Infection control procedures | Isolation | Isolation | • Isolation • Supportive care |
Recommendations from the Fred Hutchinson Cancer Research Center, University of Washington, and Seattle Children’s Hospital (the Seattle Cancer Care Alliance).
Due to recent price increases, restrictions have been put in place for the use of aerosolized ribavirin.
Benefit and dosing of oral ribavirin in pediatric patients is not clearly understood.
Exceptions may be made in certain immunodeficiencies.
Ongoing clinical trials for treatment of respiratory viral infections on selected agents. *Studies ongoing in patients with hematologic malignancy or HCT recipients.
Ongoing clinical trials for treatment of respiratory viral infections on selected agents. *Studies ongoing in patients with hematologic malignancy or HCT recipients.
Respiratory viruses in transplant candidates
Identification of respiratory viral infection pretransplantation raises difficult management questions, as in case 1. International guidelines recommend the deferral of conditioning therapy in patients with respiratory infections in the setting of planned allogeneic HCT transplants,19,20 with low strength of evidence. The decision to delay transplant may be impacted by concern for underlying disease progression and donor availability. Small retrospective studies have provided limited data on the impact of transplant delay for viral infections.69-72 A large prospective study evaluated clinical outcomes associated with respiratory virus detection prior to allogeneic HCT.22 Various respiratory viruses were detected in 116 of 458 HCT patients prior to transplantation; viral detection was associated with prolonged hospitalization and lower survival at day 100. Interestingly, this risk was also present in patients with HRV alone, contrary to smaller studies suggesting pretransplant HRV detection does not impact outcomes.73 Symptomatic patients with viruses detected had an increased risk of mortality compared with those without a virus detected; this risk was not seen in asymptomatically infected patients. These data support delay of allogeneic transplantation in symptomatic patients, even those with less “pathogenic” viruses such as HRV. The number of HCoV and HBoV cases in this study was too small to determine an effect. These data should ideally be validated in larger, multicenter studies. The impact of other factors such as a viral-specific risk scores and viral load need to be evaluated. Our current practice is to perform respiratory viral diagnostics on all symptomatic HCT candidates and recommend delay of transplantation if feasible (Table 2). In pediatric patients, diagnostic PCR is done on all transplant candidates regardless of symptoms due to higher rates of virus acquisition and shedding in children. Whether asymptomatic shedding requires delay of transplantation requires further study; case 1 demonstrates a problematic situation in which asymptomatic infection was associated with cardiopulmonary death. Though death was likely due to multiple factors, the respiratory infection could have contributed.
Recommendations for respiratory viral infections before transplantation
| Virus . | Recommendation for URTI . | Recommendation for LRTI . |
|---|---|---|
| RSV | Delay transplant, if possible; if not possible to delay, consider oral ribavirin* | Delay transplant; consider ribavirin if delay is not feasible (anecdotal data)* |
| Influenza virus | Delay transplant if possible and treat† | Delay transplant and treat† |
| If not possible to delay, treat† | ||
| Parainfluenza virus | Delay transplant if possible | Delay transplant; consider ribavirin if delay is not feasible (anecdotal data)* |
| If not possible to delay, supportive care | ||
| Metapneumovirus | Delay transplant if possible | Delay transplant; no data on ribavirin |
| Rhinovirus | Delay transplant if possible; no data for autologous transplant | Delay transplant, if feasible |
| Coronavirus | No data | No data |
| Bocavirus | No data | No data |
| Virus . | Recommendation for URTI . | Recommendation for LRTI . |
|---|---|---|
| RSV | Delay transplant, if possible; if not possible to delay, consider oral ribavirin* | Delay transplant; consider ribavirin if delay is not feasible (anecdotal data)* |
| Influenza virus | Delay transplant if possible and treat† | Delay transplant and treat† |
| If not possible to delay, treat† | ||
| Parainfluenza virus | Delay transplant if possible | Delay transplant; consider ribavirin if delay is not feasible (anecdotal data)* |
| If not possible to delay, supportive care | ||
| Metapneumovirus | Delay transplant if possible | Delay transplant; no data on ribavirin |
| Rhinovirus | Delay transplant if possible; no data for autologous transplant | Delay transplant, if feasible |
| Coronavirus | No data | No data |
| Bocavirus | No data | No data |
Benefit and dosing of oral ribavirin in pediatric patients is not clearly understood.
See influenza treatment section in Table 1.
Effect of steroid therapy
We generally recommend reduction of immunosuppressive therapy as allowed by the particular clinical situation. However, the relative contribution of steroids and the dose effect may vary by virus (Table 3).3,11,13,14,16,45,74 Interestingly, an inverse relationship between steroid therapy (>1 mg/kg per day) and need for mechanical ventilation for influenza may exist; this observation has not been validated.14 Overall, low doses of steroids (<1 mg/kg per day) may not significantly impact progression to LRTI and/or mortality. The patient presented developed complications of HRV infection early posttransplant and thus was not on steroids for GVHD. If steroids had been initiated, without specific data on HRV, we would recommend lowering the dose to <1 mg/kg per day.
Role of corticosteroid treatment in progression of respiratory viral illnesses
| Virus . | Progression . | Mortality . | ||||||
|---|---|---|---|---|---|---|---|---|
| Steroid dose per day . | Risk . | HR (95% CI) . | P value . | Steroid dose per day . | Risk . | HR (95% CI) . | P value . | |
| RSV | >2 mg/kg | +/− | 1.4 (0.4-5.2) | .193 | >2 mg/kg | + + + | 3.3 (1.7-6.3) | <.00111 |
| Influenza | ≥1 mg/kg | +/− | 0.8 (0.2-2.4) | .6045 | ≥1 mg/kg | +/− | 1.1 (0.3-3.5) | .8745 |
| PIV | >2 mg/kg | + + + | 4.6 (1.2-17.0) | .0274 | >2 mg/kg | + + + | 3.2 (1.5-7.2) | .00413 |
| HMPV or RSV | Not reported in the combined cohort | Any steroids | + + + | 5.0 (1.8-14) | .00216 | |||
| ≥1 mg/kg | + + + + | 7.1 (2.3-22) | <.00116 | |||||
| Virus . | Progression . | Mortality . | ||||||
|---|---|---|---|---|---|---|---|---|
| Steroid dose per day . | Risk . | HR (95% CI) . | P value . | Steroid dose per day . | Risk . | HR (95% CI) . | P value . | |
| RSV | >2 mg/kg | +/− | 1.4 (0.4-5.2) | .193 | >2 mg/kg | + + + | 3.3 (1.7-6.3) | <.00111 |
| Influenza | ≥1 mg/kg | +/− | 0.8 (0.2-2.4) | .6045 | ≥1 mg/kg | +/− | 1.1 (0.3-3.5) | .8745 |
| PIV | >2 mg/kg | + + + | 4.6 (1.2-17.0) | .0274 | >2 mg/kg | + + + | 3.2 (1.5-7.2) | .00413 |
| HMPV or RSV | Not reported in the combined cohort | Any steroids | + + + | 5.0 (1.8-14) | .00216 | |||
| ≥1 mg/kg | + + + + | 7.1 (2.3-22) | <.00116 | |||||
CI, confidence interval; HR, hazard ratio.
Prevention strategies
Infection control practices
Several consensus guidelines outline specific recommendations for the prevention of respiratory viral infections through infection prevention and control practices.19,20,39,75 Patients with suspected respiratory viral infections (based on presence of symptoms) should be empirically placed on contact plus droplet precautions and diagnostic testing promptly initiated. Hand hygiene is extremely important, as most respiratory viruses are transmitted through direct contact. Symptomatic health care workers should be restricted from patient contact and symptomatic visitors should be actively excluded from visitation until symptoms are completely resolved.76 Importantly, prolonged shedding frequently occurs in immunocompromised individuals17,21,77-79 ; thus, guidelines for HCT recipients recommend appropriate isolation be maintained for at least the duration of clinical illness, hospitalization, or viral shedding to prevent transmission.19
Influenza vaccination
Inactivated influenza vaccine (IIV) is recommended for all patients ≥6 months of age with hematologic malignancies, although Infectious Diseases Society of America guidelines do not recommend vaccination in patients receiving intensive chemotherapy such as induction or consolidation chemotherapy for acute leukemia or those who have received anti–B-cell antibodies in the last 6 months.39,80 Though immune responses are generally lower in children with malignancies,81 a recent Cochrane review demonstrated reductions in respiratory infections and hospitalization; however, the quality of evidence is low.82 In adults with cancer, vaccination is associated with lower mortality, although data are observational and randomized trials are lacking.83,84 Patients >65 years of age may demonstrate reduced antibody responses to influenza vaccination and use of high-dose IIV formulations reduced laboratory-confirmed influenza in a large multicenter trial.85 We offer high-dose IIV to patients >65 years; of note, data in immunosuppressed patients are limited. Live attenuated influenza vaccine (LAIV) should not be used for immunosuppressed patients.19,80 One exception would be in an outbreak setting in which LAIV is determined to be a more effective option due to strain type. We offer quadrivalent vaccine when available.
Timing of influenza vaccination following HCT depends on local epidemiology, but immune responses are likely more effective later after HCT. HCT recipients aged ≥6 months should receive IIV annually starting 6 months after transplant or starting 4 months after transplant during a community outbreak of influenza80,86 with second doses recommended for children and considered for many adults, depending on time from transplant. Pretransplant vaccination may be appropriate in reduced-intensity HCT, where host immunity is expected to extend into the early transplant period; several studies have shown protective antibody levels with pretransplant vaccination of various pathogens including influenza.87-89 Influenza vaccination is also recommended for all family members, close contacts, and health care workers caring for immunocompromised patients. LAIV is not recommended for household contacts of HCT recipients <2 months posttransplant or with severe immunosuppression due to GVHD.80 However, if LAIV is given to persons caring for severely immunosuppressed patients, Centers for Disease Control and Prevention guidelines recommend avoidance of contact for 7 days following vaccination.90
Influenza chemoprophylaxis
Postexposure chemoprophylaxis with NAIs should be considered in immunosuppressed patients who are in close contact with confirmed influenza cases or during influenza outbreaks.19 In outbreak settings, HCT recipients should receive vaccine immediately if ≥4 months posttransplant, and chemoprophylaxis with oseltamivir or zanamivir should be initiated for 2 weeks after vaccination while immunity develops.19 Chemoprophylaxis is also recommended for patients <24 months after HCT or who are ≥24 months post-HCT and substantially immunocompromised, regardless of vaccination history.91,92 Oseltamivir resistance may emerge when using widespread prophylaxis93,94 ; drug resistance patterns of circulating strains should be considered when initiating prophylaxis and/or preemptive therapy.
Palivizumab.
Palivizumab is approved for prevention of RSV bronchiolitis in high-risk immunocompetent infants (age ≤2 years).95 Palivizumab may be considered in pediatric HCT recipients ≤2 years during RSV season; prophylaxis is not indicated in older children or adults.19,20 Palivizumab can be considered in outbreak settings96,97 but would come at substantial cost, especially in adults.
Novel therapies
Antivirals
Several antivirals are in development for treatment of RSV infection, including in immunocompromised patients and infants (Figure 2). GS-5806 is an oral RSV entry inhibitor that demonstrated reductions in viral load and clinical severity in phase 1 studies.98-100 Phase 2 trials are underway in hospitalized and adults transplant recipients.101-104 ALS-8176, a nucleoside analog targeting RSV polymerase, demonstrated reduction of viral load and decreased disease severity in a human challenge model.105 Studies in hospitalized infants are underway.106 ALN-RSV01, a small interfering RNA, was effective in a challenge model107 and reduced cumulative daily symptom scores in lung transplant recipients.108,109
Influenza antivirals continue to be an important area of investigation.110 IV zanamivir used under the US Food and Drug Administration (FDA) eIND may be useful in strains with H275Y oseltamivir resistance.111 In healthy individuals, an open-label phase 2 study demonstrated good safety and a reduction in viral load112 ; a randomized trial comparing efficacy to oseltamivir is nearing completion.113 Laninamivir (prodrug laninamivir octanoate) is a single-dose inhaled agent approved in Japan; in a large randomized trial, laninamivir octanoate reduced time to illness alleviation and was effective against oseltamivir resistant virus.114 Phase 3 results of favipiravir, an inhibitor of the RNA-dependent RNA polymerase complex, are forthcoming.115 Nitazoxanide reduced symptom duration in phase 2b/3 trials in adults and adolescents with uncomplicated influenza116 ; a phase 3 trial is underway.117
DAS181 is a recombinant fusion protein that cleaves sialic acid residues from respiratory epithelial cell surfaces and prevents influenza viral fusion and entry.118-121 A phase 2 trial demonstrated reduction in influenza viral load in healthy adults122 ; no data for immunocompromised hosts exist. DAS181 is also active against PIV and was used to treat several immunocompromised patients with PIV infection.123-126 A phase 2 randomized trial of DAS181 in immunocompromised hosts with PIV LRTI is underway.127
Several agents have shown efficacy against HRV and enteroviruses. Oral pleconaril, a capsid binder, was effective in 2 randomized trials128 ; however, its future development remains unclear after initial FDA rejection due to concerns regarding viral resistance and reduced effectiveness of oral contraceptives.129 Vapendavir, another capsid binder, decreased HRV viral load in an experimental infection model in healthy volunteers130 and decreased symptom severity in mild asthmatics131 ; a trial in moderate to severe asthmatics with HRV is underway.132 Some studies suggest HRV-C species be inherently resistant to capsid binders,133,134 which may have implications for drug development.
Antibody-based therapy
High-titer RSV immunoglobulin was evaluated in phase 2 trials in immunocompromised adults with RSV URTI and LRTI135,136 ; further study is needed to determine efficacy. Motavizumab, a humanized IgG1 monoclonal antibody with higher affinity for RSV than palivizumab, failed to show a reduction of viral load or illness severity in hospitalized infants with RSV LRTI137 but reduced health care attended visits in healthy infants.138 MEDI8897 is a monoclonal antibody and promising prophylactic agent with an extended half-life currently in phase 1b/2a trials in healthy preterm infants.139
Several monoclonals are under investigation for influenza with and without concomitant oseltamivir (Figure 2).140-143 IV hyperimmune immunoglobulin increased hemagglutination inhibition antibody responses,144 and a phase 3 trial is underway.145 High- versus low-titer anti-influenza plasma for treatment of severe influenza A is also under evaluation.146
Omalizumab, an anti-IgE monoclonal antibody FDA approved for treatment of moderate to severe allergic asthma, is in phase 2 trials in an HRV challenge model.147
Vaccines
Several candidate vaccines for RSV and PIV are being evaluated in infants, children, healthy and pregnant women, and older adults,27,148-152 including bivalent RSV/PIV vaccines.153,154 RSV vaccine trials are underway in healthy women and older adults.149 Several vaccine candidates are being evaluated in animals and humans for HMPV.155 Both donor and recipient vaccination strategies should be studied in the HCT setting based on encouraging results herpesvirus vaccines.156-159
Adoptive T-cell therapy
Adoptive T-cell therapy with pathogen-specific T cells is an emerging field.160,161 Recent evidence of broad-spectrum T cells for treatment of multiple herpes-group viruses has been described.162 As cell processing and expansion technology improves, this therapy has the potential to impact outcomes in HCT recipients with respiratory viral infections.
Future directions
Diagnostic information of respiratory virus infections is dramatically increasing due to widespread availability of multiplex PCR testing, resulting in better characterization of disease burden while simultaneously creating new challenges for clinicians as demonstrated in the cases. Although most infections are mild and self-limited, severe clinical disease causing hospitalization, critical illness, chronic lung disease, and death can occur. Although these complications are well characterized for RSV, PIV, and influenza after HCT, further study is needed for other respiratory viruses in HCT and non-HCT immunosuppressed patients. Validation of proposed severity scores, identification of laboratory biomarkers for progressive disease, and more data on the impact of pretransplant infections are critically important for respiratory viruses. Additionally, the mechanisms of acquisition and determinants of transmission need further study, and these will inform infection prevention strategies. Finally, development of new therapeutics and vaccines and systematic evaluation of new and existing treatments are needed.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Lisa Chung and Ryan Owens for their graphic design support, and Steve Pergam for additional infection prevention input.
This work was supported in part by the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (K23AI114844), and the NIH National Heart, Lung, and Blood Institute (K24HL03294).
Authorship
Contribution: A.W., J.A.E., and M.B. researched the topic and wrote the paper.
Conflict-of-interest disclosure: J.A.E. received research support from Gilead Sciences, Alios BioPharma, GlaxoSmithKline, Pfizer Inc., Roche, and Chimerix Inc., and served as consultant for Pfizer Inc. and Gilead Sciences. M.B. received research support from Gilead Sciences, Ansun Biopharma, GlaxoSmithKline, and Chimerix Inc., and served as a consultant for Gilead Sciences, Biota Pharmaceuticals, Chimerix Inc., Humab Biomed, and Pulmocide Ltd. A.W. declares no competing financial interests.
Correspondence: Michael Boeckh, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailstop E4-100, Seattle, WA 98109; e-mail: mboeckh@fredhutch.org.